The 3D genome organization and its dynamic modulate genome function, playing a pivotal role in cell differentiation and development. CCCTC-binding factor (CTCF) and cohesin, acting as the core architectural components involved in chromatin looping and genome folding, can also recruit other protein or RNA partners to fine-tune genome structure during development. Moreover, systematic screening for partners of CTCF has been performed through high-throughput approaches. In particular, several novel protein and RNA partners, such as BHLHE40, WIZ, MAZ, Aire, MyoD, YY1, ZNF143, and Jpx, have been identified, and these partners are mostly implicated in transcriptional regulation and chromatin remodeling, offering a unique opportunity for dissecting their roles in higher-order chromatin organization by collaborating with CTCF and cohesin.
- CTCF
- 3D genome
- protein partners
- RNA partners
- post-translational modifications
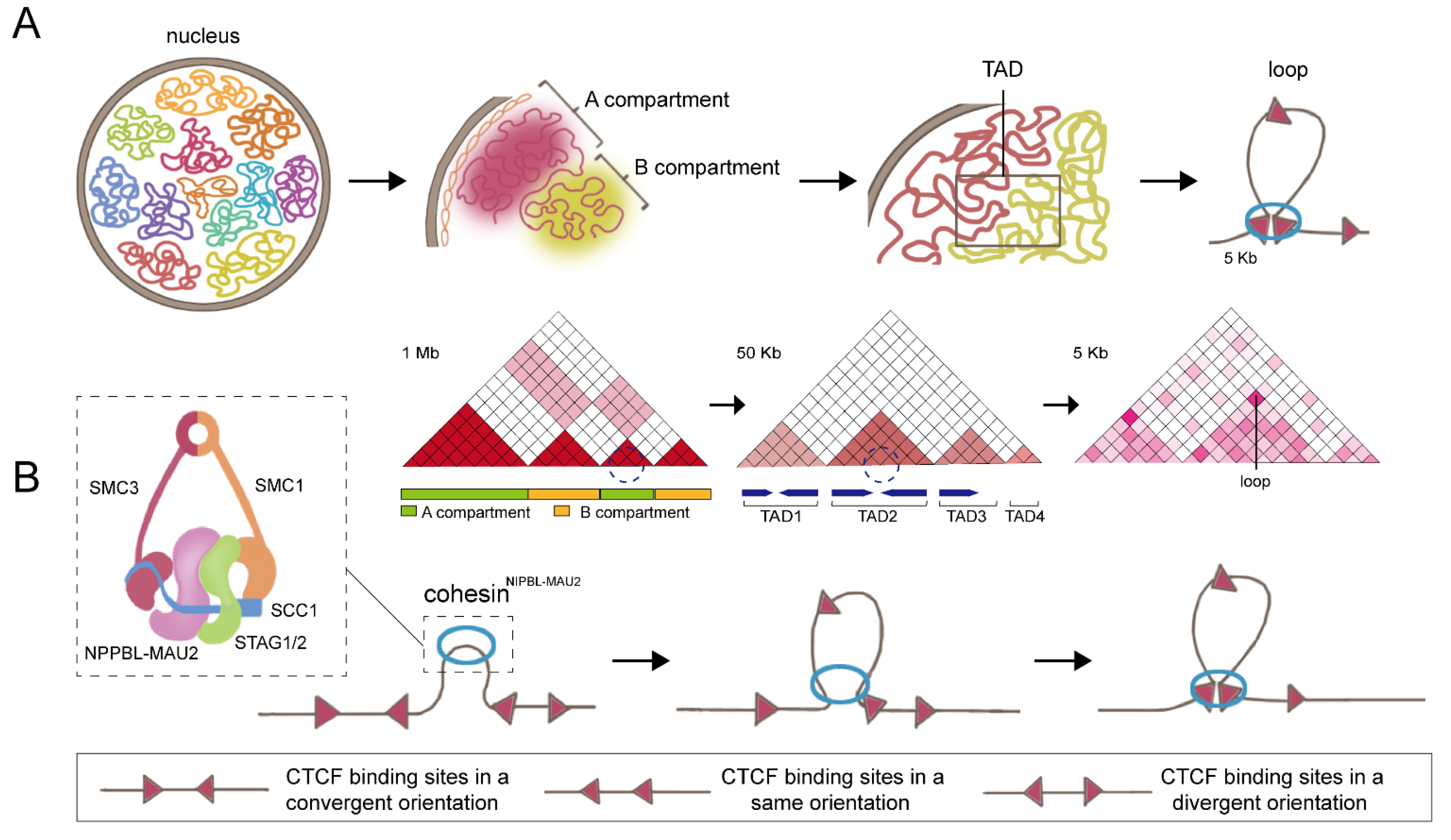
1. Cohesin: The Key Partner of CCCTC-Binding Factor (CTCF)
2. Protein Partners of CTCF
2.1. Systemic Discovery of CTCF Partners
2.2. Transcriptional Regulatory Protein Partners Related to Embryonic Stem Cell Development
2.3. Transcriptional Regulatory Protein Partners Regulating Immune Cell Development
2.4. Transcriptional Regulatory Protein Partners Associated with Muscle Cell Development
