Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Jorge Sanchez-Molina.
Inherited retinal dystrophies (IRDs) affect an estimated 1 in every 2000 people, this corresponding to nearly 2 million cases worldwide. Currently, 270 genes have been associated with IRDs, most of them altering the function of photoreceptors and retinal pigment epithelium. Gene therapy has been proposed as a potential tool for improving visual function in these patients.
- retinal gene therapy
- subretinal injection
- subretinal injection technique
1. Introduction
Intraocular delivery has become a classic route of drug administration for the treatment of eye diseases. In particular, intracameral antibiotics are used for the prevention of postoperative endophthalmitis after phacoemulsification [1] and intravitreal injections are the standard of care for the treatment of age-related macular degeneration (AMD) and other posterior pole diseases [2]. The eye offers to the practitioner certain advantages for the administration of drugs: (1) it is a privileged immune environment based on the absence of lymphatic vessels and the existence of a very tight blood–retinal barrier, (2) ocular structures are easily accessible to the clinician, (3) real-time monitoring of the structure and function of the eye is feasible, due to the large spectrum of diagnostic tools available, (4) low concentrations of drug are sufficient to achieve therapeutic doses, and finally, (5) the fellow eye can be used as a comparison group [3,4][3][4].
For diseases affecting the photoreceptor (PR) layer and the RPE, such as most inherited retinal dystrophies (IRDs), there is a need to identify a more efficient way of delivering drugs. Subretinal injection has been proposed as a suitable approach for treating these conditions. Accessing the subretinal space (SRS), the potential area between the neurosensory retina and the RPE, allows direct contact of the drug with the PR and RPE layers, optimizing the concentration of the drug in these cells. In comparison to the intravitreal space, the SRS is a safer area as it is anatomically closed and immune privileged. Moreover, smaller therapeutic drug doses are needed using the subretinal approach [7][5]. Recently, the ongoing evolving field of gene therapy for the treatment of retinal dystrophies has led to injection of adeno-associated virus (AAV) vectors into the SRS. This modality of treatment has shown efficacy in clinical trials treating RPE65-associated Leber congenital amaurosis (LCA) [8][6], CHM-associated choroideremia [9[7][8],10], CNGA3-related achromatopsia [11][9], and MERTK-associated retinitis pigmentosa [12][10]. Current cell therapies for dry AMD and Stargardt diseases are using this approach to reach the SRS [13][11].
It is essential to note that subretinal injection implies the creation of iatrogenic retinal detachment (Figure 1). Nevertheless, when performed with care, the technique is associated with minimal trauma and early retinal structure and function recovery, suggesting a good safety profile overall [14][12].
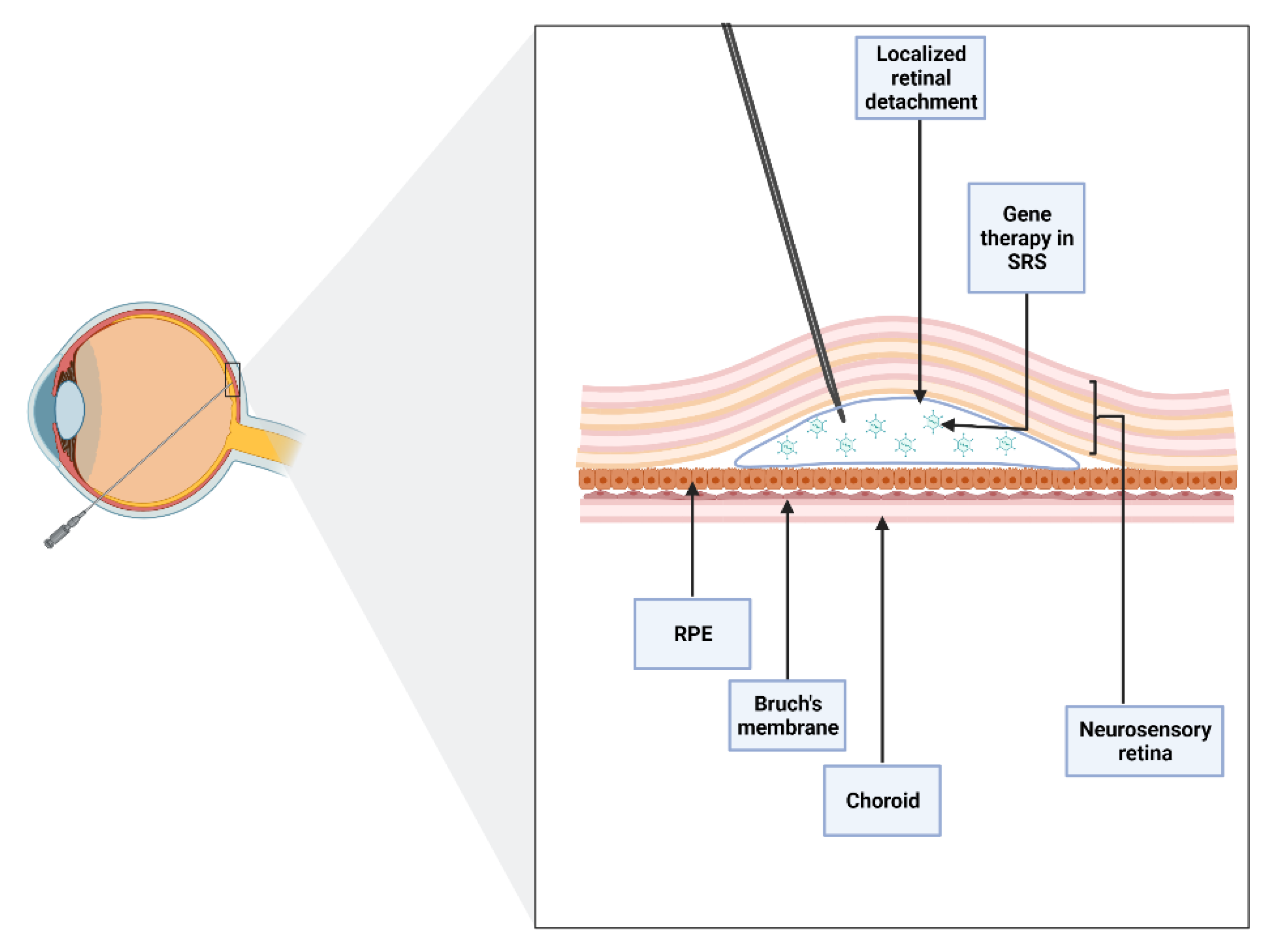
Figure 1. Schematic of subretinal injection. An iatrogenic retinal detachment is produced after accessing the subretinal space (SRS), the area between the neurosensory and the retinal pigment epithelium (RPE). Subretinal injection allows direct contact of the drug with photoreceptors and RPE layers.
2. Indications for Subretinal Injections
2.1. Cell Therapy
Primary degeneration of PRs or a malfunction of the RPE lead to retinal degeneration. Some of the most frequent types of retinal degeneration, such as AMD and IRDs (which include retinitis pigmentosa and Stargardt disease), are caused by the irreversible loss of these cells. Therefore, replacement of damaged tissue using cell therapy could be an interesting option [28,36][13][14]. Furthermore, in many cases, cell therapy could be a good treatment as it can be used in both genetic and acquired diseases where the irreversible cell loss would make gene therapy ineffective [37][15].
It is believed that a variety of progenitor and stem cells can migrate into the retinal layers and survive there in order to restore the retinal function or stimulate cell regeneration. In particular, survival and migration have been proven with human tissue, including neural and retinal progenitor cells, forebrain progenitor cells, brain-derived precursor cells, embryonic stem cell-derived retinal progenitors, RPE stem cells, bone marrow mesenchymal stem cells, and with tissues from other species including rat mesenchymal cells or progenitor cells from the swine neural retina [15][16].
