Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Marek Wiergowski.
Criminal poisonings are among the least frequently detected crimes in the world. Lack of suspicion of this type of event by police officers and prosecutors, clinical symptoms imitating many somatic diseases and technical difficulties in diagnostics, as well as high research costs make the actual frequency of these events difficult to estimate. The substance used for criminal poisoning is often characterized by: lack of taste, color and smell, delayed action, easy availability and difficulty to detect.
- criminal poisoning
- political intoxication
- ricin
- fentanyl
1. Introduction
Toxicology still refers back to the words of Paracelsus, who stated that toxic effect of each substance depends mostly on its dose. Besides the dose, the toxicity is affected by factors reliant directly on the poisonous substance, such as the route and rate of administration, hydro- and lipophilicity, physical state and formulation (e.g., liquid, gas, solid) and the clinical state of the victim, particularly including: age, sex, body mass, comorbidities and genetic predisposition. The poison used in a criminal poisoning should be characterized by such features as: colorlessness, lack of taste and smell, delayed toxic effects, difficulties in detection and easy availability (Table 1) [1].
Table 1. Properties of pharmacologically active substances facilitated criminal poisoning [1,2][1][2].
| Substance Properties | Estimated Likelihood of Use in | Effect | |
|---|---|---|---|
| Acute Poisonings | Chronic Poisonings | ||
| Tasteless and colorless | high | high | Possibility of an unnoticed administration. |
| Well soluble in water | high | high | Easy administration, quick absorption and distribution. |
Today, with the development of diagnostic techniques, the chance of revealing various types of criminal poisoning has increased. However, it is worth realizing that at the same time there has also been significant progress in planning criminal activities.
2. Criminal Poisoning Cases
WResearchers would like to outline the historical background of politically motivated crime poisonings, with an emphasis primarily on the detection of toxic substances and medical treatment. There are many poisonings in which circumstances are of a political nature but no toxic substances or their metabolites have been detected. One such example is the case of the suspected poisoning of Pyotr Verzilov, Pussy Riot activist [3]. Verzilov fell ill on 11 September 2018 in Moscow. He lost his eyesight and ability to speak, became delirious and lost consciousness. He was hospitalized in Moscow in critical condition and four days later Verzilov was flown to Berlin [4]. Staff of the Berlin Charité hospital believed that, although there were no traces of poison in Verzilov’s system, there was no other explanation for his condition [5].
Table 2 describes selected cases of politically motivated poisonings in the years 1978–2020, with particular emphasis on the circumstances of the event, symptoms of poisoning and the undertaken treatment.
Table 2. Description of political criminal poisonings in the years 1978–2020.
| Poison, Victim of Poisoning (Time, Place and Died/Survived after Poisoning) | Case Description | Symptoms of Poisoning, Treatment Undertaken, Results of Autopsy | |||
|---|---|---|---|---|---|
| Ricin, | Georgi Ivanov Markov (11 September 1978, London, UK, died) [2,6,7] | (11 September 1978, London, UK, died) [2][6][7] |
A month before his death, while visiting a friend in Germany, Markov remembered an anonymous phone call (three months ago) that had threatened him with death if he continued to write broadcasts for Radio Free Europe. The last script Markov prepared for this radio was a text from July 1978—“The Mind Under House Arrest”, in which he accused Bulgarian radio commentators of cowardice and inability to express their own opinions. On 7 September 1978 (the 67th birthday of Bulgarian leader Todor Zhivkov), at circa 1.30 PM, Markov was waiting at the bus stop at the southern end of the Waterloo Bridge. Suddenly he felt pain in the back of his right thigh. As he turned, he saw a man bending down to get a dropped umbrella. The stranger, with a foreign accent, apologized to him for his clumsiness, then took a cab away. At first, Markov didn’t notice the whole thing until someone in the office told him about the red stain on the back of his pants. When Markov looked at the leg, he saw a red dot on his thigh. He assumed that the change would soon disappear and paid no attention to it. |
Markov felt weakness 5 h after the incident. Then there was: fever and vomiting. The next day, the patient was admitted to St. James in Balhama. He had a high fever at the time and complained of abdominal pain, vomiting and diarrhea. After the examination, he was found to have enlarged, painful lymph nodes in his right groin, and a swelling of about 6 cm in diameter was observed on the posterior surface of the right thigh. The next day, the patient’s condition deteriorated—heart rate increased to 160 bpm, blood pressure dropped and increased sweating appeared. Symptoms indicated the development of septic shock. In morphology, an increase in leukocytosis was observed. Further symptoms were diuresis stopped and blood appeared in the vomit. The ECG (performed on 11 September 1978) revealed a complete atrioventricular block with numerous ventricular beats. On the same day, the patient had a cardiac arrest. During the autopsy performed on 12 September 1978, only a small mark on the back of the thigh was found. During internal examination, pulmonary edema resulting from heart failure, mild fatty liver, hemorrhagic necrosis of the small intestine and hemorrhagic lymph nodes in the right groin were found. Necrosis has also been observed in the testes, pancreas, and inguinal lymph nodes. Microscopic examination revealed small hemorrhagic foci in the myocardium. During the autopsy, a small metal ball with a diameter of about 1.5 mm was found, which fell out of the fragment of the thigh collected for examination. Two holes were drilled in the ball—one was open and the other was blind. It was found that the holes would be able to hold about 500 mg of a substance that could have contributed to Markov’s death. Already during the autopsy, it was suspected that the cause of death was not septic shock but poisoning with the toxin placed in the ball. |
|