2.5. Transcriptional Regulatory Protein Partners Involved in Multiple Developmental Processes
2.6. Transcriptional Regulatory Protein Partners Showing Potential Roles in 3D Genome Organization and Transcriptional Regulation
2.7. Chromatin Remodeling Associated Protein Partners
2.8. Interplay between Nuclear Receptor and CTCF
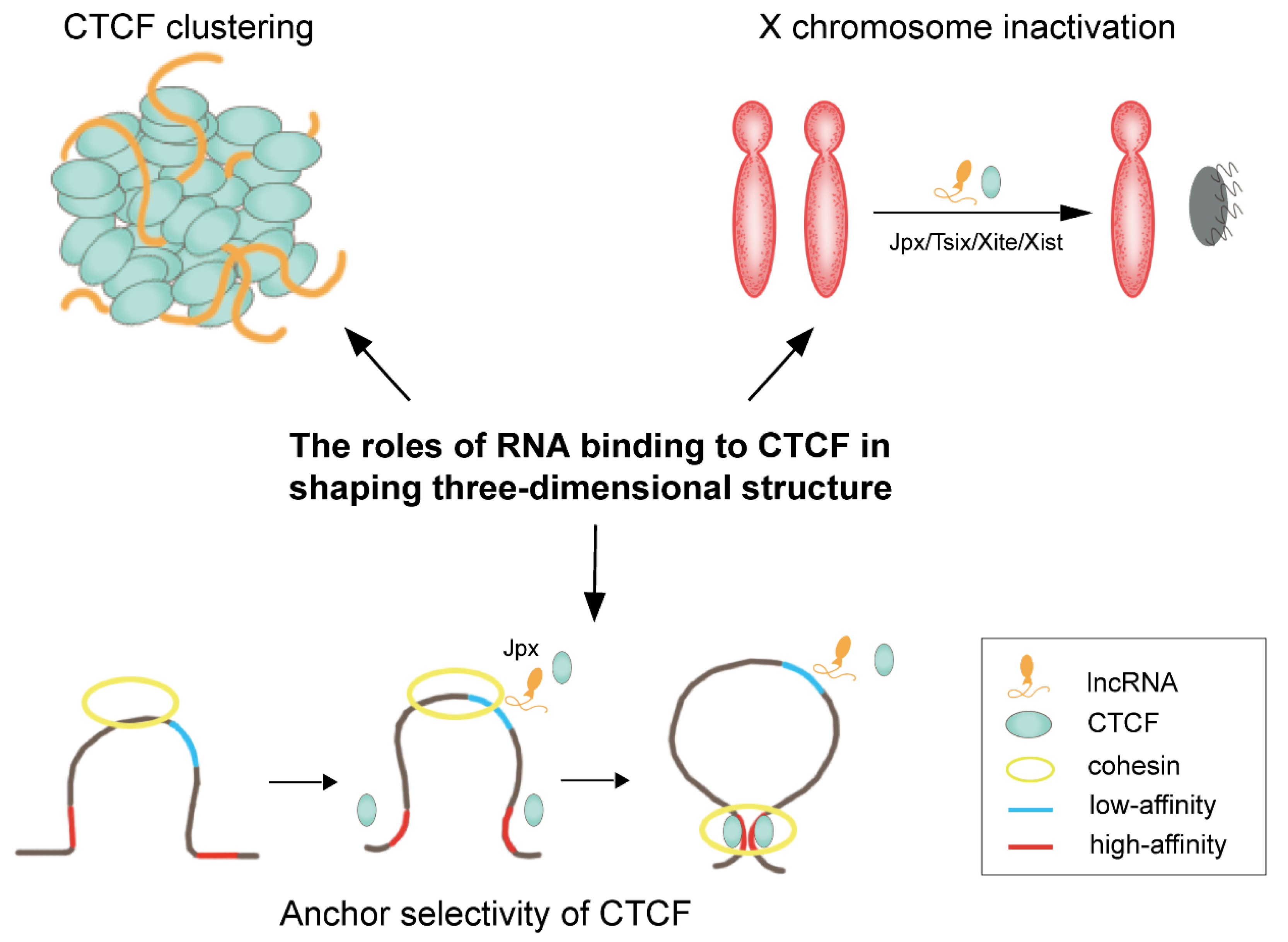
3. RNA Partners of CTCF
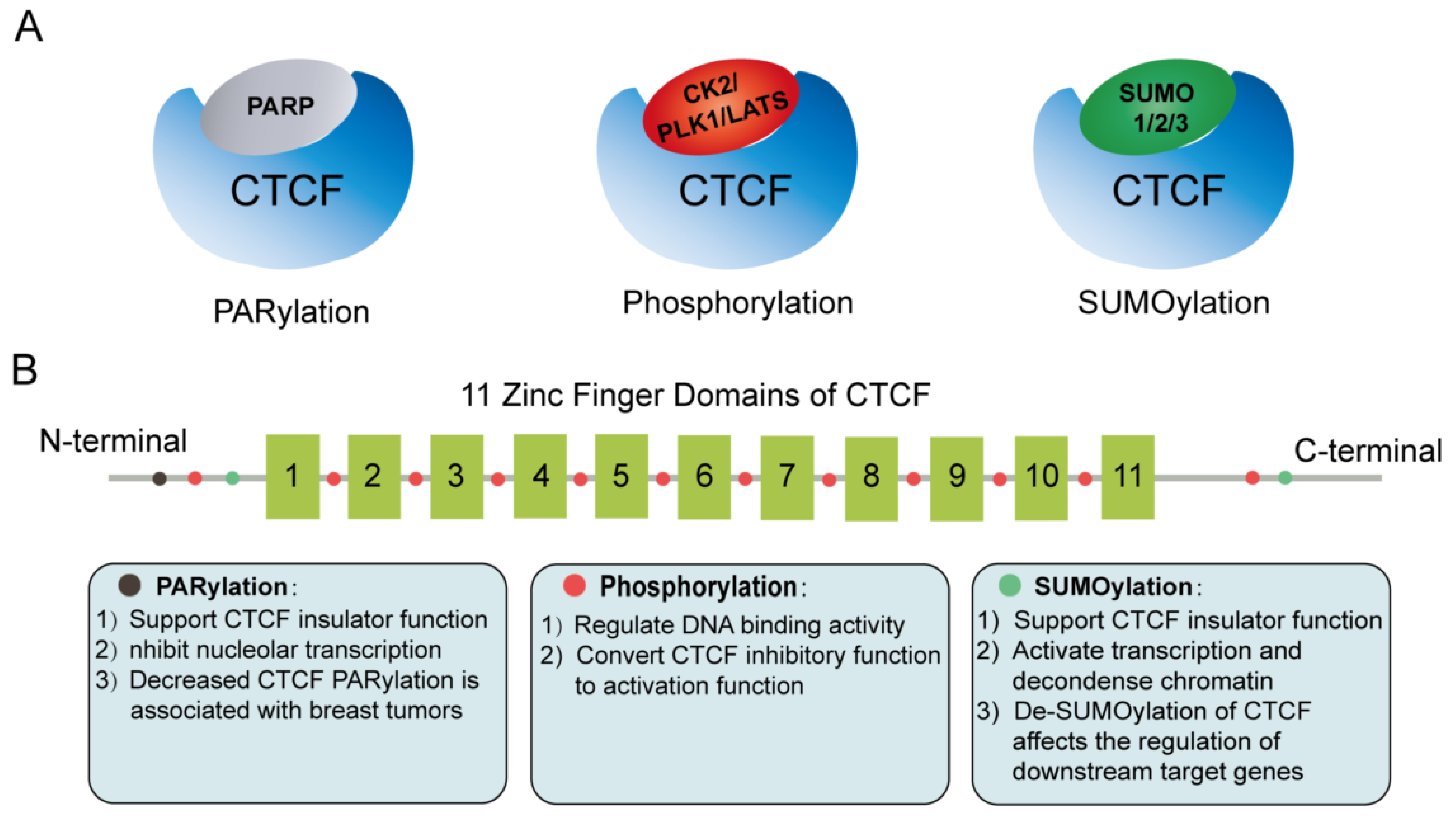
4. Post-Translational Modifications of CTCF
References
- Nasmyth, K. Disseminating the genome: Joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu. Rev. Genet. 2001, 35, 673–745.