Cell therapy is a promising treatment with interesting results obtained in animal research. The first RPE cell therapy reported took place in the early 1980s in monkeys and was later reproduced in rats [28][13]. From that point on, various sources of cells for cell therapy have been proposed. In particular, many cell types have been tested to treat pathologic retinal degeneration in animal models. Three months after subretinal transplantation, pre-induced adult human peripheral blood mononuclear cells in mice with degenerating retina survived and migrated. In rats, human fetal lung fibroblasts with expression of the ciliary neurotrophic factor gene can be used to prevent PR degeneration and to avoid laser-induced choroidal neovascularization subretinal transplantation of RPE, over-expressing fibulin-5 can be used. Subretinal injection using mini pigs as a model of hESC-RPE cells has shown its safety, with no cell migration or tumors affecting ocular or systemic structures [15][16].
2.2. Gene Therapy
The evolution and growth of gene therapy has turned into an encouraging option for the treatment of IRDs [39][17].
Due to the pathophysiology of many retinal illnesses, subretinal gene therapy has the theoretical basis to turn out to be a successful treatment. Some IRDs such as retinitis pigmentosa are considered monogenic disorders, whereas both genetic and environmental risk factors underlie in AMD, with three types of cells involved (PRs, RPE, and choriocapillaris) [40,41][18][19]. With genome editing, it is possible to modify gene expression in these diseases. AAV, a small nonpathogenic dependoparvovirus, has been the most widely used vector for delivering gene therapies to the SRS. In fact, this vector has been used for subretinal drug delivery in different animal models, showing evidence of safe and stable genetic expression [15][16].
Moreover, delivering DNA to the retina using AAV has been useful in clinical trials since 2008 [15][16]. For example, the FDA approved the first gene therapy treatment for ocular disease in 2017 for the treatment of inherited biallelic RPE65 mutation-associated retinal dystrophy by delivering AAV2-mediated VN subretinally [42][20]. This therapy allows hope for the recessive forms of the disorder. At the moment, there are no FDA-approved gene or stem cell-based therapies targeting other genetic subtypes of retinal diseases [37][15]. Currently, there are more clinical trials ongoing for treating IRDs, such as retinitis pigmentosa including the X-linked form, choroideremia [43][21], Leber hereditary optic neuropathy, and Stargardt disease [44][22], and other disorders are being targeted, including achromatopsia and Usher syndrome [45][23]. To date, although clinical trials have mainly focused on gene augmentation therapies, other studies have also tried to repair the mutation at the mRNA level [43][21].
2.3. Submacular Hemorrhage
Fovea-involving SMH is a vision-threatening ocular pathology. Experimental models have provided evidence of various pathological pathways concerning this condition. For example, toxic effects of blood located subretinally, with early PR degeneration and subsequent cell death, have been proven. Even more, blood clots are known to produce fibrin-mediated tractional damage in different retinal layers [47][24]. Exudative AMD is the most frequent cause underlying SMH, but there are others such as polypoidal choroidal vasculopathy, undifferentiated and traumatic choroidal neovascularization (CNV), proliferative diabetic retinopathy and retinal arterial macroaneurysm [47][24]. CNV can be considered the most common cause of SMH associated with AMD [20][25]. Management of SMH secondary to AMD can be challenging and the best treatment is not always clear.
For some cases, the subretinal approach could be useful, as the use of a VEGF inhibitor alone is not effective in medium- to large-sized SMH. For large SMH, pars plana vitrectomy (PPV) associated with subretinal rTPA and pneumatic displacement of dense SMH with an expansile gas could be an effective treatment option (Figure 32). In order to improve best-corrected visual acuity at 12 months and capture good quality images to plan future VEGF inhibitor therapy, different studies recommend performing the procedure in the first 2 weeks after the beginning of the symptoms [20,48,49][25][26][27].
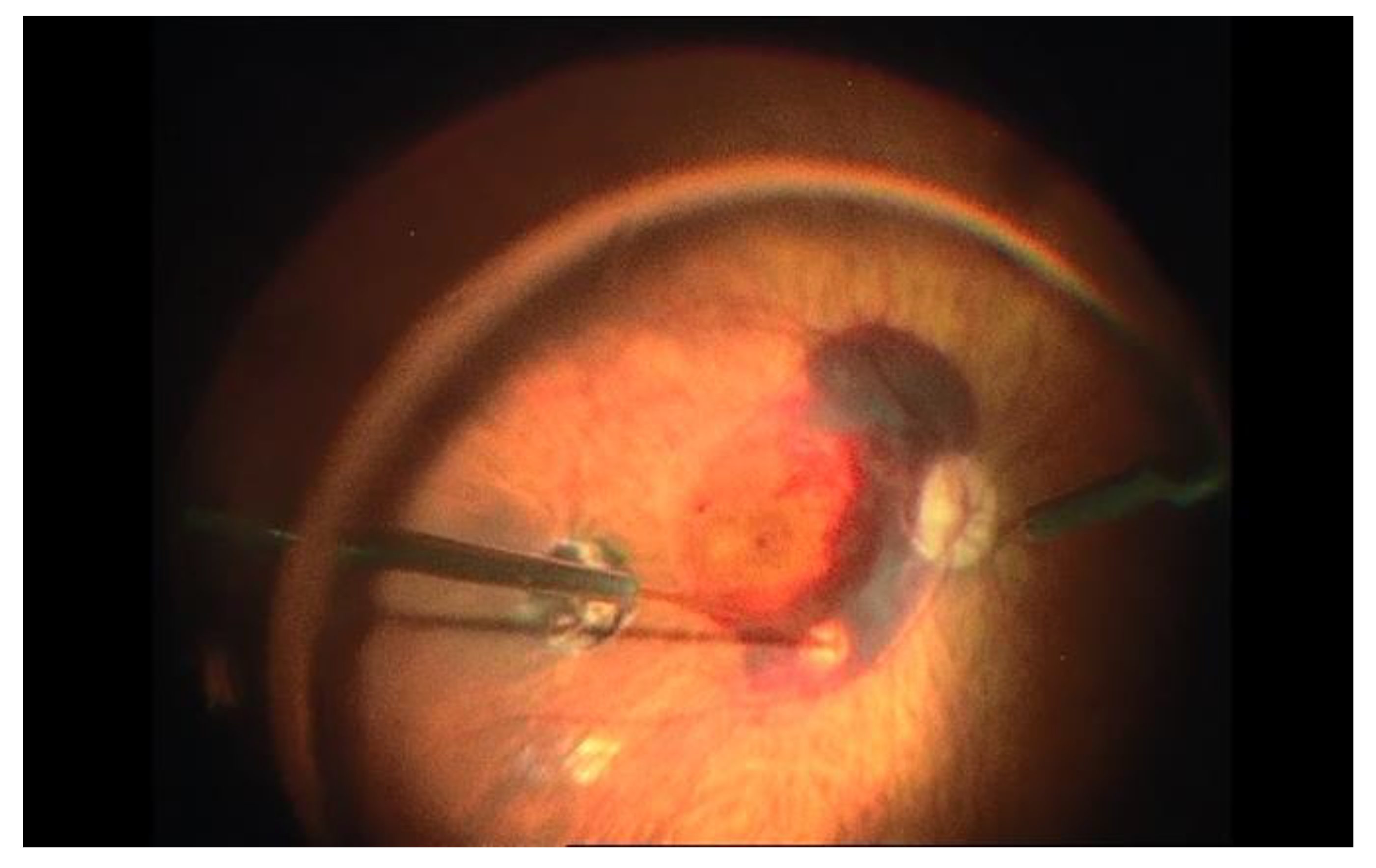
Figure 32. Subretinal injection of rTPA after pars plana vitrectomy in a case of large submacular hemorrhage associated with age-related macular degeneration. Subretinal injection is performed after internal limiting membrane (ILM) peeling. Note the blanching of the retina when reaching the subretinal space.
