Coronavirus 2019 (COVID-19) has become a global threat with a mortality rate around 6%
[1]. In adults, the clinical manifestations of COVID-19 may or may not appear for the entire duration of the incubation period (2–14 days) and beyond, or they may be severe. Children are less likely to develop symptomatic infections and are less prone to serious illness
[2], although there are reports of children who have a disease called Multisystem Inflammatory Syndrome
[3][4][3,4]. COVID-19 belongs to the beta family of Coronaviridae and is known for the spike protein used to hook and infect the host cell
[5][6][7][5,6,7], conferring COVID-19 easy transmissivity and high pathogenicity,
[8] allowing the virus to: (i) fuse cell–cell and RNA release, (ii) start the replication cycle, and (iii) spread further to infect more cells
[9][10][9,10], ensuring greater transmissibility (R0 > 2). From the ultrastructural point of view, the virus, having a higher specificity than other viruses of the same family
[11], binds to the host via the binding domains of the angiotensin 2 converting enzyme receptor (ACE2), expressed more on the luminal surface of type II alveolar epithelial cells
[12], resulting in an increase in angiotensin-2 with relative increase in pulmonary vascular permeability and subsequent severe acute respiratory syndrome or multiorgan dysfunction
[13]. This clinical picture is due to the presence of acute inflammation, mainly CD4 and CD8 positive T lymphomonocytes, responsible for the recall of cytokines (interleukins such as IL-1β, IL-2R, IL-6, as well as interferon and tumor necrosis factor) and chemokines such as CCL-2, CCL-3, and CCL-10, which determine the hyperinflammatory state in COVID-19-positive patients
[14]. Following this process, there is therefore the desquamation of pneumocytes, the formation of fibrinoid exudates and pulmonary edema, and finally formation of hyaline membrane with consequent irreversible alveolar damage. Conversely, during the recovery phase, there is an increase in pro-coagulation factors and subsequent activation of the coagulation cascade with the formation of small and large peripheral vascular thrombi also known as disseminated intravascular coagulation
[13][15][13,15]. In more critically ill patients, laboratory tests also show hyperferritinaemia, elevated lactate dehydrogenase, and erythrocyte sedimentation rate
[16]. This pathological structure can also be associated with further complications at the olfactory level
[17] or at the visual level (eye pain, redness, and conjunctivitis), splenic and hepatic level where drug toxicity and immune-mediated damage play a role
[18], renal, neurological, and skin level such as erythematous rashes and urticaria, lympho-haematological
[19][20][21][22][23][24][25][26][19,20,21,22,23,24,25,26], and even at the level of the cardiovascular system
[27][28][29][27,28,29] where patients with SARS-CoV-2 have an increased risk of developing acute myocardial infarction following coronary spasm, hypoxic damage, microthrombi, direct vascular endothelial damage, hypercoagulability, and instability of the atherosclerotic plaque
[30]. It is also known that the downregulation of ACE2 and the increased stimulation of the angiotensin II receptor are associated with systemic hypotension, hypokalaemia, and lung damage
[31]. As is well known, iron represents one of the main elements in the fight against pathogens, given its ability to support the immune system during the viral replication phase. In fact, since high availability of iron is required for COVID-19 to allow the hydrolysis of the ATP required in this phase, the innate immune system will modify the bioavailability of iron downwards, limiting its ability to replicate and helping the body to fight the virus. Ferritin is the most important component of iron metabolism, and its main role is to store iron during the ferric state during infections. During the inflammatory phase, the serum iron concentration decreases and ferritin increases, causing hyperferritinemia and activation of macrophages. Regulating this mechanism is hepcidin, capable of regulating the concentration of intra- and extracellular iron thus depriving the pathogen of iron for the replication of COVID-19
[32][33][32,33]. The metabolic processes of iron are also generated by the generation of reactive oxygen species (ROS)
[34], which can cause mitochondrial dysfunction favoring multiple-organ damage as occurs during COVID-19 infection
[34][35][34,35]. Alterations at this level lead to an upregulation of mitochondrial genes and genes that respond to oxidative stress with further accumulations of intracellular iron. These generate ROS and reactive nitrogen and sulphur species, further contributing to the increase in the inflammatory response in COVID-19 disease. In fact, the same mitochondrial ROS also directly activates the production of proinflammatory cytokines that can alter mitochondrial homeostasis and mitochondrial respiration thus causing various systemic alterations
[36]. The inflammatory phase also leads to a depletion of the body’s natural antioxidant agents such as vit C, which in favorable physiological conditions protects cells, decreases oxidative stress and ROS, and improves the body’s circulatory function and its immune system. In critical conditions, its concentration drops precipitously so as to require intravenous administration to reach a quantity sufficient for the body. Although still in need of robust confirmation, on this basis clinical studies have been conducted on the treatment of COVID-19-positive patients, which have shown how this supplement can improve the critical condition of some patients
[37][38][37,38]. Similarly to vit C, vit D also has the same effect on oxidative stress and provides a protective effect from COVID-19 but, also in this case, further studies should be encouraged to shed light on the effects of vit D deficiency in COVID-19-positive patients
[39][40][41][39,40,41].