| Fentanyl, | Khaled Meshal | (25 September 1997, Amman, Jordan, survived) |
Khaled Mashal was a leader of the Palestinian political-military organization Hamas, which had been battling Israel for many years (especially in the area of Gaza Strip). Numerous suicide bombings conducted by the Hamas fundamentalists had led to the order of killing the Palestinian politician given by the authorities of Israel. On 25 September 1997 Meshal was attacked by two men from an Israeli intelligence—Mossad. The attack was carried out by spraying a toxic substance into the victims’ ear. | One of the first symptoms experienced by the victim was tinnitus and a sensation of an electrical current going through his body. After approximately 2 h he started feeling nauseous, short of breath and started vomiting soon after. He was admitted to a hospital where, due to acute respiratory failure, he needed a mechanical ventilation. Meshal’s condition improved only after administering the antidote (naloxone) provided by the Israeli authorities. | |
| Well soluble in fats | high | ||||
| About 4 h after the meal, the patient complained of nausea, vomiting, abdominal pain and watery diarrhea. | The studies revealed: presence of megakaryocytes and an increased number of macrophages, enteritis, severe disseminated intravascular coagulation (DIC) with marked thrombocytopenia, cholestatic jaundice and renal failure. Arafat’s death was due to intussusception following a cerebral hemorrhage. | ||||
| On admission to Charite Hospital, the patient was additionally observed to have hypothermia, bradycardia, impaired trunk reflexes, exaggerated tendon reflexes and pyramidal symptoms. | |||||
| Initial investigations confirmed that the patient had been poisoned with a paralytic agent from the cholinesterase inhibitor group. | Laboratory evidence confirmed reduced butyrylcholinesterase levels in the patient’s blood. Based on samples sent to a specialized unit, Novichok was confirmed in blood, urine and on Navalny’s skin. The treatment included atropine and obidoxime. The patient left the hospital on his own after 33 days, including 24 days of respiratory therapy. |
The common feature of the poisoning cases described above was the alleged or proven involvement of secret services in the physical elimination of the political opponents. The symptoms were experienced by the victims almost immediately after poisoning (fentanyl, VX) or a few hours after exposure (ricin, Novichok, polonium 210Po isotope). The following describes the toxicological properties of selected toxic substances used for criminal purposes, as well as the diagnostic methods and the proposed treatment methods.
3. Toxicological Properties, Treatment and Diagnostics
The physicochemical and toxicological properties for selected criminal poisons are presented in Table 3 [16]. The most important factors determining the potency of the toxic effect were the dose and route of administration. It is worth noting that there is a very limited amount of data on poisons’ doses that are lethal to humans [17].
Table 3. Selected poisonous substances used for criminal purposes in the years 1978–2020 on a political background, their physicochemical and toxicological properties.
| Toxic Substance (CAS) | Physicochemical Properties | Toxicological Properties | Refs. | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lethal/Incapacitating Dose [mg⋅min | −1 | ⋅m | −3 | ] | Time of Death [h] | ||||||||||||||||||||||||||||||
| Ricin | (9009-86-3) |
White powder. It can be prepared in liquid/crystalline form. |
Lethal dose: |
| Several dozen hours (with p.o. possible delay in absorption up to 5 days) | [16,18,19,20] | [16][18][19][20] | ||||||||||||||||||||||||||||
|
| [16,21] | [16][21] | ||||||||||||||||||||||||||||||||
| TCDD | (2,3,7,8-tetrachlorodibenzo-p-dioxin), | Viktor Andriyovych Yushchenko | (5 August 2004, Kyiv, Ukraine, survived) [2very high |
] | Viktor Yushchenko, a political activist in opposition to pro-Russian political groups, and a candidate for president of Ukraine in the presidential election.Possible accumulation in the fatty tissue, as well as slow release from it. | ||||||||||||||||||||||||||||||
| TCDD | (108-88-3) | On the day of the incident after dinner with Igor Smeshko (head of the Security Service), Yushchenko felt bad. Viktor Yushchenko’s wife said that on the evening of poisoning she felt a strange taste of “medicine” when kissing her husband. Viktor Yushchenko survived. Dose of absorbed poison estimated at approx. 1.5–2.5 mg TCDD. |
Within hours of the exposure, Yushchenko developed nausea, vomiting and abdominal pain. On 6 August 2004, the patient was diagnosed with food poisoning. Due to the lack of improvement after treatment, the patient was transferred to the Rudolfinerhaus clinic in Vienna. In the clinic, Yushchenko was diagnosed with acute edema pancreatitis, toxic liver damage and gastrointestinal ulceration. After a week of treatment, the patient returned to Ukraine. Unfortunately, after two weeks, due to back pain and “half palsy of the face”, the patient had to be returned to the Viennese clinic. Approximately 3 weeks after the poisoning, the patient developed a characteristic chloracne on his face. Only this symptom led doctors to suspect dioxin poisoning. This suspicion was finally confirmed on 12 December 2004. |
Crystalline, colorless solid, soluble in organic solvents, hydrophobic | The concentration of TCDD in the blood of Viktor Yushchenko was 50,000 times higher than the general population’s acceptable level of the above-mentioned substance in blood. During the treatment, attempts were made to accelerate the elimination of the xenobiotic by using orally olestra—an unabsorbed fat substitute. As a result of the measures taken, the half-life of TCDD in the patient’s body was reduced from approx. 7–8 years to approx. 15.4 months. The skin changes resolved within 3–5 years. |
Lethal dose LD | 50 | : |
| From several days to several weeks | [16,22,23] | [16][22][23] | Delayed effect onset | high | very high | ||||||||||||||||||||
| Isotope of polonium | Isotope of polonium | 210Po, | Aleksander Litwinienko | (1 November 2006, London, UK, died) [2] |
Alexander Litvinenko (pseudonym Edwin Carter) was a former KGB lieutenant colonel. In 2000, he obtained asylum in Great Britain and began working as a consultant in the British intelligence services. | ||||||||||||||||||||||||||||||
| Fentanyl | (437-38-7) |
