Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Styliani Chorianopoulou and Version 2 by Amina Yu.
Sulfur (S) plays crucial roles, including in the management of the essential micronutrients (EM) metalome. Each of the EM at first exists as a free cation, which is an existence that causes undesirable actions. Efficient handling of the EM metalome requires efficient chelation, transport, and translocation, along with efficient management of these actions. For each one of these management systems toward handling each EM properly, the phytoavailability of EM, along with the proper form of S in place and in time, is of central interest. Moreover, the functional EM metalome of plants is modified by S availability.
- copper homeostasis
- manganese homeostasis
- sulfur homeostasis
1. Sulfur Homeostasis in Plants
S is a macronutrient essential for plant growth and development. It is required for the biosynthesis of cysteine (Cys), methionine (Met), and glutathione (GSH) and for several secondary metabolites, such as glucosinolates, as well as for the biosynthesis of proteins, cofactors, and vitamins. S-containing metabolites have central roles in the responses of plants to various environmental conditions [1][2][3][18,19,20].
S is taken up from the rhizosphere in the form of sulfate, via sulfate transporters localized in the cytoplasmic membranes of rhizodermal and outer cortex cells. Subsequently, sulfate will either be transferred to the root stele and loaded in the xylem to be transported to the shoot or it will be translocated into root plastids. Upon arrival of the sulfate to the aerial plant parts, it will be finally transferred into chloroplasts. At any time, depending on the needs of the plant, sulfate may be also transported into the vacuoles of the cells either of the roots or the shoot of the plant. All these transfers are made mainly through specific sulfate transporters, localized either in a cytoplasmic membrane or in a subcellular organelle’s membrane [2][4][5][19,21,22].
After entering in a root or shoot plastid, sulfate is assimilated into adenosine-5′-phosphosulfate (APS), which is then reduced into sulfite, and then sulfide, leading to cysteine biosynthesis. Cysteine is the key metabolite for subsequent biosynthesis of the S-containing organic compounds in plants, while a major pool of sulfur is GSH, a Cys-containing tripeptide. In parallel, APS will be phosphorylated to 3′-phosphoadenosine-5′-phosphosulfate, an intermediate metabolite used for sulfation reactions. Moreover, sulfite may also be used for sulfolipids biosynthesis into the plastid, or it may be transported to peroxisome, where it can be reoxidized to sulfate through the activity of sulfite oxidase [1][2][6][7][18,19,23,24].
The regulation of S homeostasis takes place predominantly during sulfate uptake and APS reduction, through transcriptional regulation of the sulfate transporters as well as of APS reductase (APR) isoforms. Several metabolites have been appointed to have roles in the control of sulfate assimilation: GSH serves as a negative regulator through feedback inhibition, while OAS is a positive regulator of the sulfate uptake and assimilation pathway [1][5][8][18,22,25]. Phytohormones also play outstanding roles in the regulation of S homeostasis, such as cytokinins as well as the “stress-related” hormones abscisic acid, jasmonic acid, and salicylic acid [1][18]. Furthermore, the roles of regulatory components of sulfate uptake and assimilation such as the transcription factor SLIM1, the sulfur-responsive element SURE, and the miR395 have been described [2][19]. Recently, an epigenetic regulatory mechanism involving the nuclear-localized MORE SULFUR ACCUMULATION1 (MSA1) methyltransferase has been identified [9][26].
2. The Biological Importance of Essential Micronutrients
EMs play central roles in the metabolism, growth and production, maintenance, abiotic and biotic stress tolerance, and as structural and functional components of metalloproteins if they are at an optimal concentration. EMs are transition metals, with Fe and Cu to undergo redox changes under biological conditions. At supraoptimal levels, they are toxic because they can cause oxidative stress due to the production of reactive oxygen species via Fenton reaction. To sustain the appropriate ion homeostasis, plants maintain equilibrium in EM homeostasis by the establishment and maintenance of stable linkages with appropriate organic ligands in a specific geometry [10][11][12][13][14][27,28,29,30,31]. The following structural and functional roles highlight the biological importance of each one of the EMs.