2. Microbiota and SARS-CoV-2 Infection
Lately, different authors have reported in literature the possibility, more than suggestive, of a connection between COVID-19 infection and intestinal microbiota dysbiosis, considering that, in COVID-19 patients with severe intestinal dysfunction, the presence of virions in fecal, oral, and gastrointestinal samples was found in a range between 2% and 36%
[42][43][44][45][46][47][48][49][50][51][42,43,44,45,46,47,48,49,50,51]. The most commonly reported intestinal symptoms related to COVID-19 could be mild as nausea and stomach discomfort or more intense such as vomiting and diarrhea
[27][52][53][54][55][27,52,53,54,55]. Moreover, variations of the intestinal microbiota, with an increase in opportunistic pathogenic germs and reduction of protective commensal bacteria, relate to fecal levels of SARS-CoV-2 and severity of symptoms from COVID-19. Furthermore, this microbiota alteration could continue even after the eradication of SARS-CoV-2 and after the remission of disease symptoms
[56]. Recent scientific evidence provided that the gravity of COVID-19 symptomatology is linked to comorbidity and advanced age since both aspects are connected to inflammation and alteration of the intestinal microbiota, in consideration of both the nature of the intestinal flora and its bacterial composition
[57]. Consequently, it has been assumed that an intervention directed at supporting the intestinal barrier and reducing the inflammatory stimulus by recommending a diet rich in fiber and fermenting foods could be suitable to reduce the infection and gastrointestinal symptoms linked to COVID-19
[58]. Therefore, it is supposed that COVID-19 infection might be capable of modifying and undermining the integrity of the gut microbiota with a consequent higher gravity of the disease and complications
[58]. Consequently, the intestinal biological components and its related dysbiosis are considered a potentially fundamental element to influence the adaptive response versus respiratory pathogens
[59]. Indeed, recent studies have reported that patients with COVID-19 infection and underlying comorbidity with consequently reduced gut microbiota diversity and greater intestinal permeability showed a worse prognosis
[52]. Confirmation of this evidence was the evaluation of how food microbes, such as probiotics or prebiotics, develop an antiviral effect against coronaviruses and could strengthen host immune functions. Moreover, a sensible decrease of Lactobacillus and Bifidobacterium species has been found in patients affected by COVID-19, even if the clinical meaning of this result is not yet defined
[60]. Therefore, intestinal microbiota
[61] could lead healthy subjects to an hyperinflammatory condition
[62], which enhances the harmful effects of COVID-19. Indeed, composition of the intestinal microbiota is involved in the production of inflammatory cytokines and has an important role in the induction, development, and correct function of the host immune system and its activity
[63]. In addition, ACE2 protein is widely distributed on the luminal surface of intestinal epithelial cell
[64][65][64,65]. Indeed, recent evidence has reported that the “cytokine storm” may be the key that aggravates the morbidity and mortality of the COVID-19 infection
[66] (
Figure 1), and there is even evidence that COVID-19 affects women less and causes a stronger T-cell response than males, leading to more effective viral clearance
[67][68][69][67,68,69].
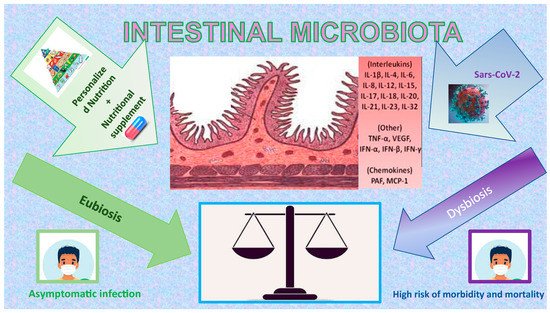
Figure 1. Impact of COVID-19, nutrition, and nutritional supplement on Intestinal Microbiota. The figure is a graphic illustration of how the correct use of macro- and micronutrients can be the ideal balance of our organism in the defence or rapid recovery to COVID-19, having a positive effect on the Intestinal Microbiota (green side), mainly for their antioxidant and anti-inflammatory properties.