Crystal-like solid, moderately water-soluble | Lethal dose: |
| 210Po, | Jasir Arafat | Impedes detection of the perpetrator and assessment of true time and place of intoxication. | ||||||||||||||||||||||||||||
(11 November 2004, Percy, France, died) [2] |
|||||||||||||||||||||||||||||||||||
| Isotope of polonium 210Po | Yassir Arafat was the president of the Palestinian Authority and leader of the liberation movement. Before his death, he was in solitary confinement at the Palestinian Authority headquarters in Ramallah for about 3 years. On 12 October 2004, about 4 h after a meal, he developed severe nausea, vomiting, abdominal pain and then watery diarrhea. On 29 October 2004, the patient was transferred to the French Percy Military Hospital in Clamart, where he was diagnosed with enteritis. Urine tests were negative for gamma-emitting radionuclides. On 11 November 2004, Arafat died of a cerebral hemorrhage. An autopsy was not performed. It was only after the murder of Litvinenko (2006) that it was suggested that the cause of Yassir Arafat’s death could have been polonium-210. As the biological samples collected during the patient’s hospitalization were destroyed in 2008, an exhumation was performed on 27 November 2012, on the basis of which it was found that the hypothesis of Arafat polonium-210 poisoning cannot be fully rejected. |
Radioactive metal, soluble in water, forming simple salts in dilute acids | Lethal dose LD | 50 | : |
| From 2–3 weeks after the onset of symptoms | Unusual and difficult to detect | very high | On 1 November 2006, Litvinenko met with a former KGB agent. He felt bad after eating sushi. He developed stomachache, vomiting and diarrhea. On 3 November 2006, he presented himself to the Emergency Department of Barnet General Hospital under the pseudonym Edwin Carter. On 10 November 2006, the presence of the toxin | very high | Clostridium difficile | Impedes crime detection. | ||||||||||||||||||||||
| [ | 16 | , | was identified. In the course of presenting the diagnosis, the patient revealed his true identity and suggested that he may have been poisoned. On 17 November 2006, the results of the screening showed a slightly elevated thallium concentration, but below the toxic dose. Despite this, the patient was treated. Alexander Litvinenko died on 23 November 2006 as a result of cardiac arrest. Shortly after his death, urine samples collected from him on 22 November 2006 were analyzed, and poisoning with polonium-210 was found. |
Day: 1–3—stomachache, vomiting, diarrhea, upper abdominal tenderness, slightly elevated urea levels. Day 11—the appearance of a fever. Day 13—alopecia, mucositis, progressive cytopenia. Day 18—jaundice with normal levels of alanine transaminase, bloody vomiting. Day 19—arrhythmias, fever, elevated markers of inflammation. Day 20–22—rapid deterioration in kidney function. Day 22—rash, progressive metabolic acidosis, oliguria, hypothermia, cardiogenic shock, impaired consciousness, cardiac arrest twice. Day 23—cardiac arrest, death. Autopsy results: blood-stained fibrous pericarditis, pleural effusion associated with bilateral pneumonia, ascites, generalized tissue autolysis in most organs, brain unchanged. Estimation of the concentration of polonium-210—4400 MBq. |
|||||||||||||||||||||||||||||||
| 24 | ] | [16][24] | ||||||||||||||||||||||||||||||||
| organophosphorus compound VX | (50782-69-9) |
Amber to transparent oily liquid, slightly soluble in water | Lethal dose (predicted): |
|
Incapacitating dose (predicted): |
| A few to several minutes—bronchospasm | [16,25,26,27] | [16][25][26][27] | Low lethal dose | very high | organophosphorus compound VX, | very high |
Kim Jong-nam | (13 February 2017, Kuala Lumpur, Malaysia, died) | Facilitates poisoning by making a low dose necessary to cause death. | |||||||||||||||||||
| [2] | Kim Jong-nam, as the eldest son of Kim Jong-Il, was originally being prepared for his successor [8]. As a result of a diplomatic scandal, his half-brother Kim Jong-Un seized power in North Korea and ordered his relative killed [9,10]. Jong-nam was poisoned at Kuala Lumpur Airport. The killing was carried out by two women. Within 7 s, each applied a poisoned handkerchief to his face [ | ||||||||||||||||||||||||||||||||||
| organophosphorus compound Novichok | (no clear identification of the compound) | 11,12,13]. | Kim Jong-nam, as the eldest son of Kim Jong-Il, was originally being prepared for his successor [8]. As a result of a diplomatic scandal, his half-brother Kim Jong-Un seized power in North Korea and ordered his relative killed [9][10]. Jong-nam was poisoned at Kuala Lumpur Airport. The killing was carried out by two women. Within 7 s, each applied a poisoned handkerchief to his face [11][12][13]. |
After the attack, Kim reported to an airport medical facility. There he observed: trembling hands, hyperhidrosis and weakness [11]. During a physical exam, Jong-nam showed symptoms of cholinergic syndrome [14]. He then received 1 mg of atropine and adrenaline. Due to respiratory failure, he was intubated and connected to a ventilator. The death occurred 20 min after the attack. The autopsy report is probably not available to the public [15] |
Liquid, fine powder, no details available | . | For A-230 (estimated for human): LC | t50 | —1.9–3 mg-min/m | 3 | LD | 50 | —7.5 × 10 | −4 | − 0.002 g/70 kg body weight Dla A-232 (estimated for human): LC | t50 | —7 mg-min/m | 3 | LD | 50 | —0.035 g/70 kg body weight Dla A-234 (estimated for human): LC | t50 | —7 mg-min/m | 3 | LD | 50 | —0.035 g/70 kg body weight | [16,28] | [16][ | Easy access to the substance | low | high | Facilitates crime. | ||
| 28 | ] | Novichok-type organophosphorus compound, | Sergei Skripal | (4 March 2018, London, UK, survived) [2] |
