Breast cancer is the most frequently diagnosed cancer and a common cause of cancer-related death in women. It is well recognized that obesity is associated with an enhanced risk of more aggressive breast cancer as well as reduced patient survival. Adipose tissue is the major microenvironment of breast cancer. Obesity changes the composition, structure, and function of adipose tissue, which is associated with inflammation and metabolic dysfunction. Interestingly, adipose tissue is rich in ASCs/MSCs, and obesity alters the properties and functions of these cells. As a key component of the mammary stroma, ASCs play essential roles in the breast cancer microenvironment. The crosstalk between ASCs and breast cancer cells is multilateral and can occur both directly through cell–cell contact and indirectly via the secretome released by ASC/MSC, which is considered to be the main effector of their supportive, angiogenic, and immunomodulatory functions.
- ASCs/MSCs
- obesity
- breast cancer
- tumor microenvironment
- cancer-associated fibroblasts
- cancer-associated stem cells
- epithelial–mesenchymal transition
- therapy resistance
1. Introduction
2. Crosstalk between ASCs and Breast Cancer Cells
The crosstalk between ASCs and breast cancer cells can occur directly via cell–cell interaction and indirectly via the secretome released by the cells. Cancer cells secrete numerous chemotaxis signals [11][59], which recruit ASCs from local adipose tissues as well as MSCs from bone marrow into malignant tissue [12][60]. Cancer-educated ASCs/MSCs may differentiate into cancer-associated ASCs/MSCs or cancer-associated fibroblasts (CAFs) [13][61]. These ASCs/MSCs in turn promote breast cancer progression [14][62]. In particular, the ASC/MSC secretome, which is composed of a large number of secreted proteins, peptides and extracellular vesicles (EVs), is considered to be the main effector of their regenerative, tropic, trophic, angiogenic, and immunomodulatory functions. Regarding the indirect manner, both breast cancer cells and ASCs/MSCs are potent in secreting a large number of soluble bioactive factors [13][15][61,63]. In particular, MSCs have the ability to migrate into malignant areas and stimulate cancer development by secreting a range of paracrine factors such as chemokines C-X-C ligand 1 (CXCL1), CXCL2, CXCL5, CXCL7, and CXCL12/stromal-cell-derived factor 1 (SDF1); cytokines such as IL6, IL8, and transforming growth factor β (TGFβ); and growth factors including epidermal growth factor (EGF), insulin-like growth factor 1 (IGF1), and vascular endothelial growth factor (VEGF) [13][16][17][58,61,64]. It has been shown that MSCs facilitate angiogenesis by paracrine secretion of angiogenic growth factors such as platelet-derived growth factor (PDGF) and VEGF [16][18][58,65]. ReseaOurchers' own studies also demonstrate that ASCs isolated from subcutaneous as well as visceral adipose tissue released numerous cytokines, chemokines, and growth factors involved in inflammation, angiogenesis, and cell migration and proliferation, such as IL6, IL8, TNFα, CXCL1/2/3, CXCL5, monocyte chemotactic and activating factor (CCL2), and EGF [19][20][21][32,48,66]. Moreover, both breast cancer cells and MSC/ASC-derived EVs are essential for the crosstalk between MSCs/ASCs and breast cancer cells [15][22][23][63,67,68]. EVs are a heterogeneous group of membrane-bound vesicles released from cells by invagination and budding. They facilitate cell-to-cell interactions via contact with neighboring cells or internalization by recipient cells, which includes fusion with membrane and endocytosis [24][69]. According to the biogenesis, biophysical properties, and function, EVs can be classified into three main subtypes, namely exosomes (30–150 nm), microvesicles (MVs) (50–1000 nm), and apoptotic blebs (1000–5000 nm) [25][70]. Among these EVs, exosomes and MVs are of particular importance in cell–cell communication [25][70]. Both of them contain lipids, proteins, and genetic material, such