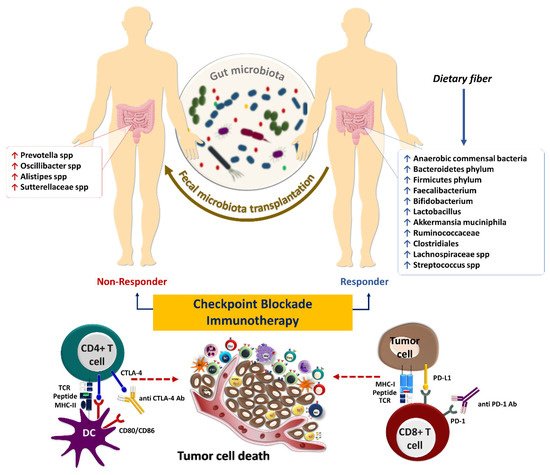
2. Gut Microbiota and Anti-Tumor Immunity
Innate and adaptive immune responses are vital components of the anti-tumor immunity against cancer. Diverse immune agents mediate tumor immune surveillance, but T-cell-mediated cytotoxicity is the principal mechanism of anti-tumor immunity. T-cells are central in anti-tumor response due to their ability to recognize specific peptides through the interaction of MHC-TCR, their cytotoxic effects, and their ability to exhibit immunological memory. Mainly, CD4
+ and CD8
+ T-cells are responsible for preventing tumor growth and cancer development. Therefore, ICIs mainly rely on T-cells for their efficacy. Notably, the gut microbiota is a tumor-extrinsic factor that can modulate anti-tumor defense mechanisms and impact the efficacy of cancer immunotherapy with ICIs (
Figure 13).
Figure 13. The influence of gut microbiota on the response to immune checkpoint inhibitors in cancer immunotherapy. Some microbes, including anaerobic commensal bacteria, which increase following sufficient dietary fiber intake, are associated with beneficial outcomes to checkpoint blockade immunotherapies such as anti-PD-1/PD-L1 and anti-CTLA4 antibodies
[9][79]. Fecal microbiota transplantation could potentially improve the response in patients who failed to respond to such therapies.
The intestinal microbiota influences innate immune cells, including neutrophils, macrophages, NK cells, and γδ T-cells. Animal studies have shown that
Bifidobacterium longum 51A could mediate the CXCL1 production and increase the accumulation of neutrophils. Moreover, oral treatment with
Bifidobacterium longum 51A could enhance the myeloperoxidase activity of neutrophils and pro-inflammatory cytokine production, such as IL-6 and TNF-α
[10][11][80,81]. Lakritz J et al. have shown that dietary administration of
Lactobacillus reuteri is associated with reduced circulatory neutrophils and increased Foxp3
+ Tregs
[12][82]. It has also been investigated that the depletion of CD4
+CD25
+ Tregs is associated with mast cell accumulations in the mammary gland, increased mammary hyperplastic, and preneoplastic lesions in human epidermal growth factor receptor 2 (HER2) transgenic mice treated with
Lactobacillus reuteri [12][82]. Furthermore, dietary administration
of Lactobacillus reuteri in animals susceptible to breast cancer and on a high-fat diet decreased systemic inflammation and enhanced tumor inhibition
[12][82].
Tumor-associated macrophages (TAM) are classified as anti-tumor M1 or pro-tumor M2 phenotypes. Microbiota perturbation following antibiotic treatment has been demonstrated to induce M2 macrophages, which partially promote tumorigenesis mediated by epithelial-mesenchymal transition (EMT) through TLR4/IL-10 signaling pathway
[13][14][83,84]. Furthermore,
Escherichia coli colonization of the colon contributes to M2 macrophage polarization through TLR-4, which could promote tumor metastasis in CRC
[15][66]. Also, M2 macrophages enhance the expression of PD-L1 in TME, potentially Reply to reviewer #suppressing the response to ICIs
[16][85]. Moreover, tryptophan metabolism by intestinal microbiota induces the immunosuppressive phenotype of TAMs, most likely related to accelerated progression and high mortality rate in pancreatic ductal adenocarcinoma (PDAC)
[17][86].
