Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Waqas Asghar and Version 8 by Jessie Wu.
Marine bioactive peptides (MBAPs) that are present in many marine species, including fish, sponges, cyanobacteria, fungi, ascidians, seaweeds, and mollusks, have gained widespread attention for their health-promoting benefits. MBAPs obtained from marine species have ameliorating potential against many health conditions, such as hypertension, diabetes, obesity, HIV, cancer, oxidation, and inflammation. Various research studies have indicated that MBAPs can be utilized as novel lead structures for the treatment of HIV in conjunction with pharmaceuticals and functional foods owing to their potential therapeutic, and antiretroviral (ARV) activities.
- bioactive peptides
- anti-HIV
- drugs
- marine organisms
- antiretroviral agents
1. Techniques Used for the Commercial Preparation and Purification of Marine Bioactive Peptides
1. Techniques Used for the Commercial Preparation and Purification of MBAPs
MBAPs have been extracted from various sources, including fish, algae, crustaceans, and mollusks, in addition to various marine waste products, such as shells, substandard muscles, viscera, trimmings, and skins [1][67]. The bioactive peptides (BAPs) are either procured from proteins during digestion and food processing or are already present in the products prior to the processing operations [2][68]. The latter consist of both ribosomal BAPs, as well as their non-ribosomal counterparts (depsipeptides, cyclic peptides, and non-natural amino acid residues) [3][69]. Figure 1 is a schematic representation of the stages involved in the preparation and purification of marine peptides.
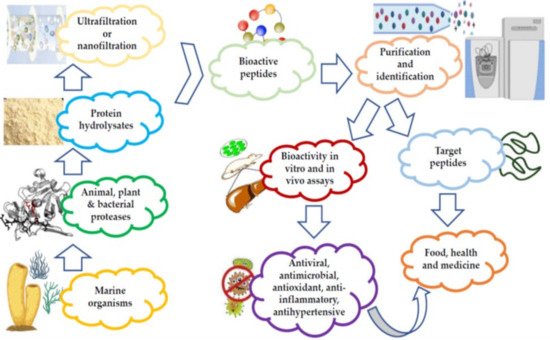
Figure 1.
Preparation and purification of marine peptides.
Different techniques have been used for the extraction of these peptides, such as organic solvents and acid/alkaline solutions. Extraction is followed by isoelectric precipitation, although, this results in the production of peptides with potentially adverse effects on the environment. Additionally, it is often accompanied by a high cost of production, leading to a longer purification process [4][5][14,70]. Recent research on antimicrobial marine peptides, or their fractions thereof, are reported to have a molecular weight <10 kDa and a high degree of antimicrobial activity [6][71]. Some of the techniques employed for the preparation and purification of MBAPs are listed in Table 1.
2. Marine Sponge-Derived Bioactive Peptides against HIV
Table 1. Summary of the techniques used for the preparation and purification of MBAPs commercially [72,73,74,75,76,77,78,79,80,81,82,83,84,85].