as DNA, messenger RNA (mRNA), microRNA (miRNA), and long non-coding RNAs (lncRNA) that can be delivered to and reprogram the recipient cells [26][71]. Studies have shown that MSC/ASC-EVs exert both inhibitory and promoting effects in several situations and different stages of breast cancer. Through the transfer of various tumor-related factors, EVs promote proliferation, angiogenesis, metastasis, and drug resistance of malignant tumor cells [27][28][72,73], as shown in breast cancer cells stimulated with Her2-loaded EVs [27][72]. These data suggest that ASCs/MSCs may secrete molecules that act in concert with the secretome of breast cancer cells to remodel the microenvironment. The direct crosstalk between ASCs and breast cancer cells is strictly dependent on their close contact that is established in the TME. Interestingly, tunneling nanotubes (TNTs) have emerged as a new important means of cell–cell communication. TNTs are thin membrane protrusions that connect cells over long distances allowing the exchange of various cellular components, including organelles, proteins, calcium ions, viruses, and bacteria [29][74]. Notably, TNTs are able to connect multiple cells forming functional cellular networks [30][75]. TNTs are therefore considered as novel bridges of intercellular communication in physiological and pathological cell processes [31][76]. Interestingly, MSCs have been shown to form TNTs and transfer mitochondria and other components to target cells [32][33][34][77,78,79]. This occurs under both physiological and pathological conditions, where cells are under stress, leading to changes in cellular energy metabolism and functions [31][76]. In this context, it is feasible to hypothesize that the protective role of ASCs/MSCs in breast cancer cell survival may be partially mediated through the formation of TNTs, in particular, when breast cancer cells are under stress from chemotherapy or radiotherapy. Moreover, the activation of several signaling pathways requires direct cell–cell contact via their membrane-bound ligands and receptors, such as the canonical Notch signaling pathway [35][80]. Notch signaling is linked to the maintenance of breast cancer stem cells [36][81] and induction of epithelial-to-mesenchymal transition (EMT) resulting in an increase in migration and invasion of breast cancer cells [37][82]. In fact, direct co-culture of obese ASCs enhanced Notch signaling in ER+ breast cancer cells co-responsible for radiation resistance [38][83]. Finally, cell–cell fusion, a process that merges the lipid bilayers of two different cells, plays a crucial role during embryonic development as well as in tissue regeneration [39][40][84,85]. Studies also provide evidence that cell–cell fusion is closely related to cancer development and metastasis [41][86]. Although this highly regulated process is not yet fully understood, bone-marrow-derived cells were reported to be able to fuse to cancer cells, and the fused hybrids acquired more malignant characteristics and enhanced self-renewal ability [42][87]. In line with this observation, MSCs were reported to fuse with diverse malignant cells to promote proliferation and metastasis, including with lung cancer cells [43][88], liver cancer cells [44][89], and gastric cancer cells [45][90]. In particular, it was demonstrated that MSCs were fused with breast cancer cells and promoted their metastatic capacity [46][91]. Recently, is has been revealed that ASCs are able to fuse spontaneously with breast cancer cells, where breast cancer stem cell (CSC) markers CD44+CD24−/lowEpCAM+ are enriched in this fused population [47][92]. These studies suggest cell fusion as a direct interaction between ASCs/MSCs and cancer cells. Further investigations are needed to explore the molecular mechanisms by which ASCs/MSCs and malignant cells are able to fuse and how this process promotes malignancy. In sum, as illustrated in Figure 1, the crosstalk between ASCs/MSCs and breast cancer cells is multilateral and majorly mediated by indirect patterns such as the secretion of soluble bioactive factors and EVs released by ASCs as well as breast cancer cells. Although observed mainly in vitro, the interaction of breast cancer cells with ASCs/MSCs may be supported by direct cell–cell contacts including the formation of TNTs, binding of membrane-bound ligands to receptors, and cell–cell fusion. These communications may reshape the TME and fuel breast cancer progression and therapy resistance.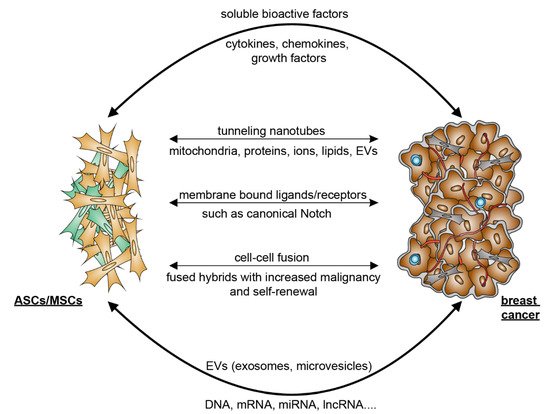