- Alipour, E.; Marko, J.F. Self-organization of domain structures by DNA-loop-extruding enzymes. Nucleic Acids Res. 2012, 40, 11202–11212.
- Sanborn, A.L.; Rao, S.S.; Huang, S.C.; Durand, N.C.; Huntley, M.H.; Jewett, A.I.; Bochkov, I.D.; Chinnappan, D.; Cutkosky, A.; Li, J.; et al. Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc. Natl. Acad. Sci. USA 2015, 112, E6456–E6465.
- Davidson, I.F.; Peters, J.M. Genome folding through loop extrusion by SMC complexes. Nat. Rev. Mol. Cell Biol. 2021, 22, 445–464.
- Yatskevich, S.; Rhodes, J.; Nasmyth, K. Organization of Chromosomal DNA by SMC Complexes. Annu. Rev. Genet. 2019, 53, 445–482.
- Sumara, I.; Vorlaufer, E.; Gieffers, C.; Peters, B.H.; Peters, J.M. Characterization of vertebrate cohesin complexes and their regulation in prophase. J. Cell Biol. 2000, 151, 749–762.
- Wendt, K.S.; Yoshida, K.; Itoh, T.; Bando, M.; Koch, B.; Schirghuber, E.; Tsutsumi, S.; Nagae, G.; Ishihara, K.; Mishiro, T.; et al. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature 2008, 451, 796–801.
- Rao, S.S.P.; Huang, S.C.; Glenn St Hilaire, B.; Engreitz, J.M.; Perez, E.M.; Kieffer-Kwon, K.R.; Sanborn, A.L.; Johnstone, S.E.; Bascom, G.D.; Bochkov, I.D.; et al. Cohesin Loss Eliminates All Loop Domains. Cell 2017, 171, 305–320.e24.
- Rubio, E.D.; Reiss, D.J.; Welcsh, P.L.; Disteche, C.M.; Filippova, G.N.; Baliga, N.S.; Aebersold, R.; Ranish, J.A.; Krumm, A. CTCF physically links cohesin to chromatin. Proc. Natl. Acad. Sci. USA 2008, 105, 8309–8314.
- Hansen, A.S.; Pustova, I.; Cattoglio, C.; Tjian, R.; Darzacq, X. CTCF and cohesin regulate chromatin loop stability with distinct dynamics. Elife 2017, 6, e25776.
- Pugacheva, E.M.; Kubo, N.; Loukinov, D.; Tajmul, M.; Kang, S.; Kovalchuk, A.L.; Strunnikov, A.V.; Zentner, G.E.; Ren, B.; Lobanenkov, V.V. CTCF mediates chromatin looping via N-terminal domain-dependent cohesin retention. Proc. Natl. Acad. Sci. USA 2020, 117, 2020–2031.
- Fudenberg, G.; Imakaev, M.; Lu, C.; Goloborodko, A.; Abdennur, N.; Mirny, L.A. Formation of Chromosomal Domains by Loop Extrusion. Cell Rep. 2016, 15, 2038–2049.
- Guo, Y.; Xu, Q.; Canzio, D.; Shou, J.; Li, J.; Gorkin, D.U.; Jung, I.; Wu, H.; Zhai, Y.; Tang, Y.; et al. CRISPR Inversion of CTCF Sites Alters Genome Topology and Enhancer/Promoter Function. Cell 2015, 162, 900–910.
- Gabriele, M.; Brandão, H.B.; Grosse-Holz, S.; Jha, A.; Dailey, G.M.; Cattoglio, C.; Hsieh, T.S.; Mirny, L.; Zechner, C.; Hansen, A.S. Dynamics of CTCF- and cohesin-mediated chromatin looping revealed by live-cell imaging. Science 2022, 376, 496–501.
- Kueng, S.; Hegemann, B.; Peters, B.H.; Lipp, J.J.; Schleiffer, A.; Mechtler, K.; Peters, J.M. Wapl controls the dynamic association of cohesin with chromatin. Cell 2006, 127, 955–967.