3. Nutrition in Pregnancy during COVID-19 Infection
Pregnancy represents a special biological phase of the life of a woman and determines a lot of indicatory physiological changes involving all organ systems in the body with the main aim to sustain the growing fetus
[70][92]. Nutrition represents a pivotal point for the physiological changes of pregnancy and, in this regard, childbearing causes an increased water need in order to expand maternal blood water and increase cardiac output from 4 to 6 L/min and, thus, blood flow to the kidneys and utero-placental unit, decreasing blood pressure overall
[71][93]. The increase in tidal volume requires an average increase in oxygen demand in pregnancy of about 20% of minute ventilation. Breathing and nutrition are closely related processes. Through the digestive system, oxygen is used to oxidize the nutrients obtained from ingested foods and to obtain the necessary energy. Physiological changes in respiratory function in pregnancy allow the umbilical vein blood amount to be rich enough in oxygen for the fetal cellular respiration process
[72][94]. Nutrition means fetal growth; especially in the third trimester of pregnancy, hormones of placental origin determine a state of insulin resistance in the pregnant woman; a condition of hyperglycaemia is established, which maintains fetal growth and lipolysis to satisfy maternal needs
[73][95]. The mother’s requirement for increased insulin could be a biological solution to promote fetal growth, while insulin itself can remain the main growth factor alongside conception product development
[74][96]. It is known that nutrition plays an essential role in the development and maintenance of the immune system. Its deficiency can negatively affect the risk of infections and the maternal organism’s acceptance of the product of conception, characterized by different genetic and haplotypic heritages
[75][97]. If innate immunity (NK cells and monocytes) is preserved, acquired immunity (T cells and B cells) is downregulated, harming any specific immune response, hence causing an immunosuppressed state
[76][98]. In addition, the inflammatory response differs alongside pregnancy according to the three main stages: the pro-inflammatory stage (implantation and placentation in the first trimester), the anti-inflammatory stage (fetal growth in the second trimester), and the second pro-inflammatory stage (initiation of parturition)
[77][99]. Intuitively, nutritional deficiencies can compromise the immune response by greatly interfering with the body’s response to external inputs, leading to an amplification of the pathophysiological pathways and, thus, conditioning the corresponding outcomes
[70][92]. To date, the SARS-CoV-2 infection represents one of the most interesting examples of how peculiar the interactions of the systemic range of the action pathogen are, i.e., a peculiar biological environment
[75][97]. From this point of view, nutrition might act as a synergic co-factor, which may be able to influence organism response, while taking into consideration its significance is still under scientific evaluation. It is established that macronutrient intake variably influences the immune system response and inflammatory pathways. Thus, it may be used as a predictive factor for response to SARS-CoV-2 in pregnant women. To date, only a few papers are available. Moreover, these existing papers might be used to discriminate between food and macronutrients useful or harmful in COVID-19 infection management
[78][100].