3. Mutual Interaction between ASCs/MSCs and Breast Cancer Cells
The communication between MSCs/ASCs and breast cancer cells has been an intensive research focus. The related studies are mostly performed using in vitro models to investigate the effects of ASCs/MSCs or their conditioned medium on proliferation, survival, migration, and invasion of breast cancer cell lines. Breast cancer cell lines are classified based on the status of three important cell surface receptors conventionally used for breast cancer subtyping, ER, PR, and HER2 [48][93]. Most studies used the following breast cancer cell lines: low metastatic breast cancer cell line BT474 (ER+, PR+, HER2+), MCF-7 (ER+, PR+, HER2−) and T47D (ER+, PR+, HER2−), metastatic breast cancer cell lines HCC1954 (ER−, PR−, HER2+, with wild type breast cancer gene 1 (BRCA1)), SKBR3 (ER−, PR−, HER2+, with wild type BRCA1), and MDA-MB-453 (ER−, PR−, HER2+, with wild type BRCA1), and highly metastatic breast cancer cell lines MDA-MB-231 (triple negative, with wild type BRCA1), MDA-MB-468 (triple negative, with wild type BRCA1), MDA-MB-436 (triple negative, mutated BRCA1), and SUM149 (triple negative, mutated BRCA1) [48][93]. Regarding ASCs/MSCs, while tumor adjacent cells [49][94] or “cancer-educated” MSCs [50][95] were recently used, most of the studies employed ASCs/MSCs isolated from non-breast sources, including abdominal adipose tissue, bone marrow, and peripheral blood [16][19][51][32,58,96]. It is well-known that ASCs/MSCs from different tissues and organs have distinct transcriptomic, biochemical, and secretory profiles, as well as biologic functions in tissue-specific homeostasis, immune modulation, and vasculogenesis/angiogenesis [52][97]. This, together with other diversities, such as different BMI, varied donor age, variable ASC/MSC passages, and individual experiment settings, often leads to inconclusive results with breast-cancer-supportive and -suppressive functions [16][53][58,98], which may not reflect the situation in vivo in breast cancer tissue.3.1. ASCs/MSCs Influence Breast Cancer and Related Molecular Mechanisms
Much attention has been paid to elucidating how ASCs/MSCs impact breast cancer cells as well as their TME (Table 1). Although their exact roles are not yet completely understood, ASCs/MSCs are described as both pro- or anti-tumorigenic, depending on the type and source of ASCs/MSCs, the use of breast cancer cell lines, and the in vitro or in vivo models. The studies concerning the anti-tumorigenic impact of MSCs are limited. MSCs have been reported to exert their negative impact on breast cancer by impairing angiogenesis via secretion of exosomes [54][99], reducing migration and invasion via the release of tissue inhibitor of metalloproteinase (TIMPs) [55][100], and decreasing breast tumor growth via down regulation of the STAT3 signaling pathway [56][101]. Nevertheless, the majority of studies report pro-tumorigenic effects of ASCs/MSCs on breast cancer cells, which are multilayered, as depicted in Table 1. The related molecular mechanisms are discussed in detail.| ASC/MSC Source | Study Design | Functions and Molecular Mechanisms | Ref. | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast cancer promoting effects of ASCs/MSCs derived from human tissues | ||||||||||||||||
| Human ASCs derived from visceral and subcutaneous adipose tissue | MCF7, MDA-MB-231 BC cell lines and MCF10A in vitro |
