Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Shashank Pal and Version 2 by Conner Chen.
The resources of fossil fuels, such as crude oil, natural gas, and coal, are depleting day by day due to increasing energy demands. Plastic items have witnessed a substantial surge in manufacturing due to their wide range of applications and low cost. Therefore, the amount of plastic waste is increasing rapidly. Hence, the proper management of plastic wastes for sustainable technologies is the need of the hour.
- municipal plastic waste
- thermal pyrolysis
- catalytic pyrolysis
1. Introduction
The demand of energy is continuously increasing due to increasing population and industrialization. Higher energy demand has led to rising demand for petroleum exploration as well as more environmental pollution [1]. As per the petroleum planning and analysis cell, India consumed almost 133.05 billion litres of fuels in the 2020 financial year. The trend of the fuel market from 2011 to 2019 in India shows that the fuel market increased by 152.7 billion from 2015 to 2019, which was 3.1% higher than that reported for the 2018 financial year [2]. As a result, it is reasonable to expect that it will rise in the next few years as the population’s need for energy rises. Therefore, there will be a need to place more emphasis on alternative fuels.
In recent times, there has been a strong tendency to move away from traditional fossil fuels in favor of novel and renewable energy sources that are cleaner, safer, and inexhaustible. With the supply-demand gap expanding, it is more important than ever to diversify energy sources and look into alternative ways to meet the country’s energy demands while preserving economic growth. Growing environmental concerns present a huge issue for energy companies, emphasizing the need to move to cleaner, more sustainable energy sources, such as compressed natural gas (CNG), liquefied petroleum gas (LPG), hydrogen, biodiesel, and plastic-derived oils. Several state-of-the-art studies show that these alternative fuels are a valuable source of energy, with fewer emissions, and are being used in the energy sector [3][4][5][6][7][3,4,5,6,7], e.g., CNG is used in cars, but there is a limit on its power output [8]. Hydrogen is a clean energy source with low toxicity, but it may not be considered the safest fuel in commercial vehicles [9][10][9,10]. Similarly, the Indian government has been focusing on biofuels as they have the potential to be used in the transportation sector. However, as compared to conventional diesel, the higher oxygen concentration in biofuels enhances nitrogen oxides (NOx) emissions in the vehicle, but some controversial studies have suggested that it depends on the composition of biodiesel [11]. It can be 10% higher than petroleum-based compounds, which may dissolve in moisture in the atmosphere and generate acid rain. However, fuel produced from municipal plastic waste attracts the researcher due to its higher potential and availability [12].
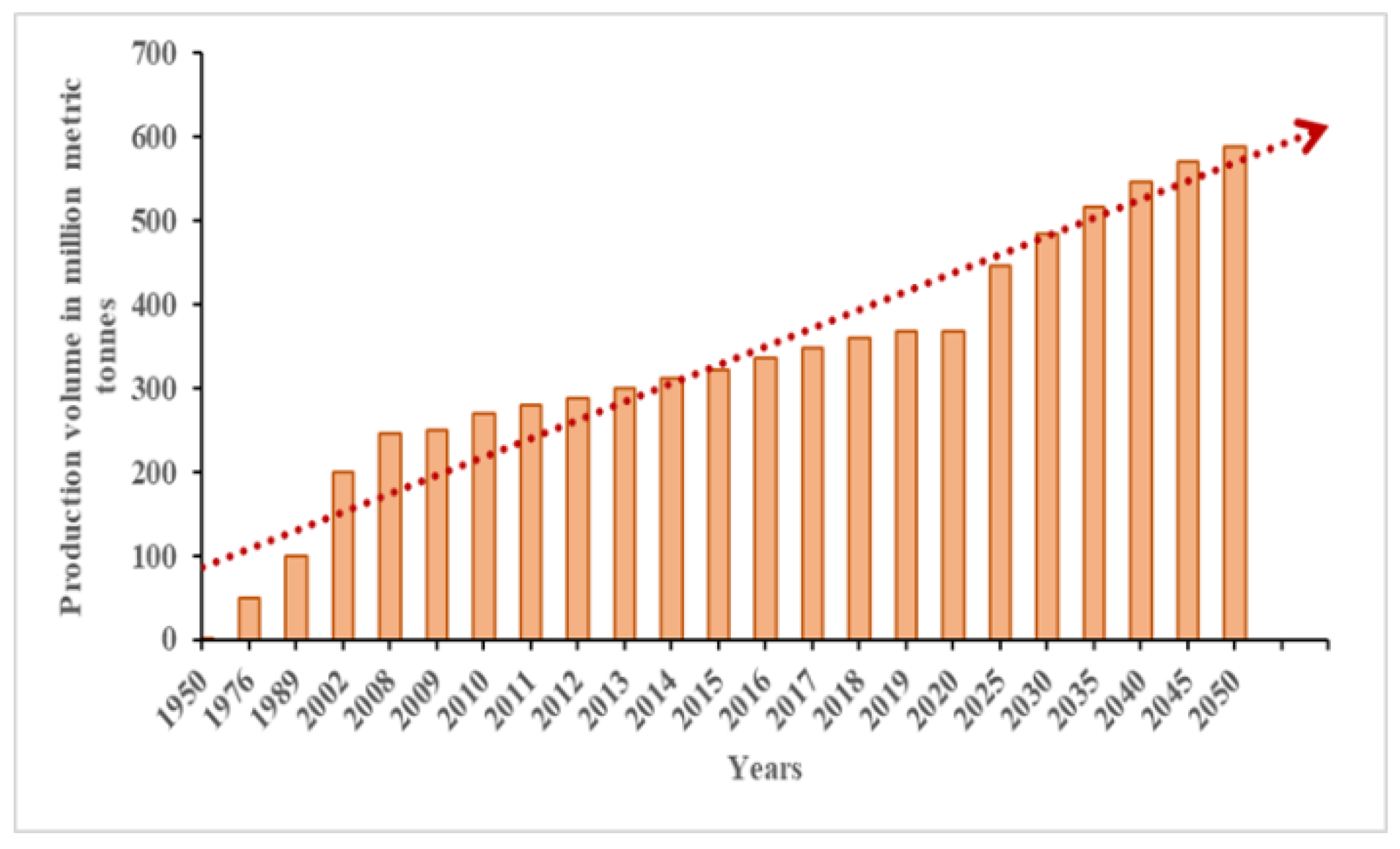
In the current scenario, plastic is one of the essential products utilized in a huge amount by the growing population due to its durability, lightweight, and ease of production, making it more viable in utilization. Figure 1 depicts the plastic production in 2020 and the plastic production forecasted from 2025 to 2050. Plastics end up as waste after being used once. The Central Pollution Control Board (CPCB) reported that 3.3 million tons of waste plastic were generated in India in 2018–19 [13] that were dumped into the nearby municipal dumping yards.