Sergei Skripal became famous as a Russian military intelligence officer who in the 1990s decided to become a double agent for the British intelligence services. He obtained secret information while working at increasingly senior levels of the GRU and later also in government institutions. In December 2004, he was arrested and convicted of treason by a Moscow military court. In 2010, as part of a spy exchange between the Russian Federation and the United Kingdom, Skripal was transported to England, and he settled in Salisbury. Despite being exposed, he continued to cooperate with Western intelligence agencies. On 4 March 2018 Sergei Skripal and his daughter Yulia were found unconscious on a park bench in Salisbury. An investigation by British services found that they were poisoned by two GRU officers, Anatoly Chepiga and Alexander Mishkin, who sprayed the Novichok agent on the doorknob and front door of Skripal’s home. Traces of Novichok were also found in the pub where the Skripals spent the afternoon. The perfume bottle used by the agents as a container for the poison was then dumped in a container for donations to the needy. It was found by a random British man, Charlie Rowley, and given to his partner Dawn Sturgess. The woman died shortly after spraying her wrists with the substance contained therein. Despite wearing a full protective suit, a police officer searching Skripal’s home was admitted to hospital in serious condition. Investigators later determined that the bottle used in the incident contained enough Novichok to kill thousands of people. Specialists identified the agent used as most likely A-234. |
According to the testimony of witnesses present at the scene, Sergei and Yulia Skripal were unconscious. Foam was coming out of Yulia’s mouth. On admission to hospital, the condition of both was described as critical. Due to the characteristic symptoms, treatment with atropine was immediately started, and anticonvulsants were included. The patients’ condition improved gradually. Yulia Skripal left the hospital on 9 April 2018, and her father on 18 May 2018. After the inspection, access to the interior of Skripal’s house was secured. Scaffolding was erected around the building, on which special protections were placed. It took about 4 months to strip the roof, clean the house and rebuild it. All vehicles involved in the incident, including ambulances and police cars, were disposed of and buried in the Cheltenham landfill. It has long been questioned how Skripals managed to survive the attack despite being exposed to such a high dose of A-234. Both one of the Novichok developers and other scientists agreed that weather played a significant role in the case. On the day of the Skripals’ poisoning in Salisbury, it was humid and foggy, and humidity reduces the harmfulness of this type of poison. It is worth noting that the weather conditions only hindered the poison’s absorption, not its toxicity per se. Skripals were also inadvertently helped by the agents who tried to liquidate them. To help Novichok stick to various surfaces, including skin, it was mixed with a gel-like substance. This agent slowed down the absorption of the poison into the body which made it possible to apply effective treatment. |
Chemically stable | ||||||||||||||||||||||||||||
| Novichok-type organophosphorus compound, | high | Alexei Navalny | very high |
(20 August 2020, Tomsk, Russia, survived) | Alexei Navalny, leader of the Russian opposition, chairman of the Russia of the Future party and founder of the Foundation for the Fight against Corruption, has for many years strongly criticized the policies pursued by Vladimir Putin and his United Russia party. On 20 August 2020, Navalny boarded a flight from Tomsk to Moscow. During the flight his well-being suddenly deteriorated. Facilitates storage and transport of the poison. |
||||||||||||||||||||||||||||||
| Therefore, the pilots decided to make an emergency landing in Omsk and transport the patient to a local hospital. | Navalny was successfully transported to the Charité hospital in Berlin on 22 August 2020. Navalny’s subsequent investigation revealed that Novichok had been sewn by FSB agents into the seams in his underwear left in his hotel room. |
The first symptoms noticed in Navalny were pallor, intense sweating, drooling, vomiting and loss of consciousness. Doctors at the Omsk hospital quickly suspected poisoning with an agent from the acetylcholinesterase inhibitor group. Navalny was put into a coma, intubated, mechanically ventilated. The patient was also administered atropine. |
Quickly degradable after death | high | high | Impedes identification of the poison and the cause of death. | |||||||||||||||||||||||||||||
| Occurring naturally within the body | high | high | Does not arouse suspicion in case of detection. | ||||||||||||||||||||||||||||||||
| Occurring naturally in the burial place | low | low | Presence within the corpse does not arouse suspicion of poisoning. | ||||||||||||||||||||||||||||||||
3.1. Ricin
3.1.1. Properties, Metabolic Pathway and Toxic Effects
Ricin is a protein found in a plant called Ricinus communis. It is one of the first lectins detected, i.e., proteins that nonenzymatically attach to membrane sugar receptors. The biological properties of ricin were first described in 1888 by Hermann Stilmark [29]. Ricin is classified as an extremely hazardous substance [30,31][30][31]. However, production of the ricin is difficult to legal control because the castor bean plant from which ricin is derived can be grown at home without any special care. In the US, scientists must register it in the Department of Health and Human Services to use ricin and investigators possessing less than 1 g are exempt from regulation [32]. Castor bean seeds, in addition to ricin itself, also contain a homologous but much less toxic agglutinin called RCA120. Ricin is composed of two RTA and RTB protein chains (having 267 and 262 amino acids, respectively), linked by a disulfide bridge. RCA120, in turn, is a protein composed of four chains, 2 RTA and 2 RTB, linked by a disulfide bridge between the A chains. Both compounds show a very high sequence homology of their amino acids. However, ricin is a potential toxic substance, while RCA120 exhibits strong hemagglutinating properties.
The RTB chain is responsible for binding with galactose. The RTB chain is responsible for the entry of ricin into the cell, which is achieved by the production of endosomes. Some of the chains are transported to the Golgi apparatus. Others to the endoplasmic reticulum, in which the RTB is detached from the endosome protein, and the RTA chain itself passes into the cytosol of the cell using the ERAD (ER-associated degradation) pathway. The RTA chain is an enzyme (RNA N-glycosidase) and is responsible for ribosome inactivation (RIP—ribosome inactivating protein). Its action is based on the removal of adenine rRNA from 28S, which prevents the attachment of the translational factor EF2 (elongation factor-2) to the ribosome. In this way, protein synthesis is inhibited, which ultimately leads to cell death [33].