Direct co-culture of ASCs promoted proliferation of BC cells with an upregulation of | AURKA | , | PLK1 | , | BCL6 | , IL6, and IL8, whereas indirect co-culture led to EMT of BC cells via STAT3 and ERK signaling. | [19] | [32] | ||||||
| Human ASCs obtained from ATCC | MCF7 and BT474 BC cell lines in vitro | Supernatant of ASCs increased BC cell proliferation and radiotherapy resistance by IGF1 secretion. BC cells overexpressed IGF1R upon radiotherapy. | [57] | [102] | ||||||||||||
| Human BM-MSCs | MCF7, T47D, and SK-Br-3 BC cell lines in vitro |
BM-MSC supernatant increased proliferation of BC cells independent of IL6 and VEGF, but both signaling proteins stimulated migration by the activation of MAPK, AKT, and p38 MAPK. | [58] | [103] | ||||||||||||
| Human BC-derived MSCs | MCF7 BC cell line in vitro | Mammary MSCs increased proliferation and cisplatin resistance of MCF7 cells by triggering the IL6/STAT3 pathway. | [59] | [104] | ||||||||||||
| Human MSCs from primary BC tissue | Co-transplantation BC xenograft mouse model of MCF7 and MSCs in vivo and in vitro |
Mammary MSCs promoted BC proliferation and mammosphere formation via EGF/EGFR/AKT signaling. | [60] | [105] | ||||||||||||
| Human ASCs from adipose tissues | MCF-7, BT-474, T-47D, and 4T1 BC cell lines in vitro and in vivo |
PDGF-D secreted by ASCs stimulated tumor growth in vivo, mammosphere formation in vitro, and EMT in BC cells. | [61] | [106] | ||||||||||||
| Human MSCs from supraclavicular lymph node (LN-MSCs) and liver (Lv-MSCs) | MDA-MB-231, –436, –468, MCF7 BC cell lines, and MCF10A cells in vitro and in vivo |
The engulfment of MSCs by BC cells increased the gene expression of | WNT5A | , | MSR1 | , | ELMO1 | , | IL1RL2 | , | ZPLD1 | , and | SIRPB1. | This further increased BC cell migration, invasion, and mammosphere formation in vitro and the tumor metastasis in vivo. | [62] | [107] |
| Human ASCs from facial or abdominal liposuction | MCF7 BC cell line in vitro | ASCs co-cultured with MCF7 stimulated EMT in BC cells. The data also suggest that EMT was induced by the cross-interactions with the TGFβ/Smad and PI3K/AKT pathways. | [63] | [108] | ||||||||||||
| Human ASCs isolated from SAT via bariatric surgery, and mammary ASCs from subcutaneous breast preadipocytes | MCF7 and SUM149 BC cell lines in vitro, and orthotopic grafting of 4T1 cells into the mammary fat pad in vivo | Both ASCs subtypes suppressed the cytotoxicity of cisplatin and paclitaxel. Depletion of ASCs by D-CAN, a proapoptotic peptide targeting specific ASCs, reduced spontaneous BC lung metastases in a mouse allograft model and a BC xenograft model, when combined with cisplatin treatment. | [64] | [109] | ||||||||||||
| Human ASCs isolated from breast adipose tissues of breast cancer patients and normal individuals underwent cosmetic mammoplasty surgery | Breast tissue and BC tissue samples in vitro | ASCs isolated from breast cancer patients displayed elevated levels of IL10 and TGFβ1, and the supernatant stimulated the expression of IL4, TGFβ1, IL10, CCR4, and CD25 in PBLs. | [65] | [110] | ||||||||||||
| Human ASCs isolated from breast tumor (T-MSC) and normal breast adipose tissue (N-MSC) | Breast tissue and BC tissue samples in vitro, PBLs in vitro | The TME altered the secretome of T-MSCs with increased secretion of TGFβ, PGE2, IDO, VEGF, and lowered secretion of MMP2/9 compared to N-MSCs. T-MSCs also stimulated the proliferation of PBLs. | [66] | [111] | ||||||||||||
| Human ASCs isolated from normal breast adipose tissue (nASCs) or that of a woman with breast cancer (cASCs) | Breast tissue and BC tissue samples in vitro, B cells and Tregs in vitro | nASCs reduced proliferation of B cells in direct co-culture, and the TNFα | + | /IL10 | + | B cells ratio decreased in all co-cultures with ASCs, to a barely significantly higher extent in cASCs. nASCs shifted the cytokine profile of B cells toward an anti-inflammatory profile. | [67] | [112] | ||||||||
