Phosphates are known to be essential for plant growth and development, with phosphorus compounds being involved in various physiological and biochemical reactions. Phosphates are known as one of the most important factors limiting crop yields.
- phosphate solubilization
- soil microbiome
- sustainable agriculture
- biofertilizer
Soil Phosphate-Solubilizing Microorganisms
Many types of soil bacteria and fungi can dissolve phosphates in vitro. Phosphate-solubilizing microorganisms increase the bioavailability of soil phosphorus for plants [5]. They dissolve insoluble inorganic (mineral) phosphorus and mineralize insoluble organic phosphates [3]. Salt-tolerant or halophilic soil microorganisms are able to dissolve insoluble phosphates, thereby contributing to the development of agriculture on saline and alkali soils [5]. The ability to solubilize phosphates was characterized in detail for mycorrhizal fungi [16] that are also proven to contribute to the crop nutrition by increasing the volume of rhizosphere and thus the volume of soil from which phosphates can be absorbed [17]. Other soil microorganisms are phosphate-solubilizing bacteria (PSB) that are generally associated with the plant rhizosphere [18]. Table 1 provides information on the genera of phosphate-solubilizing bacteria and fungi.| Bacteria | Fungi | ||
|---|---|---|---|
| Genera | Ref. | Genera | Ref. |
| Aeromonas | [19] | Achrothcium | |
| Phosphate-Solubilizing Microorganism | Ecological Niche | Predominantly Produced Acids | Ref. | ||||
|---|---|---|---|---|---|---|---|
| [ | |||||||
| Escherichia freundii | Soil | 3 | , | Lactic20] | |||
| [ | 62 | ] | Agrobacterium | [ | |||
| Aspergillus niger, Penicillium sp. | 18] | SoilAlternaria | [3,20 | Citric, glycolic, succinic, gluconic, oxalic, lactic] | |||
| [ | 62 | ] | Azotobacter | [21 | |||
| Bacillus megaterium, Pseudomonas sp., Bacillus subtilus | ,22] | Arthrobotrys | Soil rizoshpere[3,20] | ||||
| Lactic, malic | [ | 63 | ] | Bacillus | [23] | Aspergillus | [24] |
| Bradyrhizobium | [25] | ||||||
| Arthrobacter sp., Bascillus sp., Bacillus firmus B-7650 | Wheat and cowpea rhizosphere | Lactic, citric | [64] | Cephalosporium | |||
| Aspergillus sp., Penicillium sp., Chaetomium nigricolor | [ | 3 | , | Lateritic soil20] | |||
| Oxalic, succinic, citric, 2-ketogluconic | [ | 65 | ] | Burkholderia | [19,26] | Chaetomium | [ |
| A. japonicus, A. foetidus | Indian rock phosphate | Oxalic, citric, gluconic, succinic, tartaric | 3,20] | ||||
| [ | 66 | ] | Cyanobacteria | [3] | Cladosporium | [ | |
| P. radicum | Wheat rhizosphere | 3 | ,20] | ||||
| Gluconic | [ | 67 | ] | Enterobacter | [22] | Cunninghamella | |
| Enterobacter agglomerans | [ | 3 | ,20] | ||||
| Wheat rhizosphere | Oxalic, citric | [ | 68] | Erwinia | [27] | Curvularia | [3 |
| Bacillus amyloliquefaciens, B. licheniformis, B. atrophaeus, Penibacillus macerans, Vibrio proteolyticus, Xanthobacter agilis, Enterobacter aerogenes, E. taylorae, E. asburiae, Kluyvera cryocrescens, Pseudomonas aeromonassens, Chrysler | Mangrove | Lactic, itaconic, isovaleric, isobutyric, acetic | ,20] | ||||
| [ | 69 | ] | Kushneria | [5 | |||
| Penicillium rugulosum | ] | Venezuelan phosphate rocksFusarium | Citric, gluconic acid[3,20] | ||||
| [ | 70 | ] | Micrococcus | [28] | Glomus | [3,20] | |
| Enterobacter intermedius | Grass rhizosphere | 2-ketogluconic | [71] | Paenibacillus | [29] | Helminthosporium | [3,20] |
| Pseudomonas | [30,31] | Micromonospora | [3,20] | ||||
| Rhizobium | [32,33] | Phenomiocenspora | [3,20] | ||||
| Rhodococcus | [25] | Phenomiocenspora | [3,20] | ||||
| Salmonella | [25] | Phenomycylum | [34] | ||||
| Serratia | [35] | Populospora | [3,20] | ||||