Ricin poisoning is characterized by a delayed onset of symptoms and a slow action with fatal outcome. The lethal dose of ricin depends on the route of its administration (Table 3). The in vivo toxicity of the substance for humans after oral administration is 1–20 mg/kg body weight, while when administered by injection or inhalation, this dose may be slightly lower [34].
After ingestion of the toxin through the alimentary tract, nausea, vomiting, diarrhea and abdominal pain occur. Within about 4–36 h, symptoms may progress, accompanied by: arterial hypertension, renal failure and liver damage.
In the case of inhalation, symptoms usually develop within about 8 h and include: cough, shortness of breath, joint pain and fever. Occasionally, these types of patients suffer from respiratory distress and death.
In the case of injection, local swelling and redness appear at the site of injection of the toxin. The first symptoms of poisoning develop within approx. 6 h. Among them, the dominant ones are: weakness and muscle pain. After the next 24–36 h, symptoms progress, including: nausea, fever and hypotension, as well as multiorgan failure or death.
3.1.2. First Aid and Treatment
Treatment of ricin poisoning is symptomatic. It includes the intravenous administration of fluids and vasopressors. In case of oral poisoning, it is possible to administer activated charcoal. Gastric lavage is considered only in cases where the intoxicity has occurred no more than one hour earlier.
Currently, the greatest hopes in the postexposure treatment of ricin poisoning are placed in the use of neutralizing antibodies. So far, two preparations for vaccination have been tested: ricin chain deactivated with formaldehyde (ricin toxoid) and deglycosylated ricin. Research using genetic recombination methods has led to the production of the RiVax vaccine.
Animal studies were carried out, including pigs exposed to lethal pulmonary exposure and systemic ricin with the use of anti-ricin F(ab’)2 antibodies of equine origin. The results of this study showed that iIt is possible to create postexposure remedies for ricin poisoning in humans [35].
3.1.3. Diagnostics
Diagnosis of ricin poisoning is based on history, identification of ricin in biological fluids or environmental samples. In clinical samples, ricin is difficult to detect due to its strong binding to glycosylated protein. Free ricin (with a molecular weight of 164 Da) is a marker of castor bean consumption [33]. There are analytical methods that enable the identification of ricin in blood, urine and in the vitreous humor of the eye (including ELISA tests, liquid chromatography with LC-MS/MS tandem mass spectrometry). Unfortunately, the level of ricin in body fluids does not have to correlate with the severity of symptoms.
3.2. Fentanyl
3.2.1. Properties, Metabolic Pathway and Toxic Effects
Fentanyl is a synthetic compound that belongs to the opioid family. First opioid substance was isolated in 1806 by Serturner which, as a tribute to the god of sleep Morpheus, was named morphine. Because of Serturner’s strong addiction to this substance that he developed soon after, he managed to describe the consequences of its chronic abuse in great detail. Since the time of their discovery, opioids have come to be an essential drug in everyday practice of medicine, mainly as analgesics. However, due to their strong addictive potential, they have also been used widely as recreational drugs.
Fentanyl is one of the most potent opioids, being a 100 times more potent than morphine. As a strongly lipophilic substance it enters the tissue compartments with ease (especially the central nervous system) and clinically produces an opioid toxidrome with a very characteristic presentation: bradycardia, bradypnea, hypotonia [36]. Fentanyl is controlled psychoactive substance by drug law in many countries (e.g., in UK, US, Netherlands, Poland, Canada) [37] and is also applied in medicine (only by prescription) [38]. However deaths involving synthetic opioids other than methadone (primarily fentanyl) continued to rise in the US in 2015–2020 [39]. In developed countries, the illicit market for the supply of fentanyl and related substances is very large [40].
On a molecular level fentanyl acts as a μ, δ and κ receptor agonist, each of which is coupled with a Gi protein (it causes a decrease in intracellular cAMP levels). Fentanyl’s interaction with an opioid receptor causes a decrease in calcium influx in the presynaptic neuron, which then suppresses the release of neurotransmitters into the synaptic cleft, as well as hyperpolarization of postsynaptic neuron due to potassium ion the efflux. All these effects impede physiological neuronal transmission [41].
The most important organ in fentanyl metabolism is the liver (Figure 1). The first phase of its biotransformation is conducted by CYP3A4, also present in enterocytes, which determines its particularly strong first pass effect. During the second phase fentanyl’s metabolites are being conjugated with the glucuronic acid, after which they are eliminated with the urine.