Nevertheless, in these cases it has been proven that long-term vision may depend on the underlying disease. Therefore, potential risks and benefits must be carefully taken into account before opting for this treatment as some research has shown no differences between subretinal and conventional vitrectomy approaches [50][28].
3. Subretinal Injection Technique
3.1. Animal Models
Subretinal injection has been used as the delivery approach for viral vectors in multiple animal models [15][16]. To date, three different approaches for subretinal injection in animals have been described in the scientific literature:
3.1.1. Transcorneal Approach
The transcorneal approach is commonly used for subretinal injection in rodent eyes. In a study using rat eyes, the authors advanced a 33G blunt needle through the rodent nasal cornea near the limbus. To avoid further damage of the lens, it was displaced medially using the needle. After reaching the SRS, the injection was performed by an assistant, resulting in the creation of visible retinal detachment. The procedure is monitored in real time under direct microscope visualization and full mydriasis is needed to avoid complications [51][29].
After using a similar technique of transcorneal subretinal injection, Qi et al. described structural recovery of the iatrogenic retinal detachment by day 1 or 2 after the procedure [52][30]. Pang et al. observed structural and functional recovery 5 weeks postoperatively in the mouse retina after in vitro AAV vector transduction [53][31].
The transcorneal route is associated with total retinal detachment, mild corneal and lens opacity, anterior synechiae, iris haemorrhage, and damaged outer PR segments [51][29]. Neonatal mice show an immature status of the ocular structures and therefore this approach is not feasible in these cases, as it turns out to be difficult to obtain adequate pupil dilation. Overall, transcorneal subretinal injection appears to be an effective and safe procedure in adult mice when performed by experienced practitioners [51][29].
3.1.2. Posterior Transscleral Approach
The posterior transscleral approach accesses the SRS through the choroid. This route has a better safety profile as it does not access the intraocular media. A sclerotomy is created using a 22.5-degree ophthalmic blade 0.5 mm away from the optic nerve. Then, a 33 G beveled cannula is inserted through the sclerotomy, with an angulation of 5–10 degrees, to reach the SRS.
Normal structural and functional profiles are observed 4 weeks after the procedure. This approach has an elevated rate of success, low rate of exclusion from the treatment, and relatively few complications. Even more, this route does not require perforation of the retina and vitreous access, which makes it a safer approach for subretinal injection [54][32].
3.1.3. Anterior Transscleral Approach
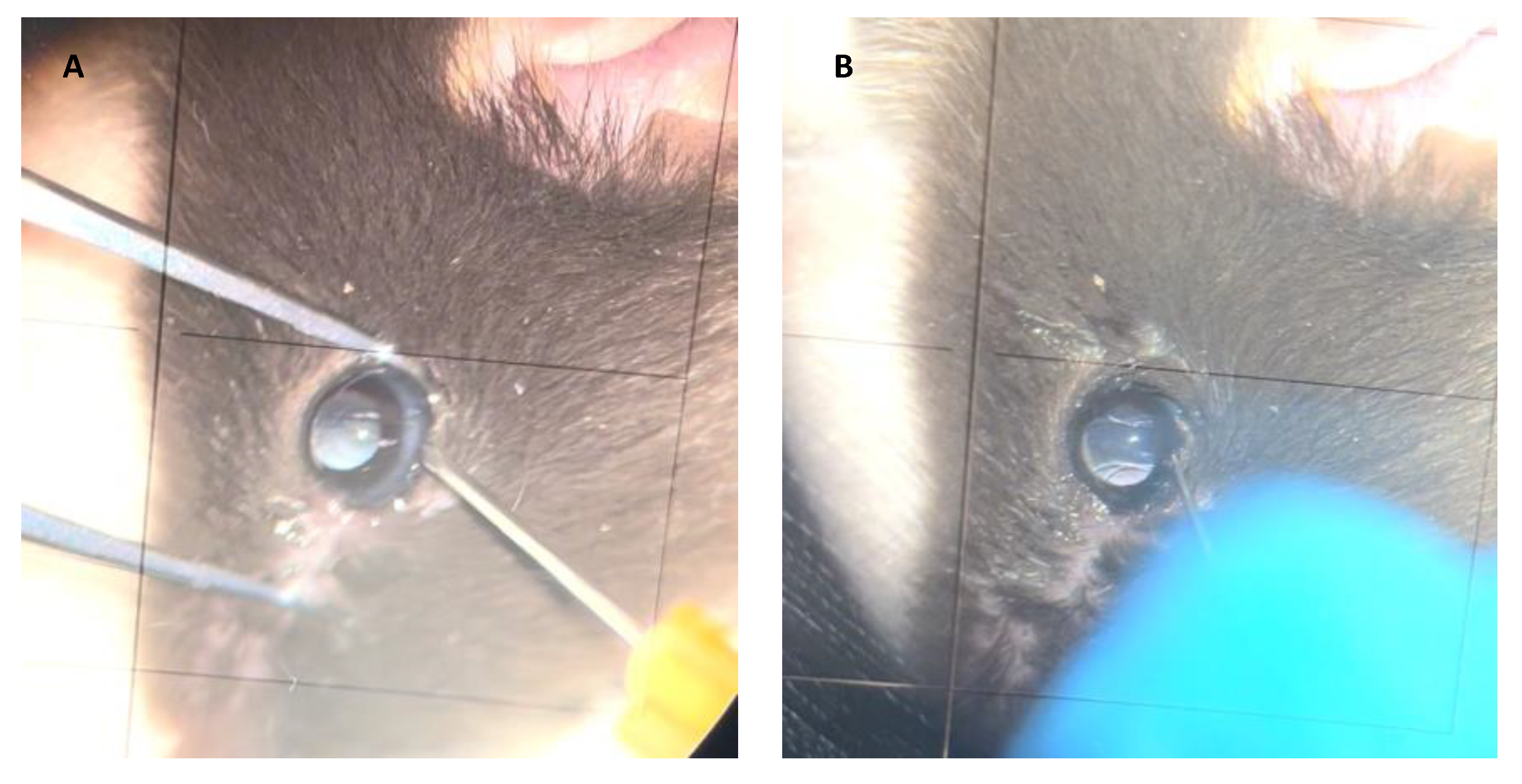
The most popular route for subretinal injection in animal models is the anterior transscleral approach (Figure 43). Using this route, intraocular media is accessed through a sclerotomy in the limbus or pars plana [55,56,57][33][34][35]. In a non-inferiority study comparing the safety of subretinal and intravitreal injection of viral vectors into cynomolgus monkeys, Ochakovski et al. employed a two-step procedure for subretinal injection using an anterior transscleral approach. First, a retinotomy is created and balanced salt solution (BSS) is injected subretinally with a 41G cannula (DORC, Zuidland, The Netherlands). Afterwards, a viral vector solution is introduced through the already existing retinotomy into the subretinal bleb [58][36]. The use of 25G 3-port PPV with or without ILM removal before subretinal injection in monkey retinas has also been described in the literature [59,60][37][38]. This route has been shown to be safe and effective for the delivery of viral vectors into the SRS in different animal models [61,62][39][40].

Figure 43. Subretinal injection technique in animal models. Anterior transscleral approach. (A) A retinotomy is performed in the limbus. (B) A blunted cannula used for subretinal injection.
New elements are being integrated to enhance the success of subretinal injection technique in animal models. The use of smaller needles protects against reflux at the retinotomy site. Pressure-regulated microinjectors allow for more accurate and constant injection volumes and hence a more controlled way of creating the subretinal bleb. Intraoperative optical coherence tomography (OCT) is now adopted as a diagnostic tool to help confirm the subretinal location of the needle and monitor the creation of the bleb [63][41].