| Techniques | General Properties | Type of Peptides |
Sources | Solvents/ Chemicals Applied |
|---|---|---|---|---|
| Preparation Techniques | ||||
| Solvent extraction | Less effective and time-consuming | Collagen peptides | Cod skin, marine crab, hemolymph | Ethanol, methanol, acetone, ethyl acetate, hexane butanol, and methanol |
| Microwave-assisted extraction (MAE) | Uses electromagnetic radiations of 300 MHz to 300 GHz, enhanced yield, rapid and selective extraction | Applied to fish, shrimp, brown seaweed, and oyster | Utilizes a series of solvents from heptane to water | |
| Chemical Hydrolysis | Simple and inexpensive | Fish protein hydrolysates | Applied to fish, bycatch fish | Sulfuric acid, hydrochloric acid, nitric acid, malic acid, oxalic acid, and phosphoric acids are majorly used |
| Enzyme Hydrolysis | Alcalase, flavourzyme, neutrase, trypsin, pepsin, papain, and bromelain are primarily used, more controllable and reproducible method, temperature, time, pH, enzyme concentration, and water/matter ratio are the major variables | Two purified dipeptides Tyr-Arg (337.2 Da) and Ile-Arg (287.2 Da), crude protein hydrolysates, papain hydrolysates, YVMRF peptide | Marine algae, marine sponge (Stylotella aurantium), Bellerochea and Nitzschia species, Stolephorus chinensis | |
| Increased cost and fouling are the main problems, MF (pore size is 0.1–10 µm), UF (pore size is 0.001–0.1 µm) | UF is used for nonhydrolyzed proteins and macro peptides | Generally applied to mackerel, shrimp, Spirulina platensis | ||
| Gel Filtration Chromatography | Simple and mild method, separate based on size, varying elution conditions, higher selectivity, and resolution, sample time consumption is the major limitation | Applied to fish and marine plants | Aqueous buffer (pH 6–8) | |
| Ion-Exchange Chromatography | It captures target protein and bulk impurities, Capto, MacroCap, and Monobeads are some of the mediums used | Applied to the mussel | Aqueous solutions or buffers containing organic solvents such as methanol and acetonitrile | |
| * HPLC including RP-HPLC, MS, LC-MS/MS, ESI, MALDI-TOF, HPLC-ELSD, UHPLC-MS/MS and RRLC–MS. | Higher resolution and sensitivity, ease of operation, rapid, expensive, and environment unfriendly | SAITAPGGAM peptide, collagen peptides, cyclic heptapeptide Euryjanicin A, ALGPGPT, LVPPLA, LAPPTM, GVLIG and GHPVL, ILTLAALGGL, IITGGL, AAPSTVL, and TVAPPGA | Applied to cyanobacteria, fish, sponge, snail and Palmaria palmata, cod skin, Prosuberites laughlini, Stonefish, Dunaliella salina | Choline-oxalic acid based on eutectic solvent |
* HPLC (High-performance liquid chromatography), reverse-phase—high-performance liquid chromatography, RP-HPLC, mass spectrometry (MS), liquid chromatography followed by tandem mass spectrometric detection (LC-MS/MS), electrospray ionization (ESI), matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF), high-performance liquid chromatography with evaporative light scattering detection (HPLC-ELSD), ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS), and liquid chromatography-tandem mass spectrometry (RRLC–MS).
2. Marine Sponge-Derived Bioactive Peptides against HIV
Marine sponges are soft-structured, filter-feeding, bioactive component-rich, aquatic invertebrate parazoans from the phylum Porifera, and act as diversified habitats for numerous marine species [21][86]. Sponges contain diverse biomolecules of varying chemical and structural characteristics that exhibit various bioactive attributes [22][87]. This is proven by the fact that out of nine FDA-approved marine drugs, four are contributed by sponges [23][88]. Research reports suggest that many of the bioactive metabolites isolated from sponges are generated by their functional enzyme clusters and the microorganisms associated with these sponges [24][89].
Koshikamides from the sponges of Theonella sp. have been reported to exhibit anti-HIV activity. In this regard, relative to their linear counterparts, the cyclic koshikamides F and H inhibited HIV entry with IC50 values of 2.3 and 5.5 µM, respectively, when tested in a single round HIV-1 infectivity assay against a CCR5-using viral envelope [25][26][90,91]. Additionally, the peptides were evaluated for their cytotoxicity against various target cell lines, including a control kidney cell line (BSC-1), a human colon tumor cell line (HCT-116), and the target cell line TZM-bl. Favorably, neither koshikamide F nor koshikamide H exhibited cytotoxicity toward any of the target cell lines at these concentrations [25][90]. Although, koshikamide H was found to exhibit moderate cytotoxicity against the HCT-116 target cell line with an IC50 value of 10 µM [27][92]. The anti-HIV activity of the cyclic koshikamides F and H has been attributed to the presence of the 10 AA residue lactone ring in their structures, a characteristic absent in the linear analogs of these peptides [28][55]. Furthermore, koshikamide F has been proven to be slightly more potent in terms of anti-HIV activity, owing to the distinctive macrolactone conformation induced by the presence of the unsaturated pyrrolidinone residue Apdp [25][90].