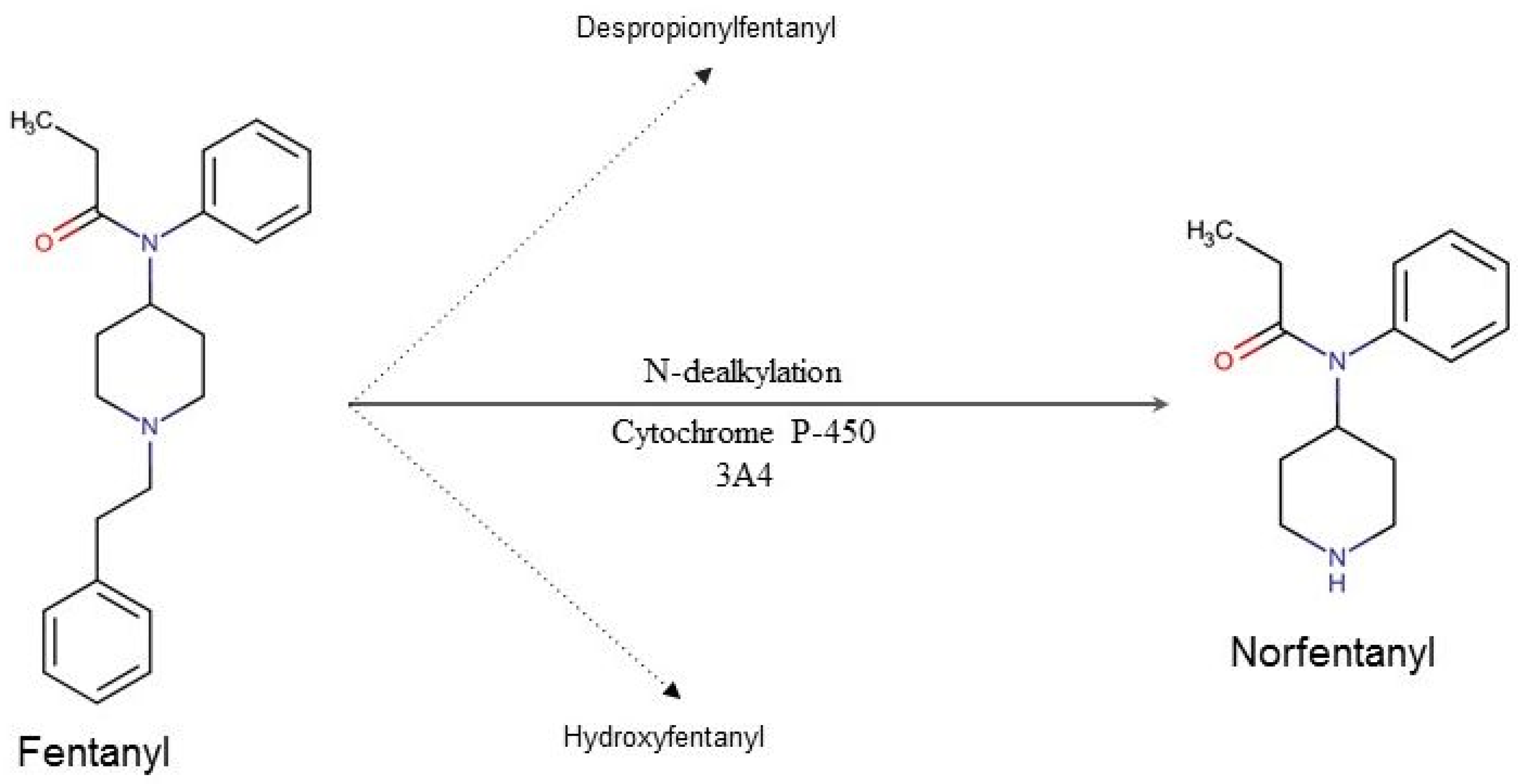
Figure 1. Fentanyl’s metabolic pathway in humans. Ninety-nine percent of the metabolized fentanyl is converted into norfentanyl. The remaining 1% is converted to despropionyl fentanyl and hydroxyfentanyl.
3.2.2. First Aid and Treatment
In spite of a fairly characteristic clinical presentation of an opioid toxidrome, fentanyl intoxication may be sometimes difficult to diagnose, even for an experienced clinician (Figure 2). The most important steps in the treatment are: securing patient’s airways, administering the antidote—naloxone—and providing ventilation support if needed. Naloxone is administered in fractioned doses, with the first one being usually 0.4 mg. After administering naloxone, the patient’s condition should be monitored closely for 2–3 min, and in the absence of improvement, the dose should be increased in a gradual way. Special care must be taken when administering naloxone in patients with an opioid addiction. Overdosing naloxone in such individuals may cause a severe opioid withdrawal syndrome, which can be life-threatening.
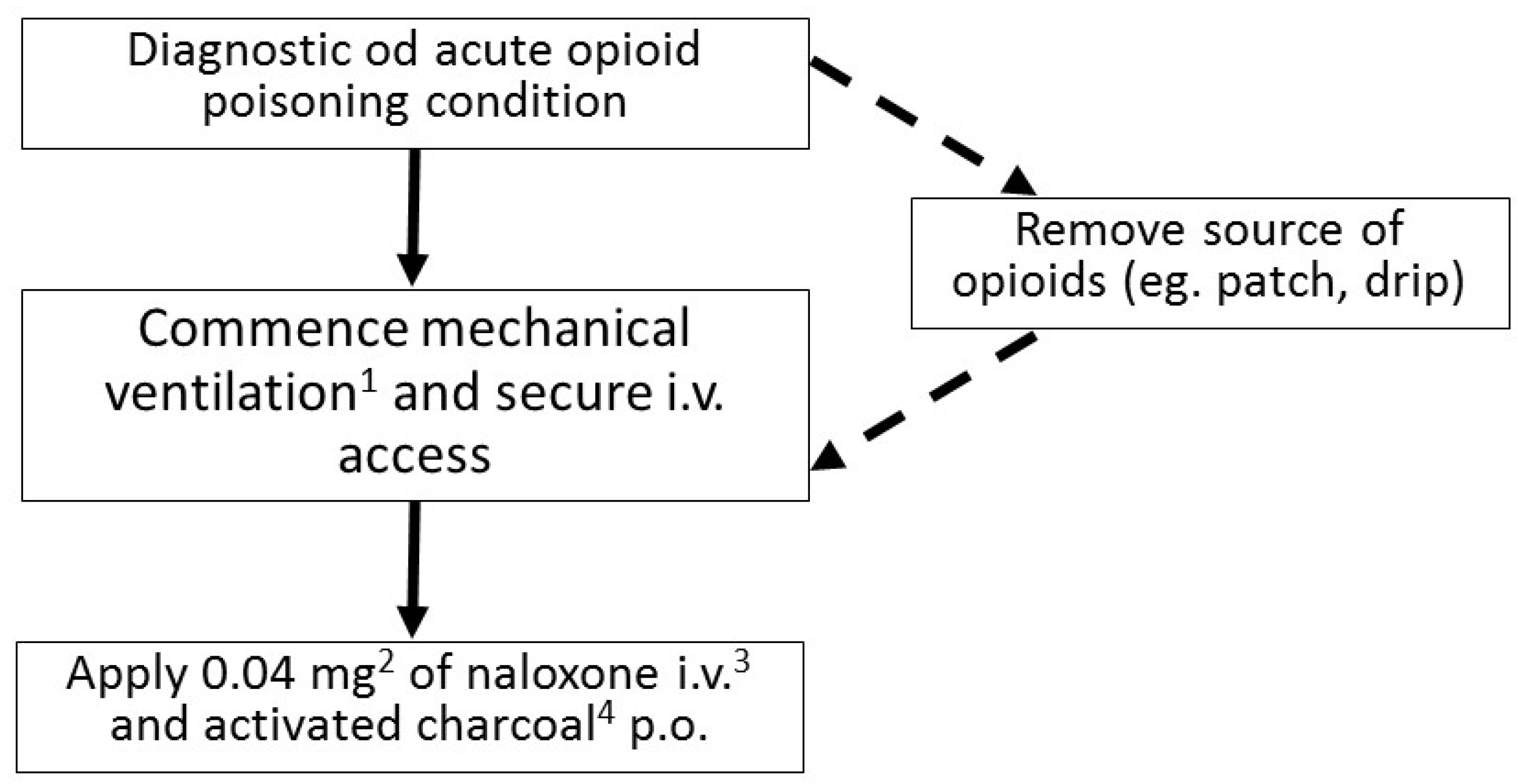
Figure 2. Flowchart describing the management of acute opioid poisoning in an adult (1 Most commonly noninvasive; if the GCS < 8 endotracheal intubation is indicated. 2 If no improvement occurs after 2–3 min the dose can be increased first to 0.5 mg, then, after another 2–3 min, to 2 mg, then to 4 mg, 10 mg, and up to the maximum dose of 15 mg. 3 If securing an i.v. access is impossible, naloxone can be administered i.m. or intranasally. 4 Recommended in absence of contraindications).
References
- Trestrail, J.H. Criminal Poisoning, Investigation Guide For Law Enforcement, Toxicologist, Forensic Scientists, and Attorneys; Humana Press: Totowa, NJ, USA, 2007.
- Nepovimova, E.; Kuca, K. The history of poisoning: From ancient times until modern ERA. Arch. Toxicol. 2019, 93, 11–24.
- Gessen, M. We Now Know More About the Apparent Poisoning of the Pussy Riot Member Pyotr Verzilov. The New Yorker. 2018. Available online: https://www.newyorker.com/news/our-columnists/we-now-know-more-about-the-apparent-poisoning-of-the-pussy-riot-member-pyotr-verzilov (accessed on 20 July 2022).
- Monroe, J. Pussy Riot Member Seized by Police and Accused of Organizing Riots. Pitchfork. 2020. Available online: https://pitchfork.com/news/pussy-riot-member-seized-by-police-and-accused-of-organizing-riots/ (accessed on 30 June 2022).