- Haarhuis, J.H.I.; van der Weide, R.H.; Blomen, V.A.; Yáñez-Cuna, J.O.; Amendola, M.; van Ruiten, M.S.; Krijger, P.H.L.; Teunissen, H.; Medema, R.H.; van Steensel, B.; et al. The Cohesin Release Factor WAPL Restricts Chromatin Loop Extension. Cell 2017, 169, 693–707.e14.
- Rhodes, J.; Mazza, D.; Nasmyth, K.; Uphoff, S. Scc2/Nipbl hops between chromosomal cohesin rings after loading. Elife 2017, 6, e30000.
- Hu, G.; Dong, X.; Gong, S.; Song, Y.; Hutchins, A.P.; Yao, H. Systematic screening of CTCF binding partners identifies that BHLHE40 regulates CTCF genome-wide distribution and long-range chromatin interactions. Nucleic Acids Res. 2020, 48, 9606–9620.
- Chernukhin, I.; Shamsuddin, S.; Kang, S.Y.; Bergstrom, R.; Kwon, Y.W.; Yu, W.; Whitehead, J.; Mukhopadhyay, R.; Docquier, F.; Farrar, D.; et al. CTCF interacts with and recruits the largest subunit of RNA polymerase II to CTCF target sites genome-wide. Mol. Cell. Biol. 2007, 27, 1631–1648.
- Shukla, S.; Kavak, E.; Gregory, M.; Imashimizu, M.; Shutinoski, B.; Kashlev, M.; Oberdoerffer, P.; Sandberg, R.; Oberdoerffer, S. CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature 2011, 479, 74–79.
- Donohoe, M.E.; Silva, S.S.; Pinter, S.F.; Xu, N.; Lee, J.T. The pluripotency factor Oct4 interacts with Ctcf and also controls X-chromosome pairing and counting. Nature 2009, 460, 128–132.
- Wang, F.; Han, J.; Wang, L.; Jing, Y.; Zhu, Z.; Hui, D.; Wang, Z.; Wang, Y.; Dong, Y.; Tan, T. CCCTC-Binding Factor Transcriptionally Targets Wdr5 to Mediate Somatic Cell Reprogramming. Stem Cells Dev. 2017, 26, 743–750.
- Kim, L.K.; Esplugues, E.; Zorca, C.E.; Parisi, F.; Kluger, Y.; Kim, T.H.; Galjart, N.J.; Flavell, R.A. Oct-1 regulates IL-17 expression by directing interchromosomal associations in conjunction with CTCF in T cells. Mol. Cell 2014, 54, 56–66.
- Li, L.; Leid, M.; Rothenberg, E.V. An early T cell lineage commitment checkpoint dependent on the transcription factor Bcl11b. Science 2010, 329, 89–93.
- Wang, W.; Chandra, A.; Goldman, N.; Yoon, S.; Ferrari, E.K.; Nguyen, S.C.; Joyce, E.F.; Vahedi, G. TCF-1 promotes chromatin interactions across topologically associating domains in T cell progenitors. Nat. Immunol. 2022, 23, 1052–1062.
- Pikarsky, E.; Porat, R.M.; Stein, I.; Abramovitch, R.; Amit, S.; Kasem, S.; Gutkovich-Pyest, E.; Urieli-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004, 431, 461–466.
- Rudnicki, M.A.; Braun, T.; Hinuma, S.; Jaenisch, R. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell 1992, 71, 383–390.
- Megeney, L.A.; Kablar, B.; Garrett, K.; Anderson, J.E.; Rudnicki, M.A. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996, 10, 1173–1183.
- Sabourin, L.A.; Girgis-Gabardo, A.; Seale, P.; Asakura, A.; Rudnicki, M.A. Reduced differentiation potential of primary MyoD-/- myogenic cells derived from adult skeletal muscle. J. Cell Biol. 1999, 144, 631–643.
- Wang, R.; Chen, F.; Chen, Q.; Wan, X.; Shi, M.; Chen, A.K.; Ma, Z.; Li, G.; Wang, M.; Ying, Y.; et al. MyoD is a 3D genome structure organizer for muscle cell identity. Nat. Commun. 2022, 13, 205.
- Vella, P.; Barozzi, I.; Cuomo, A.; Bonaldi, T.; Pasini, D. Yin Yang 1 extends the Myc-related transcription factors network in embryonic stem cells. Nucleic Acids Res. 2012, 40, 3403–3418.
