You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Lei Zhang and Version 2 by Conner Chen.
Cellular uptake of biologically active molecules is a significant obstacle to developing drug design and controlled drug delivery. For instance, it is hard for the monoclonal antibodies to penetrate the cell membrane and enter the cell due to their large molecular weight. Cell-penetrating peptides (CPPs) have been discovered to deliver chemical drugs, nucleic acids, and macromolecules to permeate cell membranes, creating a novel route for exogenous substances to enter cells.
- CPPs
- arginine-rich peptide
- non-covalent interaction
1. Introduction
Cellular uptake of biologically active molecules is a significant obstacle to developing drug design and controlled drug delivery. For instance, it is hard for the monoclonal antibodies to penetrate the cell membrane and enter the cell due to their large molecular weight. Hence, they are limited to being used to identify and target secretory protein targets on the cell surface. However, the application of monoclonal antibodies is severely constrained by the fact that many potential targets for disease therapy are located inside cells. In addition, emerging therapeutic technologies such as gene therapy also need to address the issue of cellular uptake of nucleic acids (DNA, RNA) and other biomolecules. Viral vectors [1][2][3][1,2,3], and methods such as electroporation, microinjection, and liposome encapsulation [4][5][6][7][8][9][4,5,6,7,8,9], have successfully delivered a wide range of therapeutic agents, including proteins, peptides, and oligonucleotides, to target cells. Still, these methods have certain shortcomings, including inefficient drug delivery, cellular toxicity, poor specificity, etc. Against this backdrop, cell-penetrating peptides (CPPs) are coming into view as promising candidates for drug delivery applications [10][11][12][13][10,11,12,13]. CPPs are a class of small molecules with strong membrane permeability, which can carry peptides, proteins, nucleic acids, and other macromolecules into cells, opening a new pathway for exogenous substances to enter cells. As a typical kind of CPP, the cationic peptide is distinguished by the presence of basic amino acids such as arginine (Arg) and lysine (Lys) [14]. The essential amino acids are positively charged in physiological pH; thus, they can interact with negatively charged drug molecules and cell membranes through non-covalent interaction, including electrostatic interactions [15].
Of all the cell-penetrating peptides, arginine-rich peptides have attracted the most scientific attention as cationic peptides [16][17][18][19][16,17,18,19]. Examples include the first discovered membrane penetrating peptide, Tat, a typical arginine-rich membrane penetrating peptide. Human immunodeficiency virus type 1 (HIV-1) Tat protein, secreted from infected cells, can deliver several proteins, including ovalbumin, β-galactosidase, and horseradish peroxidase into cells [20][21][22][20,21,22]. The primary domain in Tat protein responsible for crossing the plasma membrane, the protein transduction domain (PTD), is rich in arginine and lysine residues. Jin et al. [23], Know et al. [24], and Nagahara et al. [25] verified that the protein could serve as a carrier to direct the uptake of heterologous proteins into cells by generating genetic in-frame PTD fusion proteins. Moreover, Park et al., showed that the smallest structural domain of the Tat protein that acted as a membrane penetrator was the amino acid sequence at positions 49–57 (residues 49–57: RKKRRQRRR), which was a 9-amino acid sequence containing 6 arginines [26].
Numerous investigations have been conducted to investigate how CPPs transport substances into cells. The two primary recognized cellular uptake mechanisms are endocytosis and the pore formation model. [13][27][28][29][30][31][32][33][34][35][13,27,28,29,30,31,32,33,34,35] (Figure 1A). The ratio between endocytosis and direct cell entry is critical in the cellular uptake of cell-penetrating peptides, and the modification of the CPPs will affect the ratio between endocytosis and direct translocation. While the mechanisms of how the modification affects the ratio and the effect of the following details have not been fully understood, Zhang et al. [36] speculated that the positively charged arginine on the periphery of the NP1 peptides could greatly facilitate their direct translocation through the negatively charged plasma membrane via electrostatic interaction instead of via endocytosis, which provides a more efficient uptake pathway. On the other hand, the arginine and lysine residues also significantly impact the transmembrane function of cationic CPPs [14]. However, there are several hypotheses about the involvement of arginine and lysine in membrane penetration.