- BBC News, Pussy Riot’s Pyotr Verzilov Blames Russia for ‘Poisoning’. 2018. Available online: https://www.bbc.com/news/world-europe-45658983 (accessed on 30 June 2022).
- Nehring, C. Umbrella or pen? The murder of Georgi Markov. New facts and old questions. J. Intell. Hist. 2017, 16, 47–58.
- Warner, V.S. ‘Global Dissident’: Georgi Markov as a Cold War Playwright and Exile Rising to the Top: Markov’ s Literary Career in the Early 1960s. Studia Hist. Gedanensia 2014, 2014, 73–94.
- Ryall, J. Profile: Who Was Kim Jong-nam, the Exiled Half-Brother of North Korean Dictator Kim Jong-un? The Telegraph. 2017. Available online: https://www.telegraph.co.uk/news/2017/02/14/profile-kim-jong-nam-exiled-half-brother-north-korean-dictator/ (accessed on 30 June 2022).
- Otto, B.; Ngui, Y. How the Hit Team Came Together to Kill Kim Jong Nam. Wall Str. J. 2017. Available online: https://www.wsj.com/articles/emerging-details-point-to-north-korean-plot-in-scions-killing-1487787023 (accessed on 30 June 2022).
- Sang-Hun, C. Kim Jong-nam, the Hunted Heir to a Dictator Who Met Death in Exile-The New York Times. Wall Str. J. 2017. Available online: https://www.nytimes.com/2017/02/15/world/asia/kim-jong-nam-assassination-north-korea.html (accessed on 30 June 2022).
- Ng, E. Post-Mortem: VX Poison Killed Brother of North Korean Leader. AP NEWS. 2017. Available online: https://apnews.com/article/90e425dbaf1e44d1ba77e2eea890fc67 (accessed on 30 June 2022).
- Amend, N.; Niessen, K.V.; Seeger, T.; Wille, T.; Worek, F.; Thiermann, H. Diagnostics and treatment of nerve agent poisoning-current status and future developments. Ann. N. Y. Acad. Sci. 2020, 1479, 13–28.
- The Chosun Ilbo (English Edition): Kim Jong-nam Says N. Korean Regime Won’t Last Long. 2012. Available online: http://english.chosun.com/site/data/html_dir/2012/01/17/2012011701790.html (accessed on 30 June 2022).
- Leong, T. Kim Jong Nam Had ‘Constricted, Pinpoint Pupils’ and Other Symptoms of VX Poisoning, Court Hears. SE Asia News & Top Stories-The Straits Times. The Straits Times. 2017. Available online: https://www.straitstimes.com/asia/se-asia/kim-jong-nam-had-no-pulse-when-he-arrived-at-klia-airport-clinic-doctor-tells-court (accessed on 30 June 2022).
- Nakagawa, T.; Tu, A.T. Murders with VX: Aum Shinrikyo in Japan and the assassination of Kim Jong-Nam in Malaysia. Forensic Toxicol. 2018, 36, 542–544.
- ChemIDplus (National Library of Medicine). National Library of Medicine. 2022. Available online: https://chem.nlm.nih.gov/chemidplus/name (accessed on 30 June 2022).