- Donohoe, M.E.; Zhang, X.; McGinnis, L.; Biggers, J.; Li, E.; Shi, Y. Targeted disruption of mouse Yin Yang 1 transcription factor results in peri-implantation lethality. Mol. Cell. Biol. 1999, 19, 7237–7244.
- Wang, J.; Wu, X.; Wei, C.; Huang, X.; Ma, Q.; Huang, X.; Faiola, F.; Guallar, D.; Fidalgo, M.; Huang, T.; et al. YY1 Positively Regulates Transcription by Targeting Promoters and Super-Enhancers through the BAF Complex in Embryonic Stem Cells. Stem Cell Rep. 2018, 10, 1324–1339.
- Dong, X.; Guo, R.; Ji, T.; Zhang, J.; Xu, J.; Li, Y.; Sheng, Y.; Wang, Y.; Fang, K.; Wen, Y.; et al. YY1 safeguard multidimensional epigenetic landscape associated with extended pluripotency. Nucleic Acids Res. 2022, gkac230.
- Donohoe, M.E.; Zhang, L.F.; Xu, N.; Shi, Y.; Lee, J.T. Identification of a Ctcf cofactor, Yy1, for the X chromosome binary switch. Mol. Cell 2007, 25, 43–56.
- Mehra, P.; Gerasimova, T.; Basu, A.; Jha, V.; Banerjee, A.; Sindhava, V.; Gray, F.; Berry, C.T.; Sen, R.; Atchison, M.L. YY1 controls Eμ-3’RR DNA loop formation and immunoglobulin heavy chain class switch recombination. Blood Adv. 2016, 1, 15–20.
- Medvedovic, J.; Ebert, A.; Tagoh, H.; Tamir, I.M.; Schwickert, T.A.; Novatchkova, M.; Sun, Q.; Huis In ‘t Veld, P.J.; Guo, C.; Yoon, H.S.; et al. Flexible long-range loops in the VH gene region of the Igh locus facilitate the generation of a diverse antibody repertoire. Immunity 2013, 39, 229–244.
- He, Y.; Dupree, J.; Wang, J.; Sandoval, J.; Li, J.; Liu, H.; Shi, Y.; Nave, K.A.; Casaccia-Bonnefil, P. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron 2007, 55, 217–230.
- Beagan, J.A.; Duong, M.T.; Titus, K.R.; Zhou, L.; Cao, Z.; Ma, J.; Lachanski, C.V.; Gillis, D.R.; Phillips-Cremins, J.E. YY1 and CTCF orchestrate a 3D chromatin looping switch during early neural lineage commitment. Genome Res. 2017, 27, 1139–1152.
- Xiao, T.; Li, X.; Felsenfeld, G. The Myc-associated zinc finger protein (MAZ) works together with CTCF to control cohesin positioning and genome organization. Proc. Natl. Acad. Sci. USA 2021, 118, e2023127118.
- Talbert, P.B.; Henikoff, S. Histone variants at a glance. J. Cell Sci. 2021, 134, jcs244749.
- Cairns, B.R. Emerging roles for chromatin remodeling in cancer biology. Trends Cell Biol. 2001, 11, S15–S21.
- Cairns, B.R. Chromatin remodeling: Insights and intrigue from single-molecule studies. Nat. Struct. Mol. Biol. 2007, 14, 989–996.
- Rege, M.; Subramanian, V.; Zhu, C.; Hsieh, T.H.; Weiner, A.; Friedman, N.; Clauder-Münster, S.; Steinmetz, L.M.; Rando, O.J.; Boyer, L.A.; et al. Chromatin Dynamics and the RNA Exosome Function in Concert to Regulate Transcriptional Homeostasis. Cell Rep. 2015, 13, 1610–1622.
- Wen, Z.; Zhang, L.; Ruan, H.; Li, G. Histone variant H2A.Z regulates nucleosome unwrapping and CTCF binding in mouse ES cells. Nucleic Acids Res. 2020, 48, 5939–5952.
- Porter, B.A.; Ortiz, M.A.; Bratslavsky, G.; Kotula, L. Structure and Function of the Nuclear Receptor Superfamily and Current Targeted Therapies of Prostate Cancer. Cancers 2019, 11, 1852.
- Warwick, T.; Schulz, M.H.; Gilsbach, R.; Brandes, R.P.; Seuter, S. Nuclear receptor activation shapes spatial genome organization essential for gene expression control: Lessons learned from the vitamin D receptor. Nucleic Acids Res. 2022, 50, 3745–3763.