Collection and segregation of the municipal mixed plastic waste from the dumping yard is again a challenging step, and it should be cost-effective to implement the technology for waste to energy conversion [16][14]. Technically, it is possible to separate municipal mixed waste into distinct streams; however, this may be more expensive due to the infrastructure requirements for efficient collection and separation. It is noted that existing technology such as tribocharging and electrostatic separation technology can separate the different compositions of waste plastic: high-density polyethylene (HDPE)/polypropylene (PP), low-density polyethylene (LDPE)/PP, and polyethylene terephthalate (PET)/ polyvinyl chloride (PVC) [17][15]. Other technologies, such as biological treatment to separate nanoparticles from plastic waste and a hierarchical classification strategy to separate LDPE and HDPE, are similar [18][16]. Thus, different technologies are available to separate municipal mixed plastic waste (MPW), but the employment of the technology to handle the waste plastic may not be economically viable. Sharuddin et al. discussed, in a review study, that different kinds of plastic (PET, PVC, HDPE, LDPE, and MPW) have the potential to be converted into energy as valuable liquid products. However, they concluded that PET and PVC both have a lower tendency to convert into valuable liquid products than other plastics. For instance, thermal degradation of the polyvinyl chloride (PVC) releases hydrochloric acid, adversely affecting liquid product quality and yield [19][17]. Moreover, contamination of PVC in polyethylene terephthalate (PET) will decompose the resin of PET by becoming brittle and yellowish, which further requires reprocessing. As a result, MPW treatment via the thermal degradation process is not worthwhile, with impurities necessitating some additional operation for waste plastic segregation based on colors, transparency, and resin [20][18].
A study conducted on the pyrolysis of mixed plastic waste contained 46 wt.% LDPE, 30 wt.% HDPE, and 24 wt.% PP at 650 °C temperature via catalytic pyrolysis (1% of Z–N catalyst), and the ratios of reaction products (gas/liquid/solid) were 6.5/89.0/4.5 wt.%, respectively [21][19]. On the other hand, some studies have shown that the pyrolysis of municipal plastic waste produces a lower liquid yield of less than 50%, while single plastic feedstocks yielded liquid yields of 97.0 wt.%, 93.1 wt.%, 82.12 wt.%, 80.88 wt.%, 23.1 wt.%, and 12.79 wt.%, respectively, for PS, LDPE, PP, HDPE, PET, and PVC [22][23][24][25][26][27][28][20,21,22,23,24,25,26]. Similarly, Cepeliogullar et al. explored the pyrolysis of PET waste by using the fixed-bed reactor at a temperature of 500 °C and a heating rate of 10 °C/min, where they observed 23.1 wt.% liquid yields and 76.9 wt.% gaseous with no solid product [26][24]. However, lower liquid yield indicates low availability of volatile content in PET at around 86.83% compared to the other plastics (>90%) [26][24]. Salem et al. performed thermal pyrolysis of the reclaimed plastic wastes from unsanitary landfill sites in an auger pyrolysis system at a temperature of 500 °C. The process mass balance was developed on a dry basis, and the yields of pyro-oil, light wax, heavy wax, and gases were 5.5, 23.8, 69.4, and 1.3 wt.%, respectively [29][27]. The result proves that when increasing the temperature, the liquid yield will increase up to a certain limit, but too-high temperature will reduce the liquid yield and increase the gaseous product. This may be due to the reason that lower temperature leads to volatile formation at a slow rate, which allows sufficient time for volatiles to crack into lower hydrocarbons inside the reactor before leaving to the condensation section, whereas at higher temperature, the volatile formation is rapid due to higher temperature, which does not provide the same residence time for the volatiles to stay inside the reactor to crack down into more smaller hydrocarbons [30][28]. Furthermore, at higher temperatures, the reaction mechanism is dominated by the inter- and intramolecular hydrogen transfer, followed by b-scission, producing more lighter hydrocarbons. Table 1 shows the volatiles present in different types of plastics.