Depsipeptides (also termed cyclodepsipeptides) with unique non-proteinogenic amino acid combinations inherently incorporated into their structures are isolated from various species of marine sponges and are of particular interest as powerful molecules aimed at drug development against HIV [23][88]. Callipeltin A (source: Callipelta sp. and Latrunculia sp.) and neamphamide A (source: Neamphius huxleyi) have also been proven to prevent the replication of HIV [29][93]. Callipeltin A, in an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay, inhibited HIV-1-induced (Lai strain, X4 tropic) cytopathic effects in CEM4 lymphocytic cell lines at an EC50 value of 0.01 μg/mL, and a TC50 value of 0.29 μg/mL [30][94]. Additionally, the structural similarities to the potent antiviral family of compounds called didemnins might also imply that callipeltin A possesses anti-HIV activity [31][95]. Similarly, neamphamide A demonstrated HIV-inhibitory activity in a 2,3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)-2H-Tetrazolium-5-Carboxanilide (XTT) cell viability assay in a human T-cell line CEM-SS infected with HIV-1RF at an IC50 value of 28 nM, and a TC50 value of 260 nM [30][94]. Furthermore, homophymine A, a depsiundecapeptide from a New Caledonian species of marine sponges, Homophymia sp. demonstrated cytoprotective activity against HIV infection. The anti-HIV activity was evaluated by an XTT cell viability assay in HIV-1-infected cells (IIIB strain). An assessment at seven days post-infection indicated that the compound inhibited HIV-1 infection production with an IC50 value of 75 nM [32][33][96,97]. Also, a TC50 value of 1.19 μM was recorded against host cells when direct cytotoxic measurements of homophymine A were undertaken [32][33][96,97].
Anti-HIV activity has also been recorded for mirabamides E–H derived from the marine sponges Stelletta clavosa, along with the already recognized mirabamides A–D isolated from the sponge Siliquariaspongia mirabilis. The activity of mirabamides was tested using HIV-1 neutralization assays against two viral strains, HXB2 (T-cell-tropic) and SF162 (macrophage-tropic), in addition to an HIV-1 envelope-mediated cell fusion assay to ascertain whether these peptides act to prevent the entry of the virus during the initial stages of the infection [30][34][94,98]. Mirabamides A, C, and D exhibited significant inhibitory activity against HXB2 infection of the TZM-bl host cells (involved in the expression of CXCR4, CCR5, and CD4) with IC50 values of 140 nM, 140 nM, and 190 nM, respectively, while mirabamide B was relatively less effective with an IC50 value of >50 μM [30][34][94,98]. Comparatively, mirabamides A, C, and D were relatively less potent towards SF162 (IC50 values of 400 nM, 1 μM, and 1 μM, respectively), while mirabamide B once again exhibited weak inhibitory activity toward SF162 [30][34][94,98]. As mirabamides A, C, and D have been found to inhibit HIV in both the aforementioned neutralization and fusion assays, it may be surmised that these peptides can act at the initial stages of HIV entry into the host cell, preventing viral entry and subsequent fusion [30][34][94,98]. Likewise, pseudotyped viruses with an enveloped HIV-1 strain when tested in single-round infectivity assays against the new cyclodepsipeptides mirabamides E–H (isolated from the hairy olives sponge S. clavosa), in parallel with mirabamide C, exhibited significant antiviral replication activity in genital epithelial cells (expressing CCR5 and CD4 HIV-1 coreceptors) with IC50 values of 121, 62, 68, and 41 nM, for mirabamides E–H, respectively. Therefore, these compounds also seem to be inhibitors of viral entry during the initial stages of infection by virtue of their binding capabilities to the HIV-1 envelope glycoprotein for inhibition of viral fusion into the host cell membrane [35][99].