- Fiorito, E.; Sharma, Y.; Gilfillan, S.; Wang, S.; Singh, S.K.; Satheesh, S.V.; Katika, M.R.; Urbanucci, A.; Thiede, B.; Mills, I.G.; et al. CTCF modulates Estrogen Receptor function through specific chromatin and nuclear matrix interactions. Nucleic Acids Res. 2016, 44, 10588–10602.
- Achinger-Kawecka, J.; Valdes-Mora, F.; Luu, P.L.; Giles, K.A.; Caldon, C.E.; Qu, W.; Nair, S.; Soto, S.; Locke, W.J.; Yeo-Teh, N.S.; et al. Epigenetic reprogramming at estrogen-receptor binding sites alters 3D chromatin landscape in endocrine-resistant breast cancer. Nat. Commun. 2020, 11, 320.
- Kung, J.T.; Kesner, B.; An, J.Y.; Ahn, J.Y.; Cifuentes-Rojas, C.; Colognori, D.; Jeon, Y.; Szanto, A.; del Rosario, B.C.; Pinter, S.F.; et al. Locus-Specific Targeting to the X Chromosome Revealed by the RNA Interactome of CTCF. Mol. Cell 2015, 57, 361–375.
- Saldana-Meyer, R.; Rodriguez-Hernaez, J.; Escobar, T.; Nishana, M.; Jacome-Lopez, K.; Nora, E.P.; Bruneau, B.G.; Tsirigos, A.; Furlan-Magaril, M.; Skok, J.; et al. RNA Interactions Are Essential for CTCF-Mediated Genome Organization. Mol. Cell 2019, 76, 412–422.e15.
- Hansen, A.S.; Hsieh, T.-H.S.; Cattoglio, C.; Pustova, I.; Saldaña-Meyer, R.; Reinberg, D.; Darzacq, X.; Tjian, R. Distinct Classes of Chromatin Loops Revealed by Deletion of an RNA-Binding Region in CTCF. Mol. Cell 2019, 76, 395–411.e313.
- Kuang, S.; Wang, L. Identification and analysis of consensus RNA motifs binding to the genome regulator CTCF. NAR Genom. Bioinform. 2020, 2, lqaa031.
- Quinodoz, S.A.; Ollikainen, N.; Tabak, B.; Palla, A.; Schmidt, J.M.; Detmar, E.; Lai, M.M.; Shishkin, A.A.; Bhat, P.; Takei, Y.; et al. Higher-Order Inter-chromosomal Hubs Shape 3D Genome Organization in the Nucleus. Cell 2018, 174, 744–757.e24.
- Lee, J.T.; Bartolomei, M.S. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell 2013, 152, 1308–1323.
- Sun, S.; Del Rosario, B.C.; Szanto, A.; Ogawa, Y.; Jeon, Y.; Lee, J.T. Jpx RNA activates Xist by evicting CTCF. Cell 2013, 153, 1537–1551.
- Oh, H.J.; Aguilar, R.; Kesner, B.; Lee, H.G.; Kriz, A.J.; Chu, H.P.; Lee, J.T. Jpx RNA regulates CTCF anchor site selection and formation of chromosome loops. Cell 2021, 184, 6157–6173.e24.
- Yao, H.; Brick, K.; Evrard, Y.; Xiao, T.; Camerini-Otero, R.D.; Felsenfeld, G. Mediation of CTCF transcriptional insulation by DEAD-box RNA-binding protein p68 and steroid receptor RNA activator SRA. Genes Dev. 2010, 24, 2543–2555.
- Saldana-Meyer, R.; Gonzalez-Buendia, E.; Guerrero, G.; Narendra, V.; Bonasio, R.; Recillas-Targa, F.; Reinberg, D. CTCF regulates the human p53 gene through direct interaction with its natural antisense transcript, Wrap53. Genes Dev. 2014, 28, 723–734.
- Ghafouri-Fard, S.; Dashti, S.; Taheri, M. The HOTTIP (HOXA transcript at the distal tip) lncRNA: Review of oncogenic roles in human. Biomed. Pharmacother. 2020, 127, 110158.
- Thomas, C.; Tulin, A.V. Poly-ADP-ribose polymerase: Machinery for nuclear processes. Mol. Asp. Med. 2013, 34, 1124–1137.
- Yu, W.; Ginjala, V.; Pant, V.; Chernukhin, I.; Whitehead, J.; Docquier, F.; Farrar, D.; Tavoosidana, G.; Mukhopadhyay, R.; Kanduri, C.; et al. Poly(ADP-ribosyl)ation regulates CTCF-dependent chromatin insulation. Nat. Genet. 2004, 36, 1105–1110.