| Human ASCs isolated from the breast adipose tissue of reduction mammoplasty patients with different BMI | MCF7 and SUM159PT BC cell lines and HMEC breast cell line in vitro | Supernatant of all analyzed ASCs stimulated proliferation, migration, and invasion of breast cancer cells and increased the number of lipid droplets in their cytoplasm. This was mechanistically associated with the upregulated expression of the fatty acid receptor CD36, presenting the capacity of ASCs to induce metabolic reprogramming via CD36-mediated fatty acid uptake. | [68] | [113] | ||||||||||||
| Human primary subcutaneous pre-adipocytes (pre-hASCs, Lonza) | MCF7, T47D, ZR-75-1, SK-BR-3 BC cell lines and murine 3T3-L1 pre-adipocytes in vitro | Conditioned medium of ASCs stimulated proliferation and migration of MCF7, T47D, SK-BR-3, and ZR-75-1 cells. Additionally, supernatant of ASCs upregulated the expression of S100A7 and its knockdown abrogated the tumorigenic effect of ASCs on the tested breast cancer cells. | [69] | [114] | ||||||||||||
| Breast cancer promoting effects of ASCs/MSCs derived from murine tissue | ||||||||||||||||
| Murine MSCs derived from spontaneous lymphomas, mouse bone marrow, and mouse ears | Syngeneic tumor transplantation mouse model in vivo | TNFα dependent monocyte/macrophage recruitment led to increased tumor volume upon co-injection with MSCs, associated with CCR2 dependent immunosuppression of neutrophils, monocytes, and macrophages. | [70] | [115] | ||||||||||||
| Murine BM-MSCs and MSCs isolated from murine lung cancers | 4T1 BC mouse model in vivo |
BM-MSCs and MSCs from lung cancers were able to recruit CXCR2 | + | neutrophils into the tumor by TNFα via activation of CXCL1, CXCL2, and CXCL5 and promoted tumor metastasis. | [71] | [116] | ||||||||||
| Murine BM-MSCs | Murine mammary cancer cell lines PyMT-Luc, 17LC3-Luc and LLC in vitro |
Secretion of CXCL5 by BM-MSCs increased, but without significance, while proliferation of murine BC cell lines was unchanged, whereas CXCL1 and CXCL5 promoted BC cell migration. | [72] | [117] | ||||||||||||
| Murine and human BM-MSCs | 4T1 BC mouse model in vivo and in vitro |
Both types of BM-MSCs stimulated 4T1 BC cell proliferation in vivo and in vitro upon direct cell–cell contact. BM-MSCs also promoted vessel formation of HUVECs in vitro and in vivo in DU145 tumors via TGFβ, VEGF, and IL6 release. | [73] | [118] | ||||||||||||
| Murine ASCs isolated from abdominal cavity | 4T1 BC mouse cell line in vitro and CT26 murine colon cancer cell line in vitro |
Co-culture of ASCs induced stemcellrelated genes in cancer cells such as | SOX2 | , | NANOG | , | ALDH1, | and | ABCG2 | . ASCs accelerated tumor growth. Secretion of IL6 regulated stemcellrelated genes and activated JAK2/STAT3 in murine cancer cells. | [74] | [119] | ||||
| Breast cancer promoting effects of obese ASCs/MSCs | ||||||||||||||||
| Human ASCs isolated from breast cancer tissue of lean and obese patients | Human BC patient-derived xenograft cells in vivo | Adipsin secreted by obese ASCs stimulated factor B and C3a, which induced BC proliferation and expression of CSC genes | CD44 | , | CXCR4 | , | SNAI2 | , | SNAI1 | , | ZEB1, | and | BMI1. | [75] | [120] | |
| Human lean and obese ASCs isolated from abdominal lipo- aspirates of subcutaneous adipose tissue |
MCF7, ZR75, or T47D BC cell lines in vitro and MCF7 xenograft mouse model in vivo |
Leptin secreted from obese ASCs enhanced BC proliferation and increased the expression of EMT and metastasis-related genes such as | Serpine1 | , | MMP2, | and | IL6 | . | [76] | [121] | ||||||
| Human lean (ln) and obese (ob) ASCs from abdominal lipo- aspirates of subcutaneous adipose tissue |
MCF7 and MDA-MB-231 BC cell lines in vitro |
Increased proliferation of BC cells by leptin expression via estrogen stimulation and increased protein levels of CDKN2A, GSTP1, PGR, and ESR1 in BC cells co-cultured with ob-ASCs. | [77] | [122] | ||||||||||||
| Human and murine ASCs isolated from lean and obese individuals |
Tumor and stromal cell transplantation in a mammary mouse xenograft model in vivo and MCF7 BC cell line in vitro |