Table 1.
Proximate analysis of different type of plastic wastes.
| Type of Plastic | Fixed Carbon (%) | Moisture Content (%) | Ash (%) | Volatile Matter (%) | References |
|---|---|---|---|---|---|
| PET | 14.5 | NA | 0.7 | 84.8 | [31][29] |
| PET | 13.9 | NA | NA | 84.1 | [32][30] |
| HDPE | NA | NA | 0.8 | 97.15 | [32][30] |
| HDPE | 16.85 | NA | NA | 83.15 | [33][31] |
| HDPE | 0.00 | 0.00 | 0.01 | 99.99 | [34][32] |
| LDPE | 0.68 | 0.30 | 3.37 | 95.61 | [35][33] |
| LDPE | 0.051 | 0.11 | 0.023 | 99.816 | [36][34] |
| PP | 1.62 | 0.16 | 4.45 | 93.77 | [37][35] |
| PP | 1.0 | NA | NA | 96.9 | [32][30] |
| PP | 0.5 | NA | 0.00 | 99.5 | [38][36] |
| PP | 0.43 | 0.29 | 0.00 | 99.28 | [39][37] |
| PS | 1.05 | 0.32 | 0.09 | 98.54 | [40][38] |
| PS | 0.22 | 0.00 | 0.00 | 99.78 | [41][39] |
| PS | 0.071 | 0.09 | 0.025 | 99.814 | [36][34] |
| PE | 0.07 | NA | NA | 99.93 | [42][40] |
| PE | 0.00 | 0.2 | 0.4 | 99.4 | [43][41] |
| PVC | 1.97 | 0.65 | 0.11 | 97.92 | [44][42] |
| PVC | 4.10 | 0.00 | 0.01 | 95.89 | [45][43] |
| PA | 0.69 | 0.00 | 0.00 | 99.7 | [46][44] |
| ABS | 0.04 | 0.10 | 0.99 | 98.87 | [47][45] |
| ABS | 9.6 | 0.00 | 13.6 | 76.8 | [48][46] |
| PBT | 2.88 | 0.16 | 0.00 | 97.12 | [47][45] |
| MPW | 5.34 | 0.86 | 1.21 | 93.45 | [13] |
| MPW | 3.5 | 0.00 | 3.3 | 93.2 | [49][47] |
2. Thermal Decomposition of Plastic Waste
Conventional Pyrolysis Process
Conventional pyrolysis of plastic refers to the pyrolysis process that uses an electrical heater or burner as the source of heat. Many studies have been performed on conventional pyrolysis using different types of plastics, e.g., PS, PP, PS, HDPE, LDPE, and mixed waste plastic [25][50][51][52][23,50,51,52]. The operating modes for plastic pyrolysis are batch, semi-batch, or continuous reactors, and each has pros and cons of its own. High conversion can be achieved in a batch reactor, but the inconsistent nature of the end product and the high labor costs make it unsuitable for industrial production. Although fixed-bed reactors are noted for their simplicity in design, they have a smaller surface area where reactions can take place. Fluidized-bed reactors, as opposed to fixed-bed reactors, guarantee that the catalyst and fluid are thoroughly mixed, increasing the catalyst’s usable surface area and enhancing heat transmission. Large-sized particles can be handled by conical spouted-bed reactors (CSBR), although product collection and catalyst feeding provide technological hurdles. Ghodke et al. carried out the conventional pyrolysis of virgin mixed plastic and municipal mixed plastic waste (MMPW) at 500 °C and obtained a maximum yield of 62.5 wt.% with MMPW. Heating is the main cost and bottleneck in pyrolysis. The huge cost of the conventional pyrolysis method is due to inefficient heating and heat loss. Thus, researchers have recently attempted to utilize other heating sources, for example, solar [53], microwave [54], and other renewable energy sources [55][56][55,56].