Along with the previously reported depsipeptides with HIV-inhibitory activity (such as mirabamides) from S. clavosa, two new depsipeptides, namely stellettapeptin A (EC50: 23 nmol/L) and stellettapeptin B (EC50: 27 nmol/L) were discovered, exhibiting potent HIV-inhibitory activities [23][88]. TFor this study, the anti-HIV activity of stellettapeptins was evaluated in an XTT-based cell viability assay by using the human T-cell line CEM-SS infected with HIV-1RF. After an incubation period of 6 days, the incubated compounds were found effective to inhibit the cytopathic effect of HIV-1. The toxicity of these depsipeptides against the host cells was observed with IC50 values of 367 and 373 nM, respectively [36][100].
Microspinosamide, a tridecadepsipeptide from the sponge Sidonops microspinosa, is another cyclic depsipeptide with an inhibitory effect against cytopathic action of HIV-1 infection in an in vitro XTT-based assay with an EC50 value of 0.2 μg/mL [37][101]. Similarly, theopapuamide B, isolated from the bacteria symbiosis sponges Theonella swinhoei and S. mirabilis exhibited anti-HIV activity in an in vitro single-round HIV-1 infectivity assay against HIV-1 SF162 envelope pseudotyped viruses with an IC50 value of 0.8 µg/mL [37][101]. Celebeside A from S. mirabilis has similarly been attributed with HIV-1 neutralization activity with an IC50 value of 1.9 µg/mL in a single-round infectivity assay [37][101].