| Aspergillus flavus, A. niger, Penicillium canescens | Wheat grains | Oxalic, citric, gluconic, succinic | [72] | Sinomonas | [25] | Pythium | [3,20] |
| Thiobacillus | [25] | Rhizoctonia | [3,20] | ||||
| Rhizopus | [3,20] | ||||||
| Saccharomyces | [3,20] | ||||||
| Schizosaccharomyces | [3,20] | ||||||
| Schwanniomyces | [3,20] | ||||||
| Sclerotium | [36] | ||||||
| Torula | [24] | ||||||
| Trichoderma | [24] | ||||||
| Yarrowia | [3,20] |
Biochemical Properties of the Soil Related to the Bioavailability of Phosphates
Soil phosphates exist both in inorganic and organic form. The inorganic (mineral) form of phosphate is represented by primary (apatites, strengite, and variscite) and secondary minerals of phosphorus (iron, aluminum, and calcium phosphates) [38]. The release of phosphate from these minerals is slow and regulated by several factors, particularly by soil pH [38,39]. Under acidic conditions, phosphates are adsorbed on Al/Fe oxides and hydroxides such as gibbsite and goethite. Under alkaline conditions, phosphates are precipitated with calcium. Additionally, phosphorus may be immobilized on the soil clay particles, a phenomenon strongly influenced by the type of ions adsorbed on the surface of clay minerals [40]. Soil microorganisms can immobilize phosphates from the soil. After absorption by microbial cells, phosphorus is incorporated into the cellular structures of microorganisms (e.g., nucleic acids, organic phosphorus esters, free inorganic phosphate, and coenzymes), with excess phosphorus likely to be stored as polyphosphates [41]. Microbial biomass is an important temporary immobilized phosphorus pool that can be mineralized and released into the soil solution as available phosphorus long-term [42,43]. In most microorganisms, the role of a phosphate reserve is played by inorganic polyphosphates, linear polymers of phosphoric acid containing between three and several hundred phosphate residues [44]. Many bacteria accumulate polyphosphates under unfavorable conditions [45]. For example, the accumulation of polyphosphates in E. coli cells occurs under conditions of amino acid deficiency [46]. Enzymes catalyzing the synthesis of polyphosphates are polyP kinases. The role of polyP as a phosphate reserve has been proven for many microorganisms belonging to different taxa, from archaea to fungi [45]. Polyphosphates are rich in “high energy” of anhydride bonds [47], and can be used as an energy source due to the Pi release [48]. Various enzymes are involved in the consumption of accumulated polyphosphates and the breakdown of phosphates outside the cell. Polyphosphate utilization and degradation is catalyzed by polypases, including exopolypase (PPX), and several polyphosphate-specific kinases, including polyP-glucokinase and polyP-fructokinase [45]. The biomass of soil microorganisms stores significant amounts of phosphorus, protecting it from plant adsorption [49,50]. During the biogeochemical recycling process, the phosphorus accumulated by bacteria and fungi is slowly released back into the soil and becomes available to plants, as evidenced by the correlation between phosphorus uptake by plants and phosphorus biomass in microorganisms [51]. The rate of phosphate release from microbial biomass likely depends on the amount of phosphorus available in soil [52], carbon availability [53], soil texture [54], and composition of microbial community [55]. The ability of microorganisms to balance between solubilization (mineralization) and immobilization processes determines the extent to which these bacteria and fungi can improve the availability of phosphorus in the soil–plant system. Figure 1 depicts several biochemical routes of phosphate solubilization by soil bacteria: synthesis of organic and inorganic acids; excretion of ammonia ions; synthesis and excretion of phosphatases and phytases; and synthesis of polysaccharides, cytokinins, and gibberellins.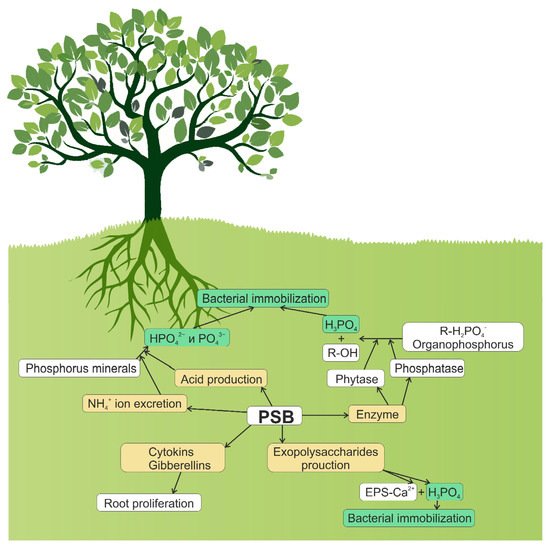