MDSCs are the hallmark of chronic inflammation in tissues. They are commonly present in tumors contributing to the immunosuppressive nature of TME. It has been shown that
Bacteroides fragilis-derived IL-17 could potentially induce MDSCs in a Th17-dependent manner to promote colon tumorigenesis in MinApc
+/− mice
[18][87]. NK cells are the principal innate immune arm involved in anti-tumor immunity through cytotoxic activity against tumor cells that escaped from CD8
+ T-cell-mediated immunity by downregulating MHC-I
[19][88]. The intestinal microbiota, such as
Fusobacterium nucleatum, produce fatty-acid-binding protein 2 (Fap2) as an outer membrane protein that potentially binds to T-cell immunoglobulin and ITIM domain (TIGIT) receptors expressed on NK cells inhibiting the cytotoxic effects of these cells
[20][89]. In addition, gut microbiota composition is positively associated with a high percentage of NK cells and a favorable response to ICIs in patients with non-small cell lung cancer (NSCLC)
[21][90]. Hence, it is reasonable to presume that the disturbance of microbiota following antibiotic treatments may result in the reduced cytotoxic effect of NK cells and their tumor immune surveillance. For example, it has been shown that administration of azithromycin downregulates cytokine production and cytotoxic effects of NK cells, negatively impacting their anti-tumor functions
[22][91].
The gut microbiota could also impact adaptive immune responses targeting tumor cells.
Bifidobacterium spp. activate tumor-specific T-cells, increase the accumulation of CD8
+ T-cells within melanoma and bladder tumors, and enhance IFN-γ production, which could slow down the growth of cancer cells by downregulating the NF-kB signaling pathway
[23][24][92,93]. Moreover,
Bifidobacterium strain enhances the efficacy of anti-tumor immune responses in colon cancer-bearing mice by increasing CD4
+ and CD8
+ T-cells, NK cells, and the CD4
+/Treg, CD8
+/Treg, and effector CD8
+/Treg ratios
[25][94]. In addition, the gut microbiota modulates the abundance of IFN-γ-producing CD8
+ T-cells to influence colitis-associated tumorigenesis
[26][95]. It has been observed that
Prevotellaceae and
Anaeroplasmataceae families are predictive of high and low tumor burdens of colon cancer, respectively
[26][95]. Li Y et al. indicated that bacterial strains, especially members of
Bacteroides and
Lactobacillus are associated with improved anti-tumor immunity and higher infiltration of tumors by tumor-specific CD45
+CD4
+CD8
+ T-cells and enhanced IFN-γ, TNF-α, and IL-2 production. This, in turn, could restrict melanoma growth in Rnf5
−/− mice
[27][96]. In general, the gut microbiota and their metabolites influence anti-tumor immunity through different mechanisms, such as the development of Th1 and Th17 cells, induction of pro-inflammatory cytokines, and activation of MDSCs and NK cells. Microbiota-derived epitopes potentially stimulate antigen-presenting cells for further T-cell development and cytokine production, improving the systemic response to cancer immunotherapy. Considering the essential role of gut microbiota in balancing anti-tumor versus pro-tumor immune responses, it is vital to develop microbiome screening and therapeutic strategies that can help tip the balance in favor of anti-tumor immunity. Given the negative role of antibiotics on some critical components of innate and adaptive immunity, it is crucial to avoid broad-spectrum antibiotics in cancer patients receiving ICI therapy as much as possible. Further clinical studies are required to determine the potential benefit of microbiome supportive therapies such as FMT from healthy donors in cancer patients that require antibiotic treatment.