One of the primary reservoirs of latent infection is the CD4+T cell containing integrated and transcriptionally silenced HIV-1 proviruses [38][102]. The activation of these resting cells, particularly after prolonged periods of dormancy, results in proviral gene expression of HIV-1 and consequent renewal of viral infection [39][103]. The increasing emphasis has therefore been on exploring therapeutic interventions aimed at complete elimination of the latent proviruses (sterilized cure) [40][104]. Of particular research, interest is what is commonly termed as the ‘shock and kill’ strategies, whereby the transcription of HIV-1 proviruses present in resting CD4+ T reservoir cells is de-repressed with the aid of small molecule drugs for the production of replicating virus, which could be inhibited through simultaneous administration of highly active antiretroviral therapy (HAART) [41][105]. The identification of various latency reversal agents (LRAs) capable of activating HIV-1 proviral gene expression from marine sponge Phorbas sp. has been a significant development in this context [39][103]. The latency reversal activity in the majority of LRAs can be attributed to their ability to activate adenosine 3′,5′-monophosphate (AMP), and protein kinase C (PKC) signaling [42][106]. The structures of sponge-derived peptides are demonstrated in Figure 2.
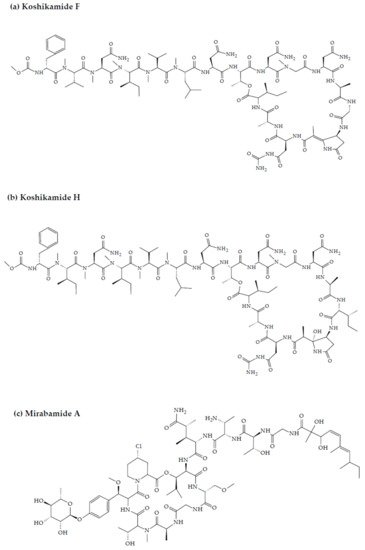
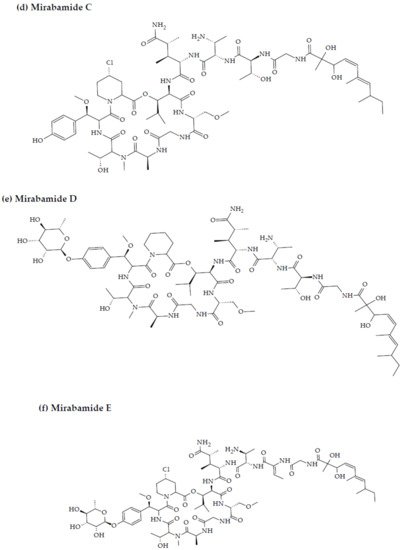
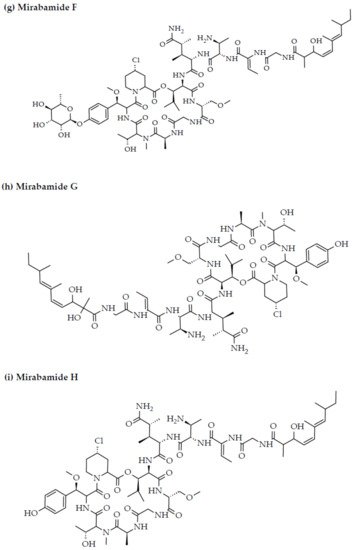
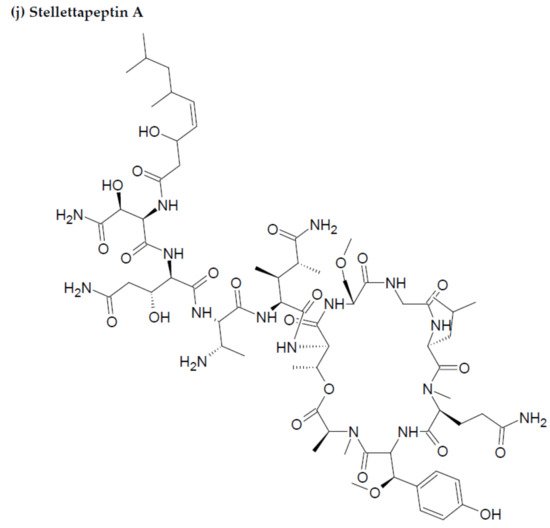
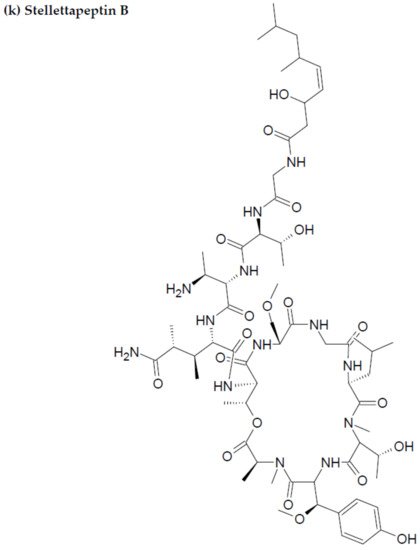
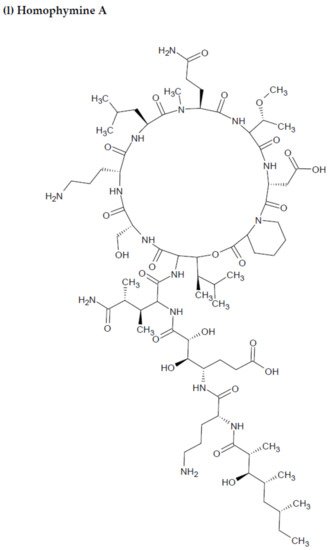
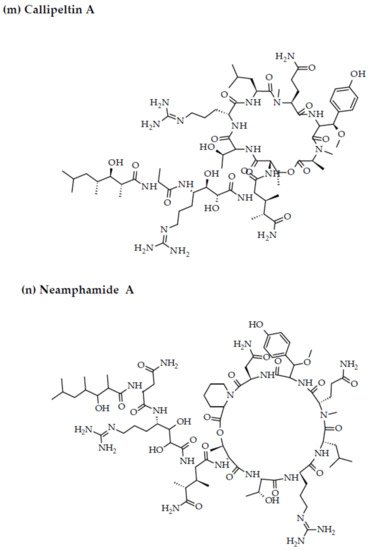
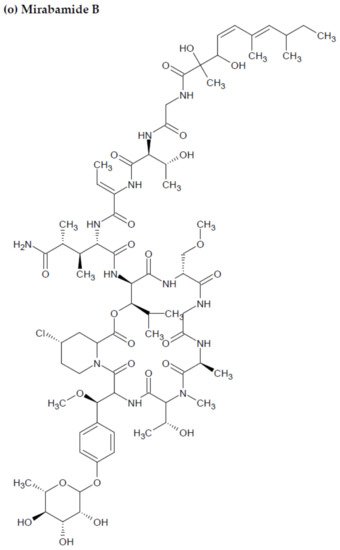
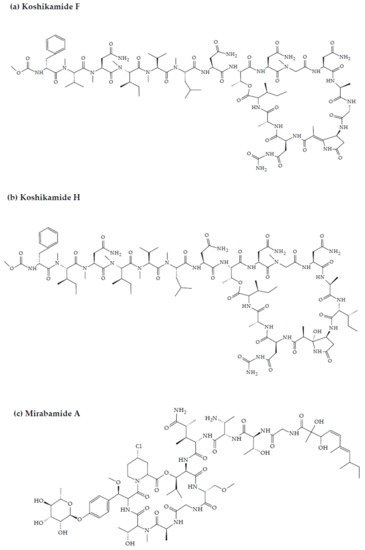
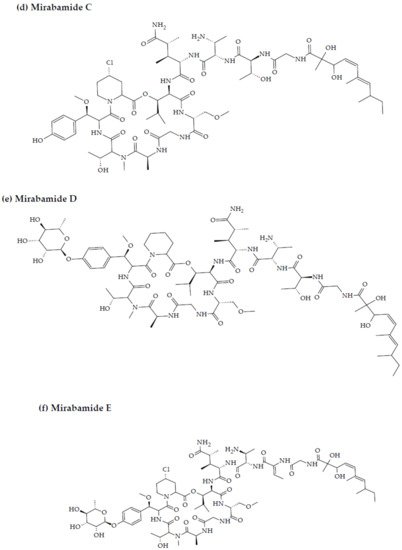
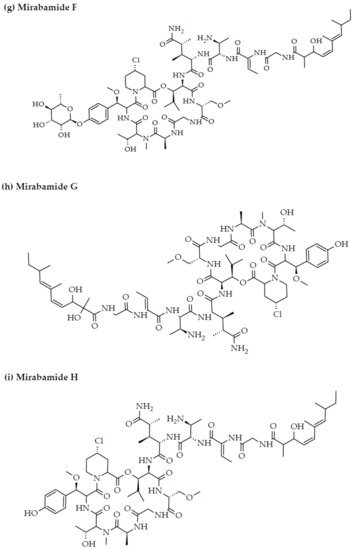
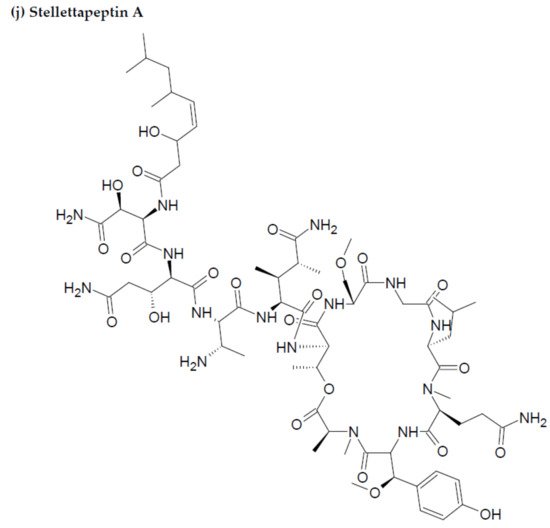
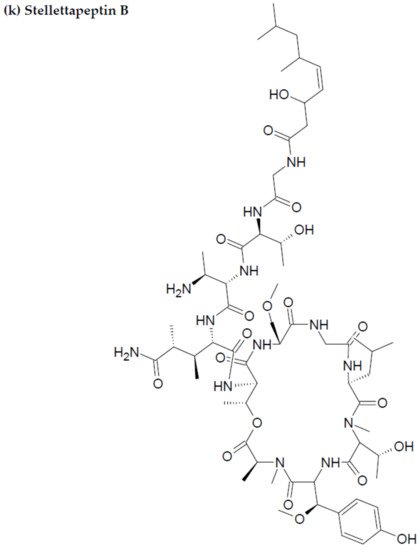
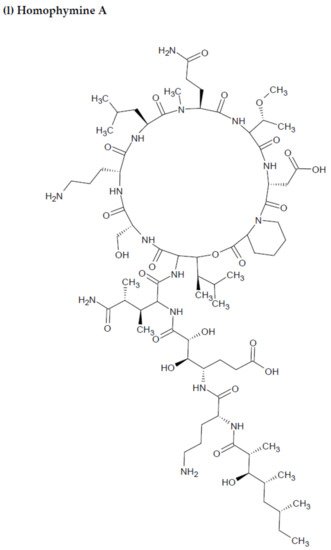
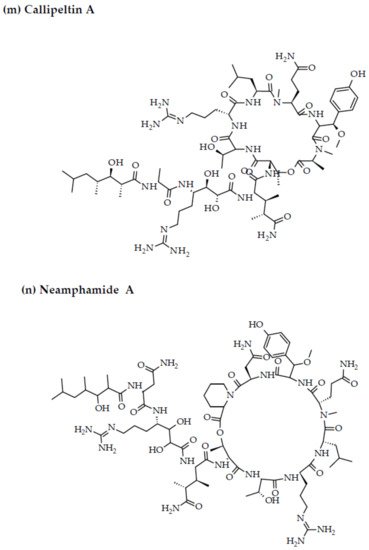
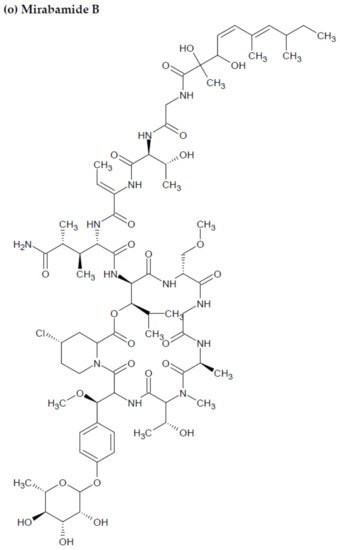
Figure 2. Chemical structures of MBAPs derived from marine sponges. (a) koshikamide F, (b) koshikamide H, (c) mirabamide A, (d) mirabamide C, (e) mirabamide D, (f) mirabamide E, (g) mirabamide F, (h) mirabamide G, (i) mirabamide H, (j) stellettapeptin A, (k) stellettapeptin B, (l) homophymine A, (m) callipeltin A, (n) neamphamide A, and (o) mirabamide B.
3. Marine Cyanobacteria-Associated Compounds with Anti-HIV Properties