3.2. Subretinal Injection via Vitrectomy
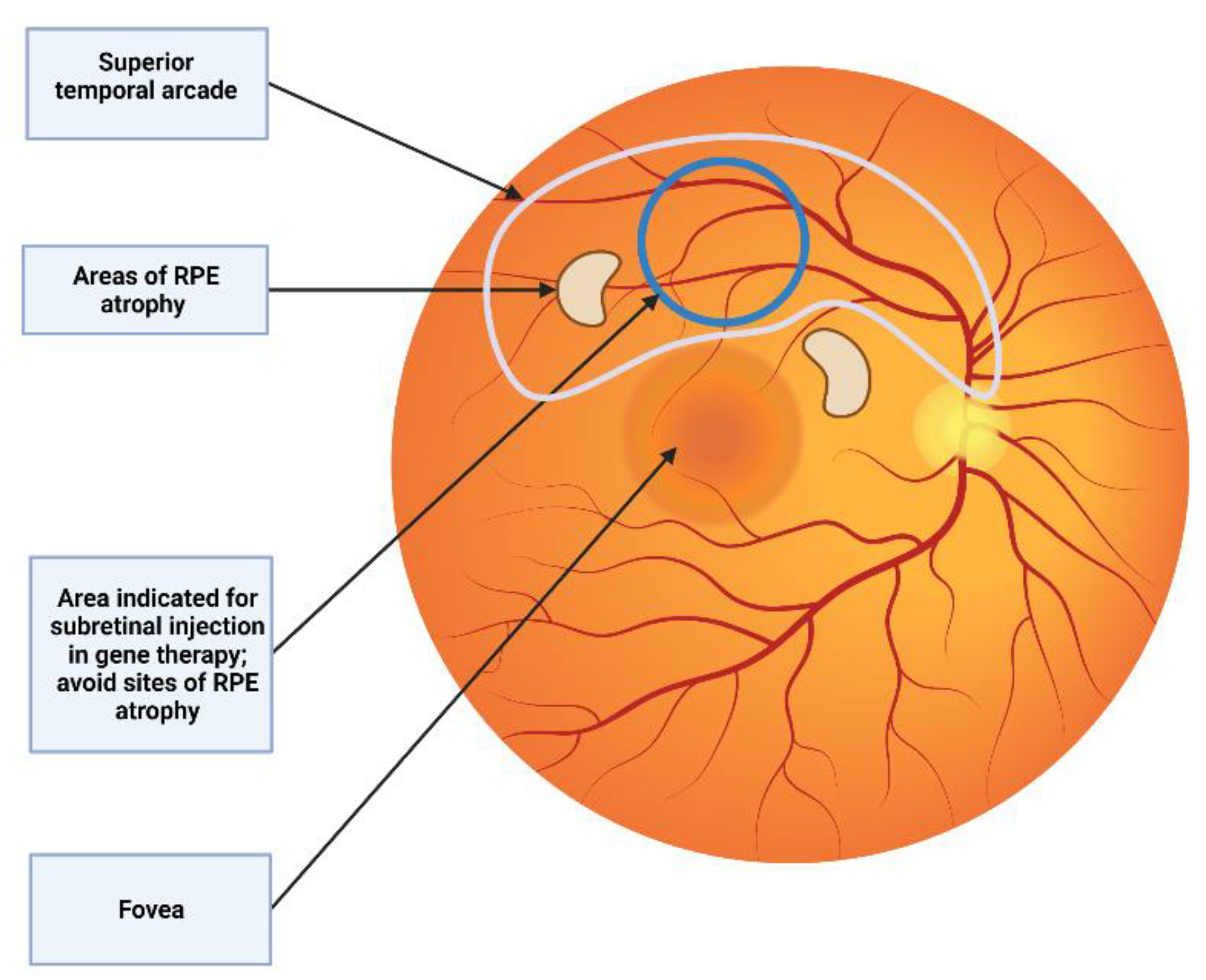
Subretinal injection is carried out under retrobulbar or general anesthesia. A 3-port PPV is made, generally using standard 23 or 25G trocar systems [57][35]. After performing core vitrectomy, posterior vitreous detachment is induced (if not already present) with or without acetonide triamcinolone for visualization of the posterior hyaloid [64][42]. After that, the peripheral vitrectomy is completed and the injection site is checked. For subretinal injection in SMH, an elevated region of the retina is recommended. To reduce the pressure needed for the needle to enter the SRS, some authors propose ILM peeling around the injection site [65][43]. The superior vascular arcade, which avoids blood vessels, is the optimum location for gene therapy. Preoperative planning using OCT and autofluorescence imaging have to be taken into account in order to avoid areas of retinal atrophy where the retina is less susceptible to detach (Figure 54) [66][44].

Figure 54. Subretinal injection location. The superior vascular arcade is the preferred location for subretinal injection. It is recommended to avoid areas of RPE atrophy where the retina is more predisposed to detach.

Then, subretinal injection is performed using a 25G cannula with a 38G or 41G tip, for example, a dual-bore 41G blunt polytetrafluoroethylene (Teflon)-tipped cannula mounted within a 23G steel shaft (DORC, Zuidland, The Netherlands) [66][44] or a 38G/25G Subretinal PolyTip Cannula (MedOne, Sarasota, FL, USA) [64][42]. A blunt or sharp tip can be used. Fan et al. used a beveled tip cannula, 45-degree angled scissors were applied for beveling the tip of the cannula [64][42]. The appearance of pseudo-schisis, which is described as momentary hydration of the outer plexiform layers on OCT, can be brought on by beveling the subretinal cannula. Therefore, the surgeon has discretion over whether to bevel the cannula or not [64][42]. (Video of surgical technique, Supplementary Video S1.)
Recently, new devices have been developed for subretinal injection. Wood et al. describe the access to the SRS without vitrectomy with a nanovitreal subretinal gateway device (Vortex Surgical, Chesterfield, MO, USA) for the management of SMH [67][45]. This device could also be used for the subretinal delivery of gene therapies, avoiding the risk associated with general anesthesia and vitrectomy, especially in pediatric patients.
Before entering the vitreous cavity, it is important to purge any air bubbles and confirm the permeability of the cannula. Non-valved trocars are recommended to prevent damaging the subretinal cannula. The technique can be performed in one or two steps. The one-step approach was described for the first time by Bainbridge et al. and consists in directly injecting the drug into the SRS [68][46]. The two-step variant used by MacLaren et al. and Fischer et al. consists of inducing a BSS subretinal bleb and secondly injecting the drug subretinally. This way, the creation of a space for the correct delivery of the gene vector reduces reflux into the vitreous cavity and the chance of losing the drug during subretinal injection. This technique is of special interest in patients with choroideremia where the retinal atrophy makes it more difficult to detach the retina [10,69][8][47]. The risks of the two-step technique include accidental opening of a second retinotomy, possible trauma at the retinotomy site, mechanical overstretching of the retina inducing a macular hole, and widening of the first retinotomy with a higher risk of vector reflux. The theorical bleb space is only filled with gene vector, keeping it within the accepted bleb height, when a single injection technique is performed. Theoretically, this might lessen both the overall requirement for blebs and the frequency of difficulties [64][42].
The injection can be performed by two surgeons (the assistant surgeon lowering the plunger of the syringe to inject the drug and the main surgeon placing the tip of the cannula subretinally) as explained by Maguire et al. and Russell et al. [8,71][6][48]. Nonetheless, the precise injection of small volumes of solution into the subretinal area by this method is a challenge: injection speed and pressure are variable, and while additional tubing systems may be used between the syringe and cannula to reduce movement, this increases the dead space requiring larger drug volumes. Recently, other methods have been proposed for single-surgeon foot-pedal-controlled automated injections using the viscous fluid injection mode of the vitrectomy system [72,73][49][50]. An automated injection system, the MicroDose Injection Device (MedOne, Sarasota, FL, USA), has been designed for low volume ophthalmic injections into the SRS; it is connected to a vitrectomy machine to allow actuation of the syringe stopper by the surgeon via a foot pedal (Figure 65). The infusion pressure is foot-pedal-controlled, with the highest limit set to the lowest that would create a constant flow of fluid instead of a stream of droplets (generally, 12–16 psi) [66][44]. The foot-pedal control helps minimize excessive retinal stretch with more controlled drug delivery.