3. Gut Microbiota and Response to Systemic Therapy, including Immunotherapy
The interaction of immune checkpoint molecules such as PD-1 and its ligand, PD-L1, suppresses T-cell function and infiltration to the TME. The PD-1/PD-L1 interaction contributes to immune tolerance and, ultimately, immune escape of tumor cells
[28][97]. The interaction of PD-1, mainly expressed on T-cells in the late phase of their activation, with its ligand PD-L1, expressed on tumor cells or other cells in the TME, inhibits the activation and effector functions of tumor-specific T-cells
[29][98]. In contrast, CTLA-4 is expressed in the early phase of T-cell activation and competes with CD28 expressed on the surface of activated T-cells, with higher affinity, in binding to CD80 and CD86 on antigen-presenting cells preventing proper co-stimulatory signals for T-cell activation. Furthermore, Tregs constitutively express CTLA-4, allowing them to inhibit the activation of conventional T-cells
[30][99]. Hence, ICIs, including anti-PD-1 (nivolumab and pembrolizumab), anti-PD-L1 (durvalumab, avelumab, and atezolizumab), and anti-CTLA-4 (ipilimumab and tremelimumab) antibodies have been developed for cancer immunotherapy
[31][100]. Most patients remain unresponsive to ICIs despite the ability of these drugs to reinvigorate tumor-reactive T-cells in clinical settings
[32][101]. Therefore, primary resistance to ICIs is an immense clinical problem that requires novel combination treatment strategies to improve the efficacy of these drugs.
Intratumoral microbes possibly mediate resistance to immunotherapy with ICIs and other forms of systemic therapy such as chemotherapy. In some cases, bacteria are found in patients’ tumors and genetically engineered mouse models of pancreatic cancer that are associated with a more immunosuppressive TME
[33][102]. It can be postulated that targeting intratumoral microbes with antibiotics could modulate chemotherapy resistance due to the direct tumor-supportive roles of intratumoral bacteria, such as bacterial-induced autophagy in tumor cells
[34][103]. Moreover, intratumoral bacteria possibly diminish the efficacy of systemic cancer therapy via metabolizing the chemotherapeutic drug to its inactive form
[35][104]. Therefore, lower chemotherapy drug concentrations can be achieved due to the presence of bacteria in human tumors than in other organs
[36][105].
Some intratumoral bacteria can negatively impact anti-tumor immunity. In contrast, others can potentially prevent cancer progression by providing bacterial antigens in the tumor that can mimic neoantigens and activate anti-tumor immunity. For example, RNA sequencing and immunopeptidomics analysis have recently identified 248 and 35 unique HLA-I and HLA-II peptides derived from 41 intratumoral bacterial species from 17 metastatic melanoma tumors. Microbial neoantigens in melanoma tumors are processed, presented, and recognized by T-cells
[36][105]. These findings confirmed that cancerous tissue could present bacterial neoantigens to tumor-infiltrating T-cells and reinforce immune TME. Furthermore, the presence of microbes in the tumor can potentially improve dendritic cell maturation. Indeed, dendritic cells stimulated with live or heat-killed commensal bacteria can express co-stimulation/maturation markers and produce pro-inflammatory cytokine/chemokine, such as IL-1β and TNF-α
[37][106]. Also, combining anti-PD-1 immunotherapy with bacterial therapy using
Clostridiales strains cleared almost all tumor cells and reduced the volume and weight of melanoma tumors
[38][107]. Therefore, clinical studies based on bacteria-based therapies in the form of complete or partial consortia are warranted to sensitize tumors to ICI therapy.