Mechanisms of Inorganic Phosphate Solubilization
In recent years, numerous studies have been done on the characteristics and mechanisms of phosphorus solubilization processes. PSBs are thought to be able to dissolve insoluble phosphates by secreting low molecular weight organic acids [56]. In alkaline soils, phosphates can precipitate to form calcium phosphates, their solubility increasing with a decrease in soil pH. PSBs increase phosphorus availability by secreting organic acids that cause a decrease in soil pH [57,58]. The increase of soil pH leads to the appearance of divalent and trivalent forms of inorganic phosphorus: HPO42− and PO43− [37]. Organic acids are produced in the periplasmic space by direct oxidation [59]. The release of these organic acids into the environment is accompanied by a decrease in pH. Surprisingly, there is no correlation between pH and the amount of solubilized phosphorus [60]. The mechanism of acidification was suggested: the release of H+ is associated with the assimilation of cations. For example, NH4+ assimilation together with H+ excretion leads to phosphate solubilization [61]. An alternative mechanism for the solubilization of mineral phosphates for producing organic acids is the release of H+ to the extracellular surface via the exchange of cation or the ATPase activity with H+ translocation [18]. Also, assimilation of NH4+ in microbial cells was reported to be accompanied by proton release, leading to the solubilization of phosphorus without any organic acids being formed [3| Pseudomonas fluorescens | |||
| Oil palms rhizosphere | |||
| Citric, malic, tartaric, gluconic | |||
| [ | |||
| 73 | |||
| ] | |||
| Aspergillus niger | |||
| Tropical and subtropical soils | |||
| Gluconic, oxalic | [ | 74 | ] |
| P. trivialis | Rhizosphere of Hippophae rhamnoides (cold Howl and Spiti deserts, Trans-Himalayas) | Lactic, formic | [75] |
| B. pumilus var.2; B. subtilis var.2; Actinomadura oligospora; Citrobacter sp. | Giant cardon cactus (P. pringlei) | Gluconic, propionic, isovaleric, heptonic, caproic, isocaproic, formic, valeric, succinic, oxalic, oxaloacetic, malonic | [76] |
| B. pumilus CHOO8A; B. fusiformis | Opuntia cholla | gluconic, oxalic, 2-ketogluconic, lactic, succinic aid, formic, citric, malic | [76] |
| Bacillus sp. SENDO 6 и | P. pringlei | Gluconic, propionic, isovaleric, formic, succinic, lactic | [77] |
| Pseudomonas putida M5TSA, Enterobacter sakazakii M2PFe и Bacillus megaterium M1PCa | Mammillaria fraileana cactus | Gluconic, propionic, acetic, formic, succinic, lactic, oxalic | [78] |
Mineralization of Organic Phosphate-Containing Compounds
The content of organic phosphorus in the soil can reach 30–50% of the total amount, with soil organic phosphorus being found primarily in the form of inositol phosphate (soil phytate). Other organic phosphorus compounds were reported, including: phosphomonoesters, phosphodiesters, phospholipids, nucleic acids, and phosphotriesters [18]. Additionally, large quantities of xenobiotics (pesticides, detergents, antibiotics, and flame retardants) that are regularly released into the environment are also known to contain organic P. Most of these organic compounds are of high molecular weight and resistant to chemical hydrolysis. For plant uptake, these compounds must be converted into soluble ionic phosphate (Pi, HPO42−, H2PO4−) or low molecular weight organic phosphates [87].