Figure 65. Left image shows the injection syringe and the high pressure extension tube when the injection is performed by two surgeons; when purging the treatment fluid there is loss of treatment in the extension tube. Right image shows the single-surgeon foot-pedal-controlled automated injection system using the viscous fluid injection mode of the vitrectomy system. MicroDose Injection Device (MedOne, Sarasota, FL, USA).
Surgical precision can be further enhanced by intraoperative OCT (e.g., using a Zeiss Rescan 7000, Carl Zeiss Meditec AG, Jena, Germany; Haag-Streit intraoperative OCT system, Haag-Streit, Switzerland; Leica Proveo microscope with integrated EnFocus OCT, Leica microsystems, Danaher, Washington DC, USA; or the Duke swept- source microscope-integrated OCT system [74][51]). This type of imaging offers a method for real-time visualization of subretinal drug delivery and allows surgeons to quantify and assess the success of the procedure [74][51]. Direct real-time imaging of the SRS allowing the evaluation of instrument depth is still challenging even with current commercially available spectral-domain intraoperative OCT systems as a consequence of shadowing artifacts secondary to intraocular surgical instruments [74][51]. On the other hand, intraoperative OCT allows monitoring of retinal detachment progression, including the foveal area, during subretinal injection [66][44]. Further, an indication of the volume of the final bleb can be obtained by measuring the intraoperative OCT scan, taken at the end of the vector injection. This would allow for the calculation of the delivered dosage. This has implications for evaluating the outcomes of clinical trials, regarding the efficacy of gene therapy [66][44].
Excessively deep penetration of the needle tip, with subsequent blanching of the retina, may result in damage to the RPE, suprachoroidal administration of the medication, bleeding, and obstruction of the cannula tip. On the other hand, too-shallow penetration of the needle tip may result in intraretinal hydration and retinoschisis during administration of the drug. In addition, the degree of retinal tissue atrophy at the injection site may interfere with such complications and be associated with more instances of hemorrhage and cannula tip blockage. Numerous efforts to create a subretinal bleb may result in localized retinal neurosensory trauma and retinotomy widening. All these features are best assessed using intraoperative OCT, reinforcing the value of this tool for subretinal injection in gene therapy surgery [75][52].
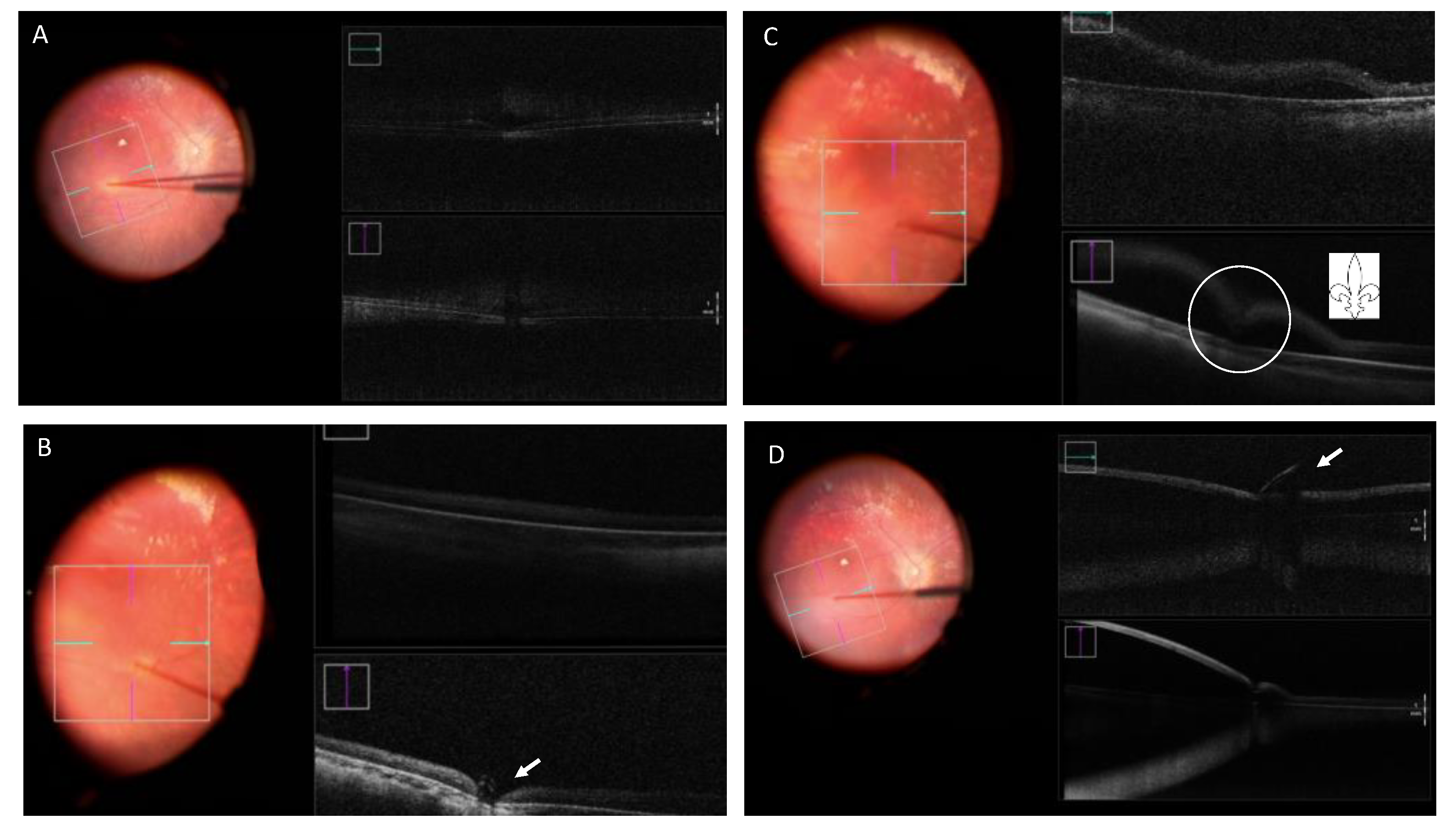
Vasconcelos et al. analyzed intraoperative OCT images during subretinal gene therapy in 19 eyes. They conclude that this type of imaging offers valuable real-time feedback on cross-sectional structure of the retina while performing subretinal gene therapy surgery. Fleur-de-lis sign identification can confirm the bleb formation onset (Figure 76). The characteristics of the material used (such as the type of needle tip), as well as the surgeon’s experience, have an effect on the creation of the bleb and the presence of visible open retinotomy; a sharp needle requires partial retina and RPE/choroid indentation and a blunt needle complete indentation to create the subretinal bleb. A sharp needle was associated with more open retinotomies.

Figure 76. Retinography and intraoperative OCT (iOCT) taken during surgery for Luxturna gene therapy. (A) Blanching of the retina and indentation in the iOCT. (B) Indentation in the iOCT just before pressing the plunger for the subretinal injection (arrow). (C) “Fleur-de-lis” sign at the beginning of the subretinal injection (circle). (D) Subretinal bubble, localized retinal detachment and blunt cannula at the edge of the retinotomy (arrow).