- Moyer, R.A.; Sidell, F.R.; Salem, H. Encyclopedia of Toxicology; Elsevier Inc.: Amsterdam, The Netherlands, 2014.
- Polito, L.; Bortolotti, M.; Battelli, M.; Calafato, G.; Bolognesi, A. Ricin: An ancient story for a timeless plant toxin. Toxins 2019, 11, 324.
- Abbes, M.; Montana, M.; Curti, C.; Vanelle, P. Ricin poisoning: A review on contamination source, diagnosis, treatment, prevention and reporting of ricin poisoning. Toxicon 2021, 195, 86–92.
- Audi, J.; Belson, M.; Patel, M.; Schier, J.; Osterloh, J. Ricin Poisoning. JAMA 2005, 294, 2342–2351.
- Peng, P.W.; Sandler, A.N. A Review of the Use of Fentanyl Analgesia in the Management of Acute Pain in Adults. Anesthesiology 1999, 90, 576–599.
- Pohjanvirta, J.; Tuomisto, R. Short-term toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals: Effects, mechanisms, and animal models. Pharmacol. Rev. 1994, 46, 483–549.
- Aylward, L.L.; Brunet, R.C.; Carrier, G.; Hays, S.M.; Cushing, C.A.; Needham, L.L.; Jr, D.G.P.; Gerthoux, P.M.; Brambilla, P.; Mocarelli, P. Concentration-dependent TCDD elimination kinetics in humans: Toxicokinetic modeling for moderately to highly exposed adults from Seveso, Italy, and Vienna, Austria, and impact on dose estimates for the NIOSH cohort. J. Expo. Sci. Environ. Epidemiol. 2005, 15, 51–65.
- Le, M.H. Polonium 210, exposed. J. Med Toxicol. 2007, 3, 82–84.
- Bide, D.J.; Risk, R.W. Inhalation Toxicity of Aerosolized Nerve Agents. 1. VX Revisited; Technical Report DRES-TR 2000-063; National Academies Press: Suffield, AB, Canada, 2000.
- Munro, N. Toxicity of the Organophosphate Chemical Warfare Agents GA, GB, and VX: Implications for Public Protection. Environ. Health Perspect. 1994, 102, 18–37.
- Gratte, J.H.; Yang, L.I. Report of the Workshop on Chemical Agent Toxicity for Acute Effects, Institute for Defense Analyses, 11–12 May 1998; National Technical Reports Library: Alexandria, VA, USA, 2001.
- Ganie, S.Y.; Javaid, D.; Hajam, Y.A.; Reshi, M.S. Mechanisms and treatment strategies of organophosphate pesticide induced neurotoxicity in humans: A critical appraisal. Toxicology 2022, 472, 153181.
- Moshiri, M.; Hamid, F.; Etemad, L. Ricin Toxicity: Clinical and Molecular Aspects. Rep. Biochem. Mol. Biol. 2016, 4, 60–65. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4986263/ (accessed on 30 June 2022).
- The Centers for Disease Control. HHS and USDA Select Agents and Toxins 7 CFR Part 331, 9 CFR Part 121, and 42 CFR Part 73. 2012; Volume 1, p. 2012. Available online: https://www.selectagents.gov/ (accessed on 30 June 2022).
- US Environmental Protection Agency. 40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities; U.S. Government Publishing Office: Washington, DC, USA, 2008; Volume 50, pp. 437–442.
- National Select Agent Registry. Permissible Toxin Amounts. 2017. Available online: https://www.selectagents.gov/sat/permissible.htm?CDC_AA_refVal=https%3A%2F%2Fwww.selectagents.gov%2FPermissibleToxinAmounts.html (accessed on 28 July 2022).
- Bozza, W.P.; Tolleson, W.H.; Rosado, L.A.R.; Zhang, B. Ricin detection: Tracking active toxin. Biotechnol. Adv. 2015, 33, 117–123.
- Coppock, R.W.; Dziwenka, M. Potential Agents That Can Cause Contamination of Animal Feedstuffs and Terror; Elsevier Inc.: Amsterdam, The Netherlands, 2015.
- Falach, R.; Sapoznikov, A.; Evgy, Y.; Aftalion, M.; Makovitzki, A.; Agami, A.; Mimran, A.; Lerer, E.; Ben David, A.; Zichel, R.; et al. Post-Exposure Anti-Ricin Treatment Protects Swine against Lethal Systemic and Pulmonary Exposures. Toxins 2020, 12, 354.
- Sisco, E.; Verkouteren, J.; Staymates, J.; Lawrence, J. Rapid detection of fentanyl, fentanyl analogues, and opioids for on-site or laboratory based drug seizure screening using thermal desorption DART-MS and ion mobility spectrometry. Forensic Chem. 2017, 4, 108–115.
- International Narcotics Control Board (INCB). Narcotic Drug Report. United Nations, 2022. Available online: https://www.incb.org/incb/en/narcotic-drugs/Technical_Reports/2021/narcotic-drugs-technical-report-2021.html (accessed on 30 June 2022).
- Dziennik Urzędowy Ministra Zdrowia. Urzędowy Wykaz Produktów Leczniczych Dopuszczonych do Obrotu na Terytorium Rzeczypospolitej Polskiej. 2016. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20180001591 (accessed on 30 June 2022).
- National Institue on Drug Abuse. Overdose Death Rates. 2022. Available online: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates (accessed on 29 July 2022).
- Drug Enforcement Agency (DEA). Fentanyl Flow to the United States (DEA-DCT-DIR-008-20). 2020; pp. 1–4. Available online: https://www.dea.gov/sites/default/files/2020-03/DEA_GOV_DIR-008-20 Fentanyl Flow in the United States_0.pdf (accessed on 30 June 2022).
- Boyer, E.W. Management of Opioid Analgesic Overdose. N. Engl. J. Med. 2012, 367, 146–155.
More