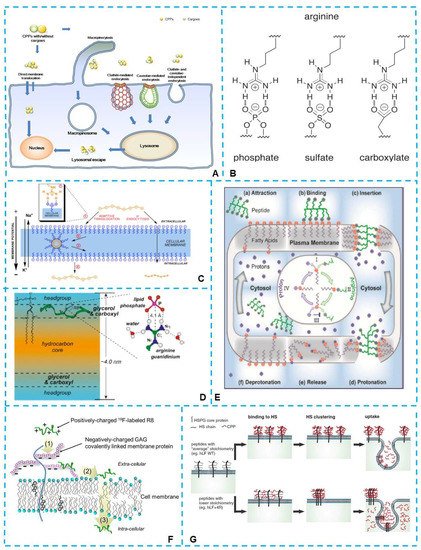
Figure 1. (A) Different cellular entry routes for either cell-penetrating peptides (CPPs) alone or CPP/cargo complexes. Direct membrane translocation and endocytosis were two major routes which had been proposed [33]. (B) Possible divalent hydrogen bond formation by the side-chain guanidino moiety of arginine with phosphate, sulfate, and carboxylate groups [37]. (C) Mechanisms of the arginine-rich CPPs uptake. The guanidinium group formed a bidentate bond with negative phosphates, sulfates, and carboxylates on the cell surface [38]. (D) Mechanism of the phosphates-related membrane penetration of arginine-rich CPPs, HIV Tat (48–60) [39]. (E) The proposed cellular uptake mechanism for arginine-rich CPPs revealed that deprotonated fatty acids were involved in the membrane penetration process [40]. (F) The mechanism for non-endocytic, energy-independent translocation of 19F-R8 into cells. The mechanism involved (1) binding of 19F-R8 to GAG at the cell surface, followed by (2) the transfer to the cell membrane and (3) the entry into the cytosol [41]. (G) Schematic of the proposed stoichiometry-dependence of uptake. The guanidine groups in arginine formed bidentate hydrogen bonds with negatively charged heparan sulfates. The cross-linking of HS was the driving force for the uptake of arginine-rich CPPs [42].
2. Cell-Surface Interactions on Arginine-Rich CPPs Allow for Internalization
2.1. Binding to Anionic Groups to Promote Uptake
2.1.1. Binding to Phosphate Anionic Groups
Numerous studies have highlighted that guanidinium-rich arginine is essential for cellular uptake. Since arginine-rich peptides preferentially bind to negatively charged molecules, the cell membrane penetration is assumed to initiate by binding to various anionic groups via non-covalent interaction, such as phosphates, carboxylates, and sulfates (shown in Figure 1B), on cell surfaces [37]. In their early work of Wender et al., the function of multiple guanidine groups in arginine-rich CPPs in membrane penetration was summarized: the guanidine group of arginine formed a bidentate bond with negative phosphates, sulfates, and carboxylates on the cell surface, and the charge-neutralized species were driven into the cell by the membrane potential [17][38][17,38] (Figure 1C). The transmembrane mechanism of arginine-rich CPPs was further investigated in the following decades.
For an exploration of the phosphates-related membrane penetration mechanism of arginine-rich CPPs, HIV Tat (48–60), a solid-state NMR technique, was applied. The results suggested that Tat was inserted into the glycerol backbone region of the membrane-water interface in anionic lipid bilayers and generated transient membrane defects, which relied on transient interactions between the arginine side chains and lipid phosphates [39] (as shown in Figure 1D). In another study, Chen et al. [43] found that steady-state water holes occurred when the guanidinium groups in the arginine-rich Tat associated with the phosphate moieties on the lipid headgroups, and Arg residues pulled down water molecules in the membrane to stabilize the insertion. Meanwhile, bidentate hydrogen bonding involving Arg residues with the lipid phosphate groups was observed in the study of Jobin et al. [44]; the non-covalent interaction could induce invagination phenomena and contribute to tubulation and internal vesicle formation.
2.1.2. Binding to Carboxylate Anionic Groups
Binding to carboxylates is also a common way of promoting the membrane penetration of the arginine-rich CPPs. In the study of Herce et al., an efficient energy-independent translocation mechanism for arginine-rich molecules was revealed [40]. A transient membrane channel was formed when the cell exterior guanidine groups were attracted and bound to the deprotonated fatty acids on the membrane. In the crossing process of the peptide−fatty acid complex, the protons from the cytosolic side competed for the binding of the guanidinium groups to fatty acids, and the high density of protons in the cytosol protonated the fatty acids, which resulted in the release of the peptide into the cytosol (shown in Figure 1E).