Visualization of a double hyperreflective sign with intraoperative OCT can also be useful to identify air bubbles. Another important feature of dynamic real-time intraoperative OCT is the ability to discriminate schisis from subretinal fluid. These examples show how intraoperative OCT may change surgeons’ performance, allowing them to have more control over the injection technique, switch to a different treatment area or continue the procedure. Future advances in intraoperative OCT technology, from better depth performance, retinal coverage, and ways to calculate bleb volume to the inclusion of other types of imaging study would help to improve and develop both the execution of gene therapy delivery techniques and the assessment of outcomes [75][52].
In the technique described by Xue et al., the retinotomies self-sealed postoperatively without complications and subretinal fluid resolved within 24 hours. Anatomical and functional recovery of the retina after iatrogenic detachment of the macula mainly occurred within the first 4 weeks after the procedure [66][44]. Simunovic et al. described recovery of structure and function after macular detachment for gene therapy in five men with a diagnosis of choroideremia; although retinal anatomy and visual acuity improved within 1 month, this was not accompanied by improvements in threshold sensitivity or color discrimination [14][12]. Future studies using advanced imaging devices, such as adaptive-optics scanning laser ophthalmoscopy and OCT, will help understand the causes of these discrepancies in visual function parameters [14][12].
3.3. Suprachoroidal Technique
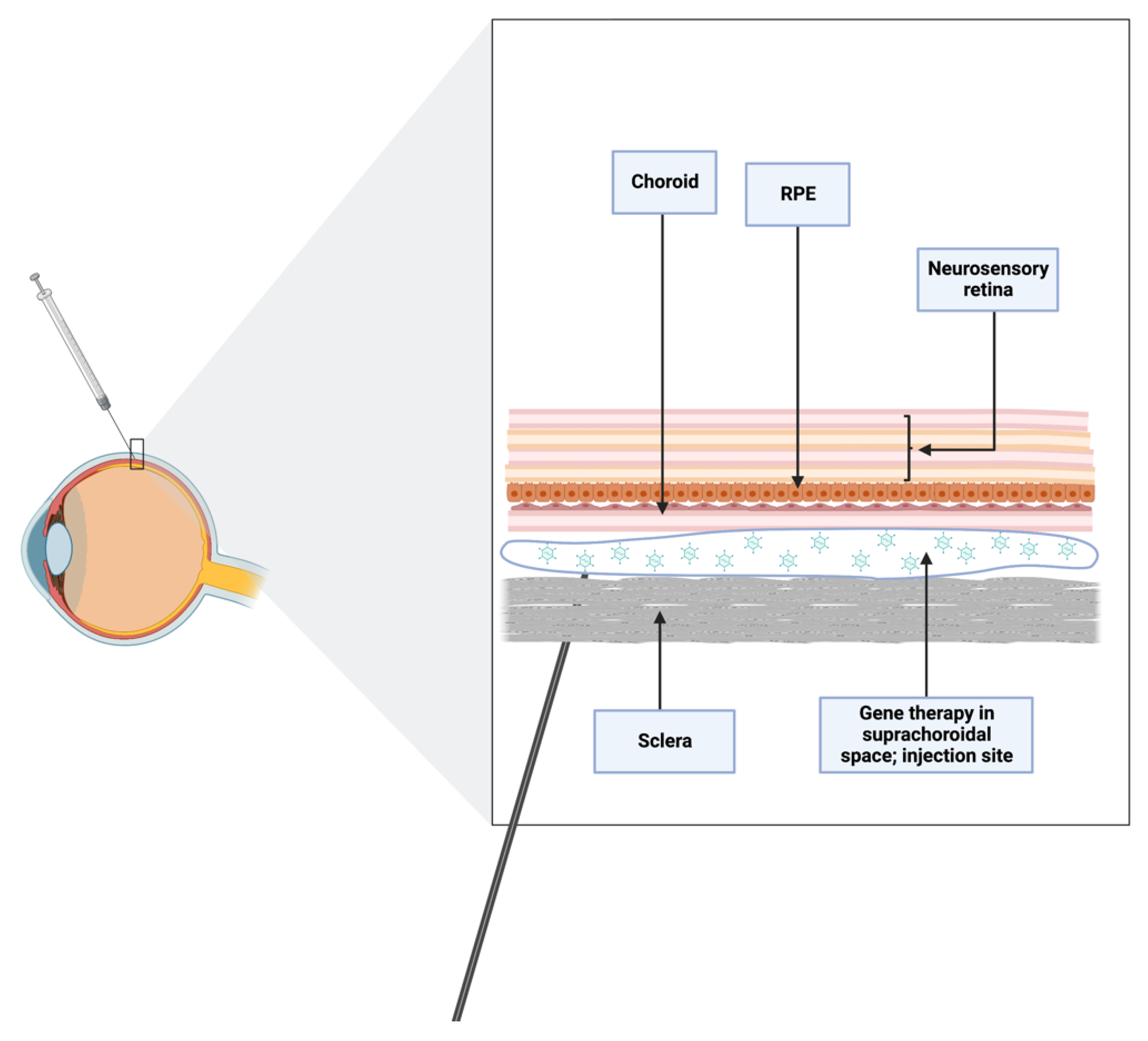
Since it directly targets the choroid, RPE, and retina, the suprachoroidal space (SCS), a potential space between choroid and sclera, is a desirable location for intraocular drug administration, as it results in high drug bioavailability in these areas while maintaining low levels elsewhere in the eye. Up until recently, a significant obstacle inhibiting the general application of this strategy was the absence of a mechanism to access the SCS safely. Sclerotomy has typically been used in preclinical settings to gain access to the SCS, and the only FDA-cleared technique is a sclerotomy with micro-cannulation (iScience catheter, Ellex Medical, Adelaide, Australia). Full-thickness scleral incisions, often made with a scalpel, can also be used to enter the SCS. Once the sclerotomy is finished and the scleral-choroidal junction is identified, a catheter can be pushed through the SCS under a microsurgical scope. This approach could be helpful when delivering chemotherapy to the SCS in cases of ocular tumors [76][53].
Needles can also be used to gain access to the SCS (Figure 87). Given the steep learning curve for this procedure, it can be difficult to establish its safety. The success rate of an injection is known to rely on the following factors: infusion pressure, gauge, particle size, and needle length [76][53]. The evidence on IOP and injection success rate is controversial; some authors postulate that there is no correlation between IOP and injection success [76][53] but others have found an elevated IOP to be associated with a higher infusion success rate, though recognizing that it is not necessary for injection or desirable in patients [77][54]. They suggested that high IOP decreased scleral surface deflection, increasing microneedle insertion depth and reducing the amount of total scleral tissue between the needle tip and the SCS [77][54].

Figure 87. Schematic of suprachoroidal injection. Needles reaching nanodimensions are optimal for accessing suprachoroidal space, avoiding iatrogenic trauma in the vitreoretinal space.
Overall, for the suprachoroidal technique to be considered an effective and safe therapy, technological advances are still needed. The introduction of microneedles, which are less than 1 mm long and may even be reaching nanodimensions, is a significant improvement in this field because they provide a less invasive and more reliable method of targeting the SCS than conventional needles. Further, a drug’s precise location in the SCS, precisely beneath the sclera and above the choroid, may avoid damage to the underlying retinal layers. For these reasons, ideally the length of the needles used for this purpose should be equivalent to the thickness of the conjunctiva and sclera [78][55], being physiologically incapable of performing an unintentional intravitreal injection deeper than the SCS [76][53].
Microneedle injection into the SCS is safe and effective, and it may be carried out in an outpatient ophthalmology clinic while the patient is under local anesthesia, according to ongoing clinical trials. By holding the microneedle perpendicular to the level of the scleral surface and setting the hard stop on the needle hub to contact the sclera/conjunctiva, the insertion depth may be precisely regulated. The microneedle may be left in place for up to one minute in order to reduce reflux from the injection site [76][53].