The resemblance of tumor-associated antigens and microbiota-derived epitopes potentially supports anti-tumor immunity. However, the intestinal microbiota has a dual effect on immunotherapy by enhancing or diminishing anti-tumor immune responses
[39][108]. For instance, bacterial species including
Faecalibacterium,
Bifidobacterium,
Lactobacillus,
Akkermansia muciniphila, and
Ruminococcaceae spp. play a considerable role in anti-tumor immune surveillance as well as the response to ICIs therapy
[40][41][109,110]. Several seminal studies have already shown that intestinal microbiota composition is perturbed during cancer progression
[42][43][44][111,112,113]. Microbiome sequencing and immune profiling of 233 patients with metastatic melanoma who received anti-PD-1 immunotherapy have shown that those with a more diverse gut microbiome had a higher ORR and improved survival outcomes. These findings indicate that a reduction in microbial diversity known as dysbiosis can result in poor response to ICIs
[45][114]. Analysis of fecal microbiome signatures of 94 melanoma patients who received anti-PD-1 immunotherapy showed that
Ruminococcus (Mediterraneibacter) torques, Blautia producta, Blautia wexlerae, Blautia hansenii, Eubacterium rectale, Ruminococcus (Mediterraneibacter) gnavus, and
Anaerostipes hadrus are increased in non-progressors. In comparison, the stool samples of progressors are enriched with
Prevotella spp.,
Oscillibacter spp.,
Alistipes spp., and
Sutterellaceae spp. Moreover, transcriptomic analyses of fecal samples of those patients have identified a remarkable upregulation of superoxide dismutase (SOD2), pro-inflammatory cytokines such as IL-1β and CXCL8, transcription factors NFKBIZ, NFKBIA, TNFAIP3, and LITAF in progressors. Fecal samples of progressor also had an abundance of inflammatory cells, including dendritic cells, monocytes, macrophages, and neutrophils
[46][115]. Furthermore, shotgun metagenomic sequencing of fecal samples from 165 non-resectable advanced (stage III or IV) cutaneous melanoma patients prior to immunotherapy with ICIs including nivolumab, pembrolizumab or ipilimumab, or a combination of ipilimumab and nivolumab revealed a significant association between the composition of the gut microbiome especially the special panel of
Bifidobacterium pseudocatenulatum,
Roseburia spp. and
Akkermansia muciniphila with ORR and PFS
[47][116].
The diversity of the gut microbiome composition is also correlated with the survival rates in response to chemoradiation in patients with cervical cancer
[48][117]. In a prospective observational study on microbial composition in patients with metastatic renal cell carcinoma (mRCC) who had received nivolumab or nivolumab plus ipilimumab, it was demonstrated that the high diversity of gut microbiome profiles is strongly linked to the benefits in treatment outcomes
[49][118]. In a randomized clinical trial of bacterial therapy combined with dual immunotherapy, fecal metagenomic sequencing of 30 mRCC patients with histology of clear cell and/or sarcomatoid and intermediate- or poor-risk disease demonstrated that ORR and PFS were significantly longer in patients who received nivolumab plus ipilimumab with CBM588 (a butyrate-producing strain of
Clostridium butyricum)
[50][119]. In addition, Jin Y et al. showed that in thirty-seven patients with metastatic, advanced stage IIIb/IV or recurrent NSCLC who were recruited from two clinical trials, CheckMate 078 [NCT02613507] and CheckMate 870 [NCT03195491] and treated with nivolumab there is a strong association between intestinal microbiome diversity and the responses to anti–PD-1 immunotherapy
[21][90]. The studies mentioned above indicate that gut microbiome diversity is associated with better outcomes. However, prospective studies that would couple microbiome profiling in cancer patients receiving systemic or localized therapy with their clinical outcome can further determine whether diversity can be used as a predictive biomarker of response to systemic or localized therapy in various cancers. Furthermore, microbiome-modifying strategies, such as complete consortia FMT from healthy donors that can potentially increase gut microbiome diversity, can be used as supportive therapy for cancer patients receiving systemic or localized treatment.