- Farrar, D.; Rai, S.; Chernukhin, I.; Jagodic, M.; Ito, Y.; Yammine, S.; Ohlsson, R.; Murrell, A.; Klenova, E. Mutational analysis of the poly(ADP-ribosyl)ation sites of the transcription factor CTCF provides an insight into the mechanism of its regulation by poly(ADP-ribosyl)ation. Mol. Cell. Biol. 2010, 30, 1199–1216.
- Zhao, H.; Sifakis, E.G.; Sumida, N.; Millan-Arino, L.; Scholz, B.A.; Svensson, J.P.; Chen, X.; Ronnegren, A.L.; Mallet de Lima, C.D.; Varnoosfaderani, F.S.; et al. PARP1- and CTCF-Mediated Interactions between Active and Repressed Chromatin at the Lamina Promote Oscillating Transcription. Mol. Cell 2015, 59, 984–997.
- Torrano, V.; Navascués, J.; Docquier, F.; Zhang, R.; Burke, L.J.; Chernukhin, I.; Farrar, D.; León, J.; Berciano, M.T.; Renkawitz, R.; et al. Targeting of CTCF to the nucleolus inhibits nucleolar transcription through a poly(ADP-ribosyl)ation-dependent mechanism. J. Cell Sci. 2006, 119, 1746–1759.
- Pavlaki, I.; Docquier, F.; Chernukhin, I.; Kita, G.; Gretton, S.; Clarkson, C.T.; Teif, V.B.; Klenova, E. Poly(ADP-ribosyl)ation associated changes in CTCF-chromatin binding and gene expression in breast cells. Biochim. Biophys. Acta Gene Regul. Mech. 2018, 1861, 718–730.
- Docquier, F.; Kita, G.X.; Farrar, D.; Jat, P.; O’Hare, M.; Chernukhin, I.; Gretton, S.; Mandal, A.; Alldridge, L.; Klenova, E. Decreased poly(ADP-ribosyl)ation of CTCF, a transcription factor, is associated with breast cancer phenotype and cell proliferation. Clin. Cancer Res. 2009, 15, 5762–5771.
- MacPherson, M.J.; Beatty, L.G.; Zhou, W.; Du, M.; Sadowski, P.D. The CTCF insulator protein is posttranslationally modified by SUMO. Mol. Cell. Biol. 2009, 29, 714–725.
- Kitchen, N.S.; Schoenherr, C.J. Sumoylation modulates a domain in CTCF that activates transcription and decondenses chromatin. J. Cell Biochem. 2010, 111, 665–675.
- Wang, J.; Wang, Y.; Lu, L. De-SUMOylation of CCCTC Binding Factor (CTCF) in Hypoxic Stress-induced Human Corneal Epithelial Cells. J. Biol. Chem. 2012, 287, 12469–12479.
- Delgado, M.D.; Chernukhin, I.V.; Bigas, A.; Klenova, E.M.; León, J. Differential expression and phosphorylation of CTCF, a c-myc transcriptional regulator, during differentiation of human myeloid cells. FEBS Lett. 1999, 444, 5–10.
- El-Kady, A.; Klenova, E. Regulation of the transcription factor, CTCF, by phosphorylation with protein kinase CK2. FEBS Lett. 2005, 579, 1424–1434.
- Sekiya, T.; Murano, K.; Kato, K.; Kawaguchi, A.; Nagata, K. Mitotic phosphorylation of CCCTC-binding factor (CTCF) reduces its DNA binding activity. FEBS Open Bio 2017, 7, 397–404.
- Del Rosario, B.C.; Kriz, A.J.; Del Rosario, A.M.; Anselmo, A.; Fry, C.J.; White, F.M.; Sadreyev, R.I.; Lee, J.T. Exploration of CTCF post-translation modifications uncovers Serine-224 phosphorylation by PLK1 at pericentric regions during the G2/M transition. Elife 2019, 8, 42341.
- Luo, H.; Yu, Q.; Liu, Y.; Tang, M.; Liang, M.; Zhang, D.; Xiao, T.S.; Wu, L.; Tan, M.; Ruan, Y.; et al. LATS kinase-mediated CTCF phosphorylation and selective loss of genomic binding. Sci. Adv. 2020, 6, eaaw4651.