Importance of Fe—Fe is required in several cellular processes, including photosynthesis, respiration, and sulfur assimilation. Fe exists as Fe(III) or Fe(II) under physiological conditions, participating in electron transfer reactions of the cells. The bulk of Fe in plant cells is needed in mitochondria and chloroplasts, where the major sinks of Fe are Fe-S clusters and heme are, and it is stored in ferritin or in vacuoles.
Several proteins contain Fe as a cofactor, mainly in the form of Fe-S clusters. Such proteins belong to the electron transport chains of chloroplasts and mitochondria. In the chloroplasts, the major Fe-S proteins are photosystem I (PSI), cytochrome b6f complex, ferredoxin, nitrite reductase, sulfite reductase, and adenosine 5′-phosphosulfate reductase (APR). In mitochondria, the main Fe-S proteins are the complexes I, II, and III of the respiratory chain and aconitase in the citric acid cycle. The biosynthesis of these clusters requires tightly regulated provision of chelated Fe and reduced S, in the form of cysteine [14][15][16][17][18][31,32,33,34,35].
Importance of Cu—Cu is a redox-active transition metal and under physiological conditions in vivo, it exists as Cu(II) or Cu(I) and participates in many physiological processes. Cu ions act as cofactors in a variety of enzymes such as cytochrome c oxidase, Cu/Zn-superoxide dismutase (Cu/Zn SOD), ascorbate oxidase, plastocyanin, laccase, amino oxidase, and polyphenol oxidase. At the cellular level, Cu possesses key roles in photosynthetic and respiratory electron transport chains, C and N metabolisms, biogenesis of molybdenum cofactor, Fe mobilization, protection against oxidative stress, oxidative phosphorylation, transcription protein trafficking machinery, ethylene sensing, and cell wall metabolism [12][19][20][29,36,37].
Importance of Zn—Zn forms tetrahedral complexes with cysteine residues of polypeptide chains. Known roles include its presence in enzymes involved in protein synthesis and energy production. Zn is required for the maintenance and the structural integrity of membranes via its binding to membrane phospholipid and sulfhydryl groups, resulting in the protection of membrane lipids and proteins against oxidative damage. It is involved in signal transduction pathways via mitogen-activated protein kinases, and it plays an important role in seed development [15][21][22][32,38,39].
Importance of Mn—Mn presents the oxidation states II, III, or IV, and serves as a cofactor in various enzymes within a plant cell, where it can fulfill two roles in proteins: (1) as a catalytically active metal or (2) as an enzyme activator. Mn contributes to photosynthesis, defense against oxidative stress, lipid biosynthesis, nitrogen metabolism, gibberellic acid biosynthesis, and RNA polymerase activation. Representatives for the catalytic role are the Mn-containing water splitting system of photosystem II, the Mn-containing superoxide dismutase, and the oxalate oxidase. The group of the Mn-activated enzymes consists of PEP carboxykinase, isocitrate dehydrogenase, phenylalanine ammonia lyase, and malic enzyme. Proteins of this group are known to be involved in the shikimic acid pathway, as well as the biosynthetic pathways of aromatic amino acids, flavonoids, lignins, and the indole acetic acid. Τhe role of Mn in the activation process is less specific and in many cases it can be replaced by magnesium [15][23][24][32,40,41].
3. Functional Interactions between EM and S
The EM-S bonds—The first level of interaction between EM and S is the formation of an effective EM-S bond (Figure 1). Ligands are distinguished as weakly (or hard) vs. highly (or soft) polarizable ones. The weakly polarizable ligands include the carboxylate groups with negatively charged oxygen atoms and the carbonyl groups with polar oxygen atoms. The highly polarizable ligands include the sulfhydryl groups. Aspartate (Asp) and glutamate (Glu) participate with their carboxylate groups; asparagine (Asn) and glutamine (Gln) participate with their carbonyl groups; while Cys and Met, the S-containing amino acids, are soft ligands. Histidine (His) carries the imidazole ring that contains the borderline aromatic N.