To access the SCS without vitrectomy, a microscope-integrated OCT could be used. Sastry et al. employed microscope-integrated OCT to precisely and accurately estimate the amount of subretinal blebs after administering BSS via a suprachoroidal cannula into ten porcine eyes. Eighty percent of the targeted injection volume could be delivered to the SRS using the suprachoroidal microscope-integrated OCT-guided method. Their results suggest that the use of subretinal delivery methods combined with a microscope-integrated OCT may increase the success of subretinal drug delivery [74][51].
References
- Romero-Aroca, P.; Méndez-Marin, I.; Salvat-Serra, M.; Fernández-Ballart, J.; Almena-Garcia, M.; Reyes-Torres, J. Results at seven years after the use of intracamerular cefazolin as an endophthalmitis prophylaxis in cataract surgery. BMC Ophthalmol. 2012, 12, 2.
- Parikh, R.; Ross, J.; Sangaralingham, L.R.; Adelman, R.A.; Shah, N.D.; Barkmeier, A.J. Trends of Anti-Vascular Endothelial Growth Factor Use in Ophthalmology Among Privately Insured and Medicare Advantage Patients. Ophthalmology 2017, 124, 352–358.
- Amato, A.; Arrigo, A.; Aragona, E.; Manitto, M.P.; Saladino, A.; Bandello, F.; Parodi, M.B. Gene Therapy in Inherited Retinal Diseases: An Update on Current State of the Art. Front. Med. 2021, 8.
- Stout, J.T.; Francis, P.J. Surgical Approaches to Gene and Stem Cell Therapy for Retinal Disease. Hum. Gene Ther. 2011, 22, 531–535.
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-Álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269.
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860.
- Lam, B.L.; Davis, J.L.; Gregori, N.Z.; MacLaren, R.; Girach, A.; Verriotto, J.D.; Rodriguez, B.; Rosa, P.R.; Zhang, X.; Feuer, W.J. Choroideremia Gene Therapy Phase 2 Clinical Trial: 24-Month Results. Am. J. Ophthalmol. 2018, 197, 65–73.
- MacLaren, R.E.; Groppe, M.; Barnard, A.R.; Cottriall, C.L.; Tolmachova, T.; Seymour, L.; Clark, K.R.; During, M.J.; Cremers, F.P.; Black, G.C.; et al. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet 2014, 383, 1129–1137.
- Fischer, M.D.; Michalakis, S.; Wilhelm, B.; Zobor, D.; Muehlfriedel, R.; Kohl, S.; Weisschuh, N.; Ochakovski, G.A.; Klein, R.; Schoen, C.; et al. Safety and Vision Outcomes of Subretinal Gene Therapy Targeting Cone Photoreceptors in Achromatopsia: A Nonrandomized Controlled Trial. JAMA Ophthalmol. 2020, 138, 643–651.
- Ghazi, N.G.; Abboud, E.B.; Nowilaty, S.R.; Alkuraya, H.; Alhommadi, A.; Cai, H.; Hou, R.; Deng, W.T.; Boye, S.L.; Almaghamsi, A.; et al. Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: Results of a phase I trial. Hum Genet. 2016, 135, 327–343.
- Schwartz, S.D.; Tan, G.; Hosseini, H.; Nagiel, A. Subretinal Transplantation of Embryonic Stem Cell–Derived Retinal Pigment Epithelium for the Treatment of Macular Degeneration: An Assessment at 4 Years. Investig. Opthalmol. Vis. Sci. 2016, 57, ORSFc1–ORSFc9.
- Simunovic, M.; Xue, K.; Jolly, J.; MacLaren, R. Structural and Functional Recovery Following Limited Iatrogenic Macular Detachment for Retinal Gene Therapy. JAMA Ophthalmol. 2017, 135, 234–241.
- Ben M’Barek, K.; Monville, C. Cell Therapy for Retinal Dystrophies: From Cell Suspension Formulation to Complex Retinal Tissue Bioengineering. Stem Cells Int. 2019, 2019, 4568979.
- Coco-Martin, R.; Pastor-Idoate, S.; Pastor, J. Cell Replacement Therapy for Retinal and Optic Nerve Diseases: Cell Sources, Clinical Trials and Challenges. Pharmaceutics 2021, 13, 865.
- Singh, M.S.; Park, S.S.; Albini, T.A.; Canto-Soler, M.V.; Klassen, H.; MacLaren, R.E.; Takahashi, M.; Nagiel, A.; Schwartz, S.D.; Bharti, K. Retinal stem cell transplantation: Balancing safety and potential. Prog. Retin. Eye Res. 2020, 75, 100779.
- Peng, Y.; Tang, L.; Zhou, Y. Subretinal Injection: A Review on the Novel Route of Therapeutic Delivery for Vitreoretinal Diseases. Ophthalmic Res. 2017, 58, 217–226.
- Narayanan, R.; Dhurandhar, D.; Sahoo, N.; Mariappan, I. Gene therapy in retinal diseases: A review. Indian, J. Ophthalmol. 2021, 69, 2257.
- Jones, M.K.; Lu, B.; Girman, S.; Wang, S. Cell-based therapeutic strategies for replacement and preservation in retinal degenerative diseases. Prog. Retin. Eye Res. 2017, 58, 1–27.
- MacLaren, R.E.; Bennett, J.; Schwartz, S.D. Gene Therapy and Stem Cell Transplantation in Retinal Disease: The New Frontier. Ophthalmology 2016, 123, S98–S106.
- Koponen, S.; Kokki, E.; Kinnunen, K.; Ylä-Herttuala, S. Viral-Vector-Delivered Anti-Angiogenic Therapies to the Eye. Pharmaceutics 2021, 13, 219.
- Chiu, W.; Lin, T.-Y.; Chang, Y.-C.; Lai, H.I.-A.M.; Lin, S.-C.; Ma, C.; Yarmishyn, A.; Lin, S.-C.; Chang, K.-J.; Chou, Y.-B.; et al. An Update on Gene Therapy for Inherited Retinal Dystrophy: Experience in Leber Congenital Amaurosis Clinical Trials. Int. J. Mol. Sci. 2021, 22, 4534.
- Talib, M.; Boon, C.J. Retinal Dystrophies and the Road to Treatment: Clinical Requirements and Considerations. Asia-Pac. J. Ophthalmol. 2020, 9, 159–179.
- Nuzbrokh, Y.; Ragi, S.D.; Tsang, S.H. Gene therapy for inherited retinal diseases. Ann. Transl. Med. 2021, 9, 1278.
- Wilkins, C.S.; Mehta, N.; Wu, C.Y.; Barash, A.; A Deobhakta, A.; Rosen, R.B. Outcomes of pars plana vitrectomy with subretinal tissue plasminogen activator injection and pneumatic displacement of fovea-involving submacular haemorrhage. BMJ Open Ophthalmol. 2020, 5, e000394.
- Iannetta, D.; De Maria, M.; Bolletta, E.; Mastrofilippo, V.; Moramarco, A.; Fontana, L. Subretinal Injection of Recombinant Tissue Plasminogen Activator and Gas Tamponade to Displace Acute Submacular Haemorrhages Secondary to Age-Related Macular Degeneration. Clin. Ophthalmol. 2021, 15, 3649–3659.
- Jeong, S.; Park, D.-G.; Sagong, M. Management of a Submacular Hemorrhage Secondary to Age-Related Macular Degeneration: A Comparison of Three Treatment Modalities. J. Clin. Med. 2020, 9, 3088.
- Sandhu, S.S.; Manvikar, S.; Steel, D.H. Displacement of submacular haemorrhage associated with age-related macular degeneration using vitrectomy and submacular tPA injection followed by intravitreal ranibizumab. Clin Ophthalmol. 2010, 4, 637–642.
- Grohmann, C.; Dimopoulos, S.; Bartz-Schmidt, K.U.; Schindler, P.; Katz, T.; Spitzer, M.S.; Skevas, C. Surgical management of submacular hemorrhage due to n-AMD: A comparison of three surgical methods. Int. J. Retin. Vitr. 2020, 6, 1–8.