Cyanobacteria are photosynthetic bacteria abundantly observed in nature [43][107]. They contain a large variety of toxins along with several bioactive compounds with potential bioactive attributes such as antitumor, anticancer, antimicrobial, antifungal, anti-inflammatory, and protease inhibition characteristics.
Cyanovirin-N (CV-N), (Figure 2) an 11 kDa protein derived from the cyanobacteria Nostoc ellipsosporum has been tested as an anti-HIV compound (preclinical development) [44][108]. The IC50/EC50 values for native CV-N against various HIV-1 isolates range between 0.1 and 36.8 nM, while against HIV-2 isolates, the IC50/EC50 values range between 2.3 and 7.6 nM [45][46][109,110]. In a recent research study, all three forms (mixed, dimer, monomer) of a recombinant version of CV-N (rCV-N) (monomeric mass: 11.962 kDa), exhibited significant anti-HIV activity with an IC50 range of 0.5−5 nM, a therapeutic index (TI) of ~1000–10,000 in vitro, negligible cytotoxicity of approximately 5 μM, and low endotoxins (mixed form refers to a mixture of monomer, dimer and higher-order oligomers together). TZM-bl cells were infected with HIV-1NL4-3 for the assessment of anti-HIV activity of the rCV-N forms, while their cytotoxicity was determined by an MTT cell proliferation assay. This anti-HIV activity can be attributed to the capability of CV-N to interfere with the fusion of HIV with the CD4+ cell membrane [47][111].