Obese ASCs secreted higher levels of IGF1, promoting tumor growth and metastasis, which could be partially ameliorated by weight loss. | [78] | [123] | ||||||||||||
| Human lean and obese ASCs from abdominal lipoaspirates of subcutaneous adipose tissue | BT20, MDA-MB-231, MDA-MB-468, MCF7, and HCC1806 BC cell lines in vitro and patient-derived xenograft mouse model | Obesity increased the tumorigenic capacity of ASCs indicated by increased EMT genes | Serpine1 | , | SNAI2, | and | TWIST1 | . This effect was likely mediated via leptin, since its knockdown led to reduced pro-metastatic effects of obese ASCs. |
[79] | [124] | ||||||
| Human ASCs isolated from lipoaspirate of subcutaneous adipose tissue from lean and obese patients. | MCF7, T47D, and ZR-75 BC cell lines in vitro | Obese ASCs induced a cancer-stem-like phenotype in BC cells with elevated gene expression of | Notch1 | , | Notch3 | , | DLL1, | and | JAG2 | . This led to radioresistance and reduced oxidative stress after radiation in co-cultured BC cells mediated by leptin. |
[38] | [83] | ||||
| Human lean and obese ASCs derived from mammary adipose tissue | MDA-MB231 BC cell line and MCF10AT1 in vitro |
Obese ASCs activated BC cell migration more effectively compared to lean ASCs by direct co-culture. Obese ASCs had an increased potential for ECM remodeling. | [80] | [125] | ||||||||||||
| Human lean and obese ASCs from abdominal lipoaspirates of subcutaneous adipose tissue | MCF7 BC cell line in vitro | The known CAF markers | NG2, ACTA2, VEGF, FAP, | and | FSP | were elevated in obese ASCs. Obese ASCs were more potent in inducing the gene expression of pro-tumorigenic factors in BC cells including | Serpin1, CCL5, TARC (CCL17 | ) | , IL24, IL6, IGFBP3, adiponectin, | and | leptin | . | [81] | [126] | ||
| Human lean and obese ASCs isolated from elective liposuction | MCF7 BC cell line in vitro and patient-derived mammary xenograft (PDX) mouse model in vivo |
The increased tumor growth rate observed in obese-ASCs-enriched PDX tumors was leptin dependent. The increased metastatic capacity was leptin independent and was associated with increased gene expression of | Serpine1 | and | ABCB1 | in tumor cells. | [82] | [127] | ||||||||
3.1.1. Promoting Proliferation and Survival
3.2. Breast Cancer Cells Educate ASCs/MSCs and Related Molecular Mechanisms
While ASCs/MSCs influence breast cancer cells, numerous investigations show that breast cancer as well as its TME “educate” their surrounding cells, including fibroblasts (FBs) and ASCs/MSCs, toward pro-tumorigenic phenotypes [101][220]. The most precisely characterized cells are FBs. As depicted in Table 2, multiple cancer-associated fibroblast (CAF) phenotypes have been identified during the last decade as key components of the TME with implications in tumor growth, therapy resistance, metastasis, ECM remodeling, and immune tolerance [102][103][104][34,205,221]. Interestingly, ASCs/MSCs are morphologically indistinguishable from fibroblasts. These two cell types share many common features including their surface marker composition, proliferation pattern, differentiation capacity, immunomodulation property, and, to some extent, even their gene expression profiles [105][106][222,223]. The major difference between these two cell types seems to be their methylation profile. While the general methylation patterns of MCSs are maintained in long-term culture and aging [107][224], the methylation of fibroblasts seems to decrease with aging or prolonged culture [108][225]. Indeed, ASCs/MSCs have been proposed to be immature FBs and one of the sources for FBs [105][222]. Recently, increasing evidence highlights that ASCs/MSCs are educated and de-differentiated by cancer cells and the TME, fueling malignancy and therapy resistance [109][110][226,227]. Cancer-cell-secreted factors and direct cancer cell–ASC/MSC contacts induce a pro-tumorigenic population of ASCs/MSCs, named cancer-associated MSCs (CA-MSCs) [98][143]. CA-MSCs have the