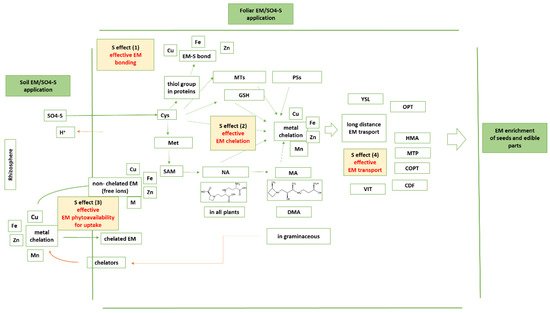
Figure 1.
The contribution of S homeostasis in essential micronutrients’ homeostasis within plants toward efficient seed EM loading.
The contribution of S in the bond—The S atom is a soft donor; hence, it prefers to bind with soft cations. In this way, it provides high redox potential to metal redox couples. The reduced form of the metal cation is softer than the oxidized one. Between two metal centers, the S-containing ligands can function as bridging ligands (M-S-M) or as monodentate ones (M=S). In proteins, S is found as thiolate or thioether. Thioethers are organic sulfides (C-S-C). Examples of sulfides are Met and biotin. Thiolates contain the thiol group (C-S-H).
The Fe-S bond—Ligands are electron donors and the metals’ electron acceptors. The softer Fe(II) catalyzes the Fenton reaction with hydrogen peroxide; hence, an efficient bonding is needed. Fe-S proteins are characterized by the presence of Fe-S clusters, which are found in a variety of metalloproteins. These clusters contain di-, tri-, and tetra-Fe centers and are sulfide-linked in variable oxidation states. Fe-S clusters contribute to the oxidation-reduction reactions of electron transport chains. There are several proteins containing Fe-S clusters that regulate gene expression. In most Fe-S proteins, the terminal ligands are thiolate sulfur centers from cysteinyl residues. The Fe centers are tetrahedral, while the sulfide groups are two- or three-coordinated [25][42].
The Cu-S bond—The harder Cu(II) can be co-ordinated by oxygen and nitrogen atoms of the harder amino acids (Tyr, Thr, His). The Cu(II)-N bonds are stable and often inert, while the Cu(II)-O bonds are more labile. The soft Cu(I) is stabilized by soft ligands. Cu(I) catalyzes the Fenton reaction with hydrogen peroxide. Cu(Ι) in proteins prefers the S atoms or ions of Cys and Met. Cu-thiolate and Cu-thioether bonds are found in a wide variety of enzymes with multifaceted co-ordination chemistry. S ligation to the metal centers provides special properties to Cu enzymes. S atoms from thiolates or thioethers act as donor ligands in a variety of Cu complexes [26][43].
The Zn-S bond—Zn is classified as borderline metal; i.e., Zn(ΙΙ) does not act as either hard or soft. Moreover, Zn does not have a strong preference for O-, N-, or S-coordination. In the various plant biological systems, Zn exists only as Zn(II), not taking part in redox reactions. In protein Zn-binding sites, the Zn(II) may be co-ordinated by the oxygen of aspartate or glutamate, the nitrogen of histidine, or the sulfur of cysteine. Among these, His is the most observed, followed by Cys, through which S plays a functional (catalytic) and a structural role in enzyme reactions. The Zn-S bond serves several roles: in metallothionein and Zn release, in thionein and Zn binding, in control of Zn transfer reactions and availability of cellular Zn in mononuclear sites, in the cellular distribution of Zn, in proteins that detect the availability of cellular Zn, in redox-active Zn proteins, in Zn thiolate cluster structures, and in Zn co-ordination dynamics [27][44].
The Mn-S bond—There are no Mn metalloenzymes containing an Mn-S co-ordination sphere, as Mn(II) establishes co-ordination with hard ligands. His is the important ligand for Mn(ΙΙ), while Cyst and Met are less likely to co-ordinate with Mn(ΙΙ) [23][40].