- Timmers, A.M.; Zhang, H.; Squitieri, A.; Gonzalez-Pola, C. Subretinal injections in rodent eyes: Effects on electrophysiology and histology of rat retina. Mol. Vis. 2001, 7, 131–137.
- Qi, Y.; Dai, X.; Zhang, H.; He, Y.; Zhang, Y.; Han, J.; Zhu, P.; Zhang, Y.; Zheng, Q.; Li, X.; et al. Trans-Corneal Subretinal Injection in Mice and Its Effect on the Function and Morphology of the Retina. PLoS ONE 2015, 10, e0136523.
- Pang, J.-J.; Lauramore, A.; Deng, W.-T.; Li, Q.; Doyle, T.J.; Chiodo, V.; Li, J.; Hauswirth, W.W. Comparative analysis of in vivo and in vitro AAV vector transduction in the neonatal mouse retina: Effects of serotype and site of administration. Vis. Res. 2008, 48, 377–385.
- Parikh, S.; Le, A.; Davenport, J.; Gorin, M.B.; Nusinowitz, S.; Matynia, A. An Alternative and Validated Injection Method for Accessing the Subretinal Space via a Transcleral Posterior Approach. J. Vis. Exp. 2016, e54808.
- Fang, Y.; Yao, X.-Q.; Niu, L.-L.; Wu, J.-H.; Thee, E.F.; Chen, D.-F.; Chen, J.-Y.; Sun, X.-H. Safety evaluation of subretinal injection of trypan blue in rats. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 2923–2933.
- Mühlfriedel, R.; Michalakis, S.; Garrido, M.G.; Sothilingam, V.; Schön, C.; Biel, M.; Seeliger, M.W. Optimized Subretinal Injection Technique for Gene Therapy Approaches. Methods Mol Biol. 2019, 1834, 405–412.
- Park, S.W.; Kim, J.H.; Park, W.J.; Kim, J.H. Limbal Approach-Subretinal Injection of Viral Vectors for Gene Therapy in Mice Retinal Pigment Epithelium. J. Vis. Exp. 2015, 102, e53030.
- Ochakovski, G.A.; Bartz-Schmidt, K.U.; Fischer, M.D. Retinal Gene Therapy: Surgical Vector Delivery in the Translation to Clinical Trials. Front. Neurosci. 2017, 11, 174.
- Dauletbekov, D.; Bartz-Schmidt, K.U.; Fischer, M.D. Subretinal and Intravitreal Retinal Injections in Monkeys. Methods Mol. Biol. 2018, 1715, 251–257.
- Takahashi, K.; Morizane, Y.; Hisatomi, T.; Tachibana, T.; Kimura, S.; Hosokawa, M.M.; Shiode, Y.; Hirano, M.; Doi, S.; Toshima, S.; et al. The influence of subretinal injection pressure on the microstructure of the monkey retina. PLoS ONE 2018, 13, e0209996.
- Mühlfriedel, R.; Tanimoto, N.; Schön, C.; Sothilingam, V.; Garrido, M.G.; Beck, S.C.; Huber, G.; Biel, M.; Seeliger, M.W.; Michalakis, S. AAV-Mediated Gene Supplementation Therapy in Achromatopsia Type 2: Preclinical Data on Therapeutic Time Window and Long-Term Effects. Front. Neurosci. 2018, 11, 292.
- Schlichtenbrede, F.C.; da Cruz, L.; Stephens, C.; Smith, A.J.; Georgiadis, A.; Thrasher, A.J.; Bainbridge, J.W.; Seeliger, M.W.; Ali, R.R. Long-term evaluation of retinal function in Prph2Rd2/Rd2 mice following AAV-mediated gene replacement therapy. J. Gene Med. 2003, 5, 757–764.
- Butler, M.C.; Sullivan, J.M. Ultrahigh Resolution Mouse Optical Coherence Tomography to Aid Intraocular Injection in Retinal Gene Therapy Research. J. Vis. Exp. 2018, e55894.
- Fan, K.C.; Yannuzzi, N.A.; Patel, N.A.; Negron, C.I.; Sisk, R.A.; Nagiel, A.; Berrocal, A.M. Surgical Techniques for the Subretinal Delivery of Pediatric Gene Therapy. Ophthalmol. Retin. 2020, 4, 644–645.
- Okanouchi, T.; Toshima, S.; Kimura, S.; Morizane, Y.; Shiraga, F. Novel Technique for Subretinal Injection Using Local Removal of the Internal Limiting Membrane. Retina 2016, 36, 1035–1038.
- Xue, K.; Groppe, M.; Salvetti, A.P.; MacLaren, R.E. Technique of retinal gene therapy: Delivery of viral vector into the subretinal space. Eye 2017, 31, 1308–1316.
- Wood, E.H.; Rao, P.; Mahmoud, T.H. Nanovitreoretinal Subretinal Gateway Device to Displace Submacular Hemorrhage: Access to the Subretinal Space Without Vitrectomy. Retina 2019.
- Bainbridge, J.W.; Smith, A.J.; Barker, S.S.; Robbie, S.; Henderson, R.; Balaggan, K.; Viswanathan, A.; Holder, G.E.; Stockman, A.; Tyler, N.; et al. Effect of Gene Therapy on Visual Function in Leber’s Congenital Amaurosis. N. Engl. J. Med. 2008, 358, 2231–2239.
- Fischer, M.D.; Hickey, D.G.; Singh, M.S.M.S.; MacLaren, R.R.E. Evaluation of an Optimized Injection System for Retinal Gene Therapy in Human Patients. Hum. Gene Ther. Methods 2016, 27, 150–158.
- Maguire, A.M.; High, K.A.; Auricchio, A.; Wright, J.F.; Pierce, E.A.; Testa, F.; Mingozzi, F.; Bennicelli, J.L.; Ying, G.-S.; Rossi, S.; et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: A phase 1 dose-escalation trial. Lancet 2009, 374, 1597–1605.
- Kwon, H.J.; Kwon, O.W.; Song, W.K. Semiautomated Subretinal Fluid Injection Method Using Viscous Fluid Injection Mode. Retina 2019, 39 (Suppl. S1), S174–S176.
- Kayikcioglu, O.R.; Mendez, T.; Morrison, V.; Freeman, W.R. A new technique for the subretinal injection of small volumes by using a modified viscous fluid injector system. Retina 2006, 26, 1089–1090.
- Sastry, A.; Li, J.D.; Raynor, W.; Viehland, C.; Song, Z.; Xu, L.; Farsiu, S.; Izatt, J.A.; Toth, C.A.; Vajzovic, L. Microscope-Integrated OCT-Guided Volumetric Measurements of Subretinal Blebs Created by a Suprachoroidal Approach. Transl. Vis. Sci. Technol. 2021, 10, 24.
- Vasconcelos, H.M., Jr.; Lujan, B.J.; Pennesi, M.E.; Yang, P.; Lauer, A.K. Intraoperative optical coherence tomographic findings in patients undergoing subretinal gene therapy surgery. Int. J. Retin. Vitr. 2020, 6, 13.
- Chiang, B.; Jung, J.H.; Prausnitz, M.R. The suprachoroidal space as a route of administration to the posterior segment of the eye. Adv. Drug Deliv. Rev. 2018, 126, 58–66.
- Patel, S.R.; Lin, A.S.; Edelhauser, H.F.; Prausnitz, M.R. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm. Res. 2011, 28, 166–176.
- Kansara, V.; Muya, L.; Wan, C.-R.; Ciulla, T. Suprachoroidal Delivery of Viral and Nonviral Gene Therapy for Retinal Diseases. J. Ocul. Pharmacol. Ther. 2020, 36, 384–392.
More