ability to differentiate into multiple cell lineages, such as fibroblasts and adipocytes, suggesting that MSCs may play a key role in the generation of most stromal components of the TME. A number of reports have demonstrated that CA-MSCs differentiate into CAFs and cancer-associated adipocytes (CAAs) in the presence of malignant cells [60][111][105,228]. While the exact mechanisms underlying the de-differentiation of CA-MSC are not yet clear, this switch resulted in a highly secretory phenotype with increased secretion of bone morphogenetic protein (BMP2), BMP4, and IL6 [112][229]. In line with this observation, there was evidence suggesting that cancer-released TGFβ was able to activate the Smad signaling pathway in MSCs, which drove differentiation into a cancer-associated phenotype [113][230]. In other tumor entities, including lymphomas [70][115], lung [114][215], and gastric cancer [115][116][231,232], IL6, IL8, IL17, IL23, and TNFα secreted by monocytes, macrophages, neutrophils, and non-MSC stromal cells were shown to be capable of promoting malignant transition of ASCs/MSCs, which was associated with significantly increased metastatic rates and tumor growth [70][110][114][115][116][115,215,227,231,232]. Other molecular mechanisms proposed for CAF activation include Notch/Eph-ephrin signaling, ECM composition in the TME, DNA damage, physiological stress, inflammatory stimuli, RTK ligands, and TGFβ-mediated signaling [102][34]. Moreover, the primary cilium, a sensory organelle with an exceptionally high receptor density [117][233], was shown to play a critical role in the de-differentiation process of adipose progenitors toward a CAF phenotype by mediating TGFβ signaling [118][234]. All these studies suggest that diverse pathways are responsible for the activation of CAFs, and the TME is likely the major player in triggering the de-differentiation of ASCs/MSCs into different CAFs, depending on their cellular context and the tumor entity.| Fibroblast/CAF Source | Study Design | Functions and Molecular Mechanisms | Ref. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Impact of cancer cells on the fibroblast phenotype | ||||||||||||
| FBs and CAFs isolated from surgical explantation and human BM-MSCs obtained from AOU Meyer Hospital (Florence) | Co-culture experiments with FBs, CAFs, and BM-MSCs with PC3, DU145, and LNCaP prostate cancer cell lines in vitro | Prostate cancer cells secreted TGFβ1 and recruited BM-MSCs into the TME. This in turn led to an elevated secretion of TGFβ1 in cancer-educated BM-MSCs. Blocking TGFβ1 reduced the recruitment of BM-MSCs into the tumor as well as their trans-differentiation. | [113] | [230] | ||||||||
| Pancreatic ductal adenocarcinoma (PDAC) tissue | Single-cell RNA sequencing to characterize CAF subpopulations in PDAC | The analysis revealed intertumoral heterogeneity between CAFs, ductal cancer cells, and immune cells in extremely dense and loose types of PDACs. A highly metabolic active subtype (meCAFs) was identified. Patients with abundant meCAFs had a significantly increased risk for metastasis and poor prognosis. These patients, however, showed a highly increased response to immunotherapy. | [119] | [235] | ||||||||
| Murine normal pancreatic and cancer tissue | Single-cell RNA sequencing to characterize CAF subpopulations (normal vs. pancreatic cancer tissue) | The analysis revealed a landscape of CAFs in pancreatic cancer during in vivo tumor development. The LRRC15 | + | CAF lineage was shown to be TGFβ-dependent and correlated with a poor patient outcome treated with immunotherapy in multiple solid tumor entities. | [120] | [236] | ||||||
| Human and murine PDAC resection specimens and normal pancreas tissue | Single-cell RNA sequencing to characterize CAF subpopulations (human and murine) | The analysis from neoplastic and TME of human and mouse PDAC tumors displayed already described myCAFs and iCAFs with distinct gene expression profiles. It further revealed a novel subtype that expressed MHC class II and CD74 called “antigen-presenting CAFS (apCAFs)”. These cells activated antigen-specific CD4 | + | T cells. These immunomodulatory CAFs were likely associated with a reduced immune response of PDAC tumors. | [121] | [237] | ||||||