2.1.3. Binding to Sulfate Anionic Groups
As described above, sulfate is also one kind of critical anionic group binding to the arginine-rich peptides. Sulfated glycosaminoglycans (GAGs), such as heparan sulfate and chondroitin sulfate localized on cell membranes, are covalently linked to core proteins at the cell surface [18][27][41][45][46][47][18,27,41,45,46,47]. Real-time in-cell NMR spectroscopy was applied to investigate the direct membrane translocation of 19F-labeled octaarginine (R8) into living cells in the work of Takechi-Haraya et al., and they found that arginine-rich 19F-R8 entered hydrophobic cell membrane after binding to GAGs [41] (Figure 1F). In the authors’ opinion, the process of the membrane penetration of 19F-R8 was non-endocytic and energy-independent. One possibility was that the charge neutralization of polyarginine with GAGs induced insoluble and energetically unstable peptide-GAG complexes, which led to the dissociation of 19F-R8 from GAGs to water or rapidly transferred to the cell membrane. In another study, Takechi-Haraya et al., investigated several arginine-rich peptides of Tat, R8, and Rev, with heparin as a GAG model; they found that the beneficial electrostatic interaction between arginine residues of peptide and anionic sulfate/carboxyl groups of heparin contributed to the favorable enthalpy gain, which served as an energy source to facilitate their cell penetration [48]. On the other hand, several studies have proven that GAG cross-linking has been associated with the activation of signaling pathways that result in endocytosis [49][50][51][49,50,51]. Zuconelli et al., investigated the role of calcium in direct cytosolic uptake of two arginine-rich peptides, nona-L- and D-arginine. They indicated that the calcium channel Orai1 played a decisive role in triggering rapid uptake of the peptides [52].
Binding to negatively charged heparan sulfates (HS) at the cell surface is considered the first step in the internalization of CPPs. Wallbrecher et al., investigated a collection of the lactoferrin-derived CPPs with respect to HS binding and uptake; the study demonstrated that the guanidine groups in arginine could form bidentate hydrogen bonds with negatively charged heparan sulfates, and the cross-linking of HS was the driving force for the uptake of arginine-rich CPPs [42] (Figure 1G). Interestingly, peptides with a low stoichiometry had a higher capacity to cross-link HS, which did not hold for other classes of CPPs [53]. Ikuhiko [54] found that the arginine-rich peptides led to the activation of small G-protein Rac1 and reorganization of actin (lamellipodia and membrane ruffling), which improved the cellular uptake of the peptides and their cytosolic translocation. In another study by Kawaguchi et al., syndecan-4, one of the heparan sulfate proteoglycans, was demonstrated to be a primary cell-surface receptor involved in the endocytosis of the octa-arginine (R8) peptide [51].
2.2. Participate in Membrane Penetration by Partitioning the Lipid Glycerol Regions
Besides binding to the anionic groups on cell surfaces, the guanidine group participates in membrane penetration by partitioning the lipid glycerol regions. According to widespread consensus, arginine-rich CPPs are extremely cationic and hydrophilic; their guanidine groups create ion and hydrogen interactions with lipid head groups to assist membrane binding. During the membrane translocation of the CPPs, the peptide backbone must pass through the lipid core region. Hydrophobic interactions between the lipid core and fewer hydrophilic peptide backbones could lead to translocation due to poorly hydrophilic methylene groups in the side chains of arginine and the peptide backbone. Umbrella sampling simulations were used in the study of Sun et al. [55]; the arginine-rich peptide octa-arginine (R8) was found to expand the surface area of the lipid bilayer due to the deep partitioning of guanidinium ions into the lipid glycerol regions. Meanwhile, R8 was also found to extend the lifetime of the transient membrane pore due to inserting an arginine side chain into the existing pore. Their study demonstrated that the arginine-rich peptide was essential in membrane pore formation and lifetimes. In addition, the study of Pourmousa et al. [56] demonstrated that H-bonds and charge-pair interactions between the bilayer and arginines and lysines were the critical interactions in the binding mode of penetrating and helped charged residues to localize in the hydrophobic region of the bilayer.