Several groups of enzymes secreted by phosphate-solubilizing microorganisms are involved in the process of phosphate mineralization. The enzymes of the first group dephosphorylate phosphor-ester or phosphoanhydride organic compounds. These are non-specific acid phosphatases (NSAP). The most studied NSAP enzymes are phosphomonoesterases, also called phosphatases [88]. These enzymes can be acidic or alkaline phosphomonoesterases [89]. The pH of the soils possessing phosphatase activity was indicated to be from acidic to neutral, indicating that acid phosphatases play a major role in this process [18].
Phytase is another phosphate-solubilizing enzyme involved in the mineralization of organic P. This enzyme is responsible for releasing phosphorus from organic compounds in the soil (plant seeds and pollen) that are stored in the form of phytate (inositol polyphosphate). Phytase releases phosphates in a form that is available to plants. While plants cannot obtain phosphorus directly from the phytate, the presence of phosphate-solubilizing microbes in the rhizosphere can compensate for the inability of plants to obtain phosphates directly from phytate [43]. Phytates are synthesized by plants and constitute a significant amount (from 60 to 80%) of organic phosphorus in the soil. However, the ester bonds in phytic acid are quite stable and their natural degradation is practically impossible. Microbial mineralization of phytate by phytase plays an essential role in the process of phosphorus recycling. Phytate can be completely hydrolyzed with the formation of one inositol and six molecules of inorganic phosphate, or partially with the formation of lower isomers of inositol polyphosphate and inorganic phosphates [92].
Among the four types of phytases identified, β-propeller phytase (BPP, EC 3.1.3.8 or EC 3.1.3.26) differs from the other three phytases (histidine acid phosphatase, cysteine phytase, and purple acid phosphatase) in that it has a neutral ∼pH 7.0 rather than an acidic pH optimum. It has been indicated that BPP is the main class of phytate degrading enzymes in nature. A typical BPP has a six-lobed propeller fold with two phosphate-binding sites (a cleavage site and an affinity site) and six calcium-binding sites, three of which are high-affinity binding sites responsible for enzyme stability and three of which are low-affinity sites, regulating the catalytic activity of the enzyme [94,95].
Thus far, only a small number of BPP have been isolated and studied, including Shewanella oneidensis MR-1 PhyS, Bacillus subtilis PhyC, Bacillus sp. DS11 Phy, B. subtilis 168 168PhyA, Bacillus licheniformis PhyL, Pedobacterobsis 5 MJ11 PhyP, and Janthinobacterium sp. TN115 PhyA115, all of which are mesophilic or thermophilic. Phosphate solubilization by acid phosphatases was reported for Pseudomonas sp., Burkholderia cepacia, Enterobacter aerogenes, E. cloacae, Citrobacter freundi, Proteus mirabalis, and Serratia marcenscens. Moreover, solubilization of organic phosphate by phytase activity was observed in Bacillus subtilis, Pseudomonas putida, and P. mendocina, and phosphatase activity was discovered in Klebsiella aerogenes and P. fluorescens. However, although P. sonchi SBR5 possesses some of these enzymes associated with phosphate solubilization, the activation of the corresponding genes was not observed when differential gene expression was analyzed under the phosphate solubilization conditions.