A multicenter and retrospective study conducted by Takada K et al. has demonstrated that probiotics are linked to beneficial clinical outcomes in patients with advanced or recurrent NSCLC treated with anti-PD-1 monotherapy
[51][120]. However, another study has reported paradoxical results in melanoma patients who took probiotic supplements while receiving immunotherapy and had a worse survival outcome. The obtained results are in line with the outcome of probiotic-treated mice, which have a lower frequency of IFN-γ-producing CD8
+ T-cells in TME and impaired anti-tumor response compared to the controls
[52][121]. A cohort study of 338 patients with NSCLC has demonstrated that intestinal
Akkermansia muciniphila is significantly accompanied by clinical benefits with increased response rates and OS following PD-1 blockade
[53][122]. Notably, the enhancement of bacterial compositions directly contributes to the efficacy of ICIs and improves clinical outcomes in cancer patients
[54][123]. Modifying the gut microbiota in immunotherapy-refractory melanoma patients sensitized their tumors to anti-PD1 rechallenge
[45][114]. FMT from patients with metastatic melanoma who had previously been treated with anti-PD-1 monotherapy and achieved complete response for at least one year to immunotherapy-refractory melanoma patients re-sensitized 30% of the treated patients to anti-PD1 treatment. FMT from patient donors modulated the immune cell infiltration and gene expression profiles in the TME
[55][22]. Additionally, in another prospective study, FMT from long-term responder melanoma patients to anti-PD-1-refractory patients sensitized patients to anti-PD-1 rechallenge, further establishing the role of the gut microbiome in modulating response to immunotherapy
[56][124]. In responding patients, the gut microbiota significantly shifted toward donor microbiota after FMT, and responders had decreased IL-8, IL-18, and CCL2 levels in their serum post-FMT
[56][124]. Furthermore, FMT from long-term survivor patients with advanced PDAC by oral gavage to a mouse model of pancreatic cancer previously treated with antibiotics demonstrated active modification of the tumor microbiota with enriched
Clostridiales, which inhibited tumor growth in an IFN-γ-producing CD8
+ T-cell-dependent manner. In contrast, FMT from PDAC short term-survivors to mice resulted in an increased CD4
+ Foxp3
+ Tregs and MDSC infiltration
[57][125].
It has been reported that intestinal microbiota composition influences the immunological complications of ICIs in solid tumors. For instance, Dubin K et al. have reported that microbiome composition in patients with metastatic melanoma who received ipilimumab treatment is significantly correlated with the development of immune-mediated colitis
[58][126]. McCulloch JA et al. have reported that the abundance of
Lachnospiraceae spp. and
Streptococcus spp. are linked to the favorable clinical response and immune-related adverse events, respectively, upon anti-PD-1 treatment of melanoma patients
[46][115]. It has also been demonstrated that increased bacterial species of
Bacteroidetes phylum in the gut is significantly associated with resistance to the development of checkpoint-blockade-induced colitis
[58][126]. Identifying the individual microbial species responsible for immunomodulation could open a new horizon toward personalized medicine in cancer immunotherapy. Hence, the microbiome has been identified as a robust predictive biomarker in response to immunotherapy, mainly the blockade of immune checkpoints. Intestinal
Bifidobacterium pseudolongum-driven inosine metabolites promote Th1 differentiation and activation, shaping a robust immune response following anti-CTLA-4 and anti-PD-L1 immunotherapy in preclinical models of melanoma and CRC
[59][127]. A preclinical study on mouse models of CRC has indicated that oral administration of
Clostridiales strains actively leads to the intratumoral infiltration and activation of CD8
+ T-cells. It has also been reported that the
Enterococcus species secrete SagA enzyme leading to degradation of the bacterial cell wall, the release of muramyl peptide fragments, and activation of the NOD2 signaling pathway, which in turn improves the response to anti–PD-L1 immunotherapy in the mouse models of melanoma and colon cancer
[60][128]. Based on these findings, it could be proposed that treatment with some bacterial strains as a limited consortium of probiotic therapy may improve the efficacy of anti-PD-1/PD-L1 therapy.