CV-N has demonstrated specific and strong interaction capabilities with the viral envelop glycoprotein gp120 through N-linked oligosaccharides with high mannose content, particularly the glycans Man-8 and Man-9 [48][112]. This consequently prevents the binding of the gp120 to the host CD4 T-cell receptor, in addition to the chemokine CCR5 and CXCR4 co-receptors, thereby inhibiting both the viral entry and the cell-to-cell fusion and transmission [49][113]. Owing to its excellent stability, lower toxicity, broad-spectrum antiviral activities, and highly specific binding to oligosaccharides, CV-N has the potential to be a class-leading compound for the prevention of sexual transmission of HIV [50][114].
Similarly, microvirin (MV-N), isolated from the bloom-forming cyanobacterium Microcystis aeruginosa, is another compound with anti-HIV potential [15][80]. MV-N is a 14.3 kDa protein and has high specificity for α(1-2)mannobiose at the termini of branched high mannose-type glycans on the viral surface [16][81]. MV-N, like CV-N, can neutralize a broad range of clinical isolates, and laboratory-adapted strains with low nanomolar potency against most HIV-1 group M clades of various subtypes and tropics [46][110] with IC50 values ranging between 2.1 and 167 nM. MV-N was much more active against HIV-1 isolates A and B when compared to CV-N [51][115]. MV-N, therefore, is active in the reduction of initiation markers, including CD25, CD69, and HLA-DR, preventing the formation of syncytium between cells infected with HIV-1, and healthy CD4+ T cells, also inhibiting viral replication [51][52][115,116]. Furthermore, as the research study by Huskens et al. evidenced, MV-N induced negligible cytotoxicity in MT4 and peripheral mononuclear blood cells (PMBCs) (CC50 > 35 μM and CC50 > 7 μM, respectively) compared to a much higher CC50 value in the case of CV-N (CC50 of 191 nM and 900 nM in MT-4 cells and PBMCs, respectively) [51][115]. It can be inferred, therefore, that MV-N has a greater potency against HIV-1 and a higher safety profile when compared to CV-N.
Scytovirin (SV-N) is a 9.71 kDa HIV-neutralizing protein initially isolated from the aqueous extracts of the cultured cyanobacterium, Scytonema varium [53][117]. The compound has a high affinity for mannose-rich oligosaccharides on the gp120 envelope, inducing blockage of the viral entry into the target cell [54][55][118,119]. Potent activity has been recorded for SV-N against various HIV-1 clinical isolates and laboratory strains, with EC50 values ranging between 0.4 and 394 nM, and 0.3 and 7 nM, respectively [56][120]. Recombinant SV-N (rSV-N) also inhibited cytopathicity induced by HIV in side-by-side in vitro XTT-based anti-HIV assays using CEM-SS host cells with an EC50 of 4.5 nM, while native SV-N EC50 values ranged between 0.3 and 7 nM [56][57][120,121]. The structures of the most active cyanobacteria-derived peptides are portrayed in Figure 3.
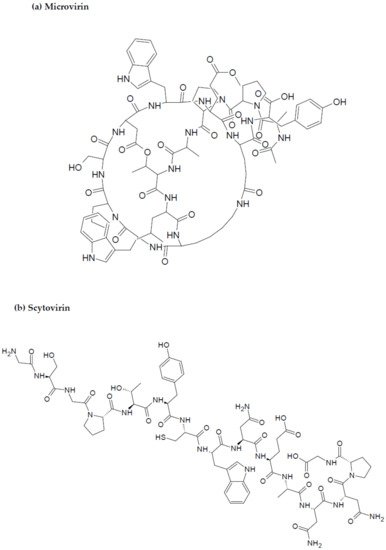
Figure 3.
Chemical structures of MBAPs derived from marine cyanobacteria. (
a
) microvirin, and (
b
) scytovirin.