The production of phosphatases by the soil microbiome was proved to be tightly controlled by the availability of inorganic phosphorus and nitrogen. The addition of nitrogen increases the phosphatase activity, while the supply of inorganic phosphorus suppresses the production and activity of phosphatases due to the negative feedback mechanism [110].
Phytase-producing fungi are, A. fumigatus, A. niger, A. parasiticus, A. rugulosus, A. terreus, Penicillium rubrum, P. simplicissimum, Pseudeurotium zonatum, Trichoderma harzianum, and Trichoderma viride. Soil Bacillus and Streptomyces spp. are able to mineralize complex organic phosphates by producing extracellular enzymes such as phosphoesterases, phosphodiesterases, phytases, and phospholipases [37].
Perspectives of use
To enhance the use of PSBs as effective and important components in sustainable soil management systems, more data in needed on the molecular mechanisms they use to increase the bioavailability of phosphates. Consumers pay attention to the health, quality, and nutritional value of agricultural products. Thus, applying phosphate-solubilizing microorganisms as biofertilizers is one option to increase food production without posing a health risk, while saving natural sources of phosphate fertilizers and developing sustainable agriculture. It is important for researchers to continue studying phosphate-solubilizing microorganisms and translate this knowledge into a form that can be easily used by farmers [137].
The papers published in the recent years indicate that the efficiency of using phosphates in agriculture could be improved by inoculation with phosphate-solubilizing bacteria, which increase the availability of phosphate without disturbing the biochemical composition of the soil. These potential biofertilizers are universal since they can be used for various crops and generally are not specific for plants. Conversely, the development of personalized, plant-specific consortia of phosphate-solubilizers is likely to increase productivity further. Inoculation of phosphate-solubilizing microorganisms into the soil appears to be an effective way to convert insoluble phosphate compounds into bioavailable forms, resulting in better plant growth, yield, and quality. In this review, we demonstrate that the bacteria of genera Bacillus, Pseudomonas, Rhizobium, Aspergillus, and Penicillium genera are regarded as the most effective phosphate-solubilizers for increasing the phosphate bioavailability in soil. PSBs cause immediate plant growth by providing phosphates in an easily absorbable form. Additionally, phosphate-solubilizing microorganisms support plant growth by increasing the efficiency of nitrogen fixation. Two main mechanisms increasing free phosphate in the soil can be distinguished: the mobilization of inorganic and organic phosphates. In general, the mobilization of inorganic phosphate is performed primarily due to the release of organic acids by bacteria, and enzymes released into the extracellular environment perform the mineralization of organic phosphates.
While most farmers rely on inorganic sources of phosphates with which to avoid nutrient deficiencies, significant amounts of these fertilizers are lost from the soil through various mechanisms and are unavailable for plant uptake. In legume cropping systems, phosphate deficiency can also lead to nitrogen deficiency and reduced crop yields. Since the PSB-based biofertilizers have indicated promising effects on plant growth and yield, we assume that the phosphate-solubilizing microorganisms may be potential substitutes for inorganic phosphate fertilizers as a method to meet plant requirements and consequently increase the yields in sustainable agriculture. Their application is an environmentally and economically sound approach.
In this review, we described the molecular mechanisms of use phosphate-solubilizing microorganisms as biofertilizers. Solubilization of inorganic phosphate in the soil is found to increase its bioavailability for the plant, thus using PSBs in the soil is to promote sustainable agriculture, improve soil fertility, and increase crop yields. We consider use Use of PSB as microbial inoculants is considered as the new frontier for increasing plant productivity. This technology can contribute to low-cost farming systems and a cleaner environment.