The need of FA is not the same for the whole population and reaches its maximum levels during pregnancy and breastfeeding. Since the 1990s, in order to prevent NTDs (neural tubal defects), a daily intake of FA (folic acid) between 400 and 600 mcgr has been highly recommended during pregnancy by most health systems around the world; this is to compensate the insufficient average dietary intake of FA. A nutrition that includes fresh fruit and vegetables is therefore essential to meet the daily requirements of folate. FA also contributes to the physiological anemia of pregnancy, promoting hematopoiesis and therefore the increase in Hb levels
[79][105]. This works only if there is enough iron deposited to be used. In a case-control study during the COVID-19 pandemic, the authors
[80][104] demonstrated that it is mandatory to screen for and treat anemia with both FA and iron. They found that among pregnant women with iron deficiency anemia, the COVID-19 group had a higher risk of puerperal infection, emergency c-section and SGA (small for gestational age). Low birth weight, prematurity, and lower APGAR scores were also more frequent in the COVID-19 group. Specifically, a daily combination of FA and iron could help to normalize weight of the newborn in this setting
[80][104]. According to the latest international guidelines, it is mandatory to increase the daily FA dose up to 4–5 mg in case of: previous NTD-affected pregnancy, neurological disease and malformations, contemporary therapy with anti-epileptic drugs, or, with malabsorption disease and high maternal BMI and/or impaired stages of glucose metabolism. This latter group might be more vulnerable to contracting the COVID-19 viral infection, as reported in (Eskenazy et. al., the INTERCOVID Study, 2021). According to th
eis study, GDM (gestational diabetes mellitus), pre-existing DM, and being overweight or obese are all independent risk factors for SARS-CoV-2. An appropriate low-calorie dietary regimen besides eventual pharmacotherapy is needed for weight control during pregnancy and to improve pregnant women’s immune-receptivity. Indeed, in the DM and GDM groups, the obese and overweight women were at a higher risk of having COVID-19 than the normal weight women, and also the insulin-dependent diabetes group was at higher risk of COVID-19 than the diet-therapy group both for DM and GDM
[73][95]. Mate et. al. emphasized the role of a well-balanced dietary regimen for pregnant woman in the SARS-CoV-2 setting, while focusing on the main nutrient useful to boost the immune system against systemic viral infection
[78][81][100,106]. It should be mandatory to adopt an adequate nutrition and eventual feeding supplement not only during pregnancy, but also from preconception to the early post-natal period. The model of “food safety” which has been proposed is based on the experience of other viral and/or respiratory infections during pregnancy, which are well-known in the relevant literature. The core concept is to base the dietary regimen on functional foods, i.e., fruit and vegetables, including sufficient amounts of recommended micronutrients such as vit A, B, D, E, Omega-3 Fatty Acids, Choline, Zn, Fe, Se, and, as mentioned above, FA. This helps the immune system and has a positive impact on pregnancy outcomes in case of viral infection, including SARS-CoV-2. Omega-3 poly-unsaturated fatty acids down-regulate the excessive inflammatory response triggered by some systemic viral infections. Choline, in the case of respiratory virus infections, improves adverse fetal effects. The main adverse effects linked to not recommended food during pregnancy in case of systemic infection are reported in the existing literature: IUGR (intrauterine growth restriction), increased infant mortality, congenital diseases, miscarriage, preeclampsia, and nervous system anomalies or dysfunction
[78][100]. It is important to mention the national cross-sectional study conducted in China by Chen et al. in 2022 on the sample of 3678 pregnant women during the COVID-19 pandemic. The Authors reported that a median daily intake of so-called functional food, like vegetables and fruit, significantly decreased to low, moderate, and high severity of pandemic, and, in this sense making immune system more vulnerable. In addition to the low quality of the foods consumed, the perinatal outcomes got worse
[82][101]. Interestingly, researchers in Northern New England have proposed a model to identify and address questionable food choices among pregnant women during the pandemic period, highlighting the need for specific food programs
[83][107]. Erol et al. discussed that vit E and Afamin are significant predictors of adverse perinatal outcomes in COVID-19 infected women. Specifically, vit E levels
[84][102] were significantly lower than in the healthy group of women. In addition, Afamin levels were significantly higher, while positively correlated with CRP (C reactive protein) levels. The higher adverse perinatal outcomes in COVID-19 groups are due to higher oxidative stress
[84][102]. A subsequent study conducted by Erol et al., 2021 enlightened readers on the possible correlation between maternal selenium status and clinical outcomes of pregnant women with the SARS-CoV-2 infection. They found that selenium levels negatively correlate with gestational week, D-dimer, and interleukin-6 (IL-6). As the infection got worse, researchers could observe lower selenium levels and higher inflammatory factors. This vicious circle leads to a higher maternal need for selenium, and it makes a selenium supplement mandatory
[84][102]. According to the study conducted by Anuk et al. on pregnant women affected by SARS-CoV-2, serum zinc levels also negatively correlate with IL-6, and with other acute phase markers, i.e., erythrocyte sedimentation rate, PCT (procalcitonin), and CRP
[85][103]. On the other hand, the serum copper level showed a positive correlation. Therefore, serum Zn/Cu levels were a reliable predictor of viral infection severity in pregnancy. Similarly, in the case when the increased serum magnesium levels were found, there were predictors of pancytopenia and higher CPR
[85][103].