| Human breast and BC tissue | Single-cell RNA sequencing to characterize CAF subpopulations (normal vs. BC tissue) | The analysis identified different CAF subpopulations in BC tissue. CAF-S1 (CD29, FAP, α-SMA, PDGFRβ, FSP1, and CXCL12) was analyzed in detail. These cells induced an immunosuppressive TME by retaining CD4 | + | CD25 | + | T cells through the signaling of OX40L, PD-L2, and JAM2, and increased CD25 | + | FOXP3 | + | T lymphocytes, and B7H3, DPP4, and CD73 signaling. | [122] | [238] |
| Human BC tissue and metastatic lymph nodes tissue (LN) | Single-cell RNA sequencing to characterize CAF subpopulations (BC and LN tissue) and co-culture experiments with MCF7, MDA-MB-231, and T47D | The analysis identified four CAF subpopulations in LN. Two had a myCAF gene expression pattern, CAF-S1 and CAF-S4, accumulated in LN and correlated with cancer cell invasion. CAF-S1 stimulated cancer cell migration by stimulating EMT, through CXCL12 and TGFβ signaling. CAF-S4 induced cancer cell invasion through Notch signaling. Patients with a high ratio of CAF-S4 cells were prone to develop late distant metastases. | [123] | [239] | ||||||||
| Murine BC tissue and normal mammary fat pad tissue | Single-cell RNA sequencing to characterize CAF subpopulations (BC compared to pancreatic cancer tissue) | The study identified six CAF subpopulations in a triple-negative syngeneic breast cancer mouse model. Among these six subpopulations, myCAFs, iCAFs, and apCAFs were found to exist in BC cancers and PDAC. The subtype expressing MHC class II proteins similar to apCAFs were also found in normal breast/pancreas tissues, indicating that this specific subtype is not TME induced. The comparison to a pancreatic tumor model suggested that similar phenotypes exist in both cancer entities without a TME-specific subtype. | [124] | [240] | ||||||||
| Murine and human BC tissue and normal mammary fat pad tissue | Single-cell RNA sequencing to characterize CAF subpopulations (murine, human BC tissue vs. normal mammary fat pad tissue) and co-culture experiments with human MDA-MB-231 as well as murine 4T1 and EO771. | A negative selection strategy was used to analyze 768 single-cell RNA sequencing transcriptome data of mesenchymal cells in a BC mouse model. In this approach, three distinct CAF subpopulations were defined. These populations were named “vascular”-CAFs, “matrix”-CAFs and “development”-CAFs. The found gene signatures were further verified on the transcriptional and protein levels in various experimental cancers. Human tumors and every CAF gene profile were correlated with distinctive molecular functions. | [125] | [241] | ||||||||
| Normal breast, BC tissue samples, and metastatic lymph nodes obtained from surgery | Comparison of multiple genome transcriptomic RNA sequencings | These approaches revealed that most of the described cancer hallmark signaling pathways were significantly upregulated in triple-negative breast cancer with a highly enriched CAF population. BGN, a soluble secreted protein, was upregulated in CAFs compared to normal cancer-adjacent fibroblasts (NAFs). The expression was negatively associated with CD8 | + | T cells and poor prognostic outcomes. | [126] | [242] | ||||||
| Human primary bladder tumor tissues and adjacent normal mucosae tissues | Single-cell RNA sequencing to characterize CAF subpopulations (bladder cancer tissue vs. normal mucosae tissue) | iCAFs were identified as poor prognostic marker with potent pro-proliferation capacities, and their immunoregulatory function in the TME of bladder cancer was further deciphered. The LAMP3 | + | dendritic cell subgroup might be able to recruit regulatory T cells, which could be a step toward an immunosuppressive TME. | [127] | [243] | ||||||
