Haze is the phenomenon of visibility degradation caused by extinction effects related to the physicochemical properties of atmospheric particulate matter (APM). Atmosphere heterogeneous reactions can alter the physicochemical properties of APM. Therefore,APM it is important to understand the atmospheric heterogeneous reactions of APM in order to reveal the cause of haze. Herein, the current situation, developmental trend, source, and compositions a general term for all kinds of solid and liquid particulate matter in the atmosphere. All kinds of APM pollution in China are reviewed. Additionally, we introduce the reaction characteristics and key chemical processes of common inorganic, organic, and mixed pollutant gases on the surface of mineral particles. The effects of mineral particulate matter on aggregation, regu are evenly dispersed in the air to form a relatively stable suspension system, that is, the aerosol system. APM can enter the human respiratory system through inhalation, and catalysis in the formation of atmospheric aerosols and the synergistic reaction mechanism of SO2, NO2, O3, causing a variety of respiratory and cardiovand VOCs on the surfaces of different mineral particles are summarized. The problems existing in the current research on heterogeneous reactions ocular diseases, thus causing harm to human health, especially in the surfaces of mineral particles are also evaluated.case of PM2.5.
- aerosol
- atmospheric mineral particulate matter
- polluted gas
- heterogeneous reaction
1. Introduction
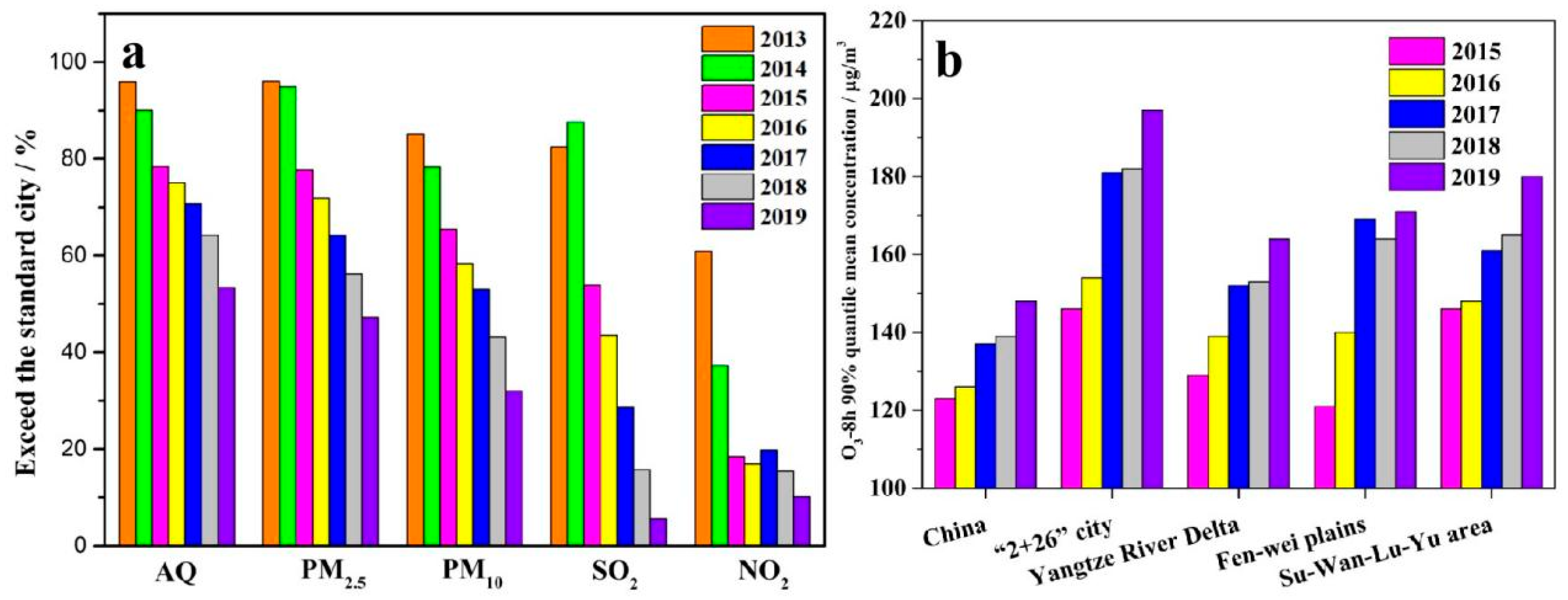
2. Source and Composition of APM
APM can be divided into primary and secondary particles [12]. Primary particulate matter is directly released into the atmosphere by natural and anthropogenic pollution sources, such as soil particles, sea salt particles, and burning soot. Secondary particulate matter refers to finer particulate matter, including primary gaseous pollutants (e.g., SO2, NOx, VOCs, and NH3) discharged by fuel combustion and industrial and automobile exhaust gas, which are converted into fine particulate matter through gas-particle transformation in the atmosphere [6,12,23][6][12][23]. Based on the composition and morphology of single particles in the atmosphere, APM can also be divided into six types: soot, fly ash, complex secondary, mineral, organic, and metal particles [24,25][24][25]. Owing to the different sources and formation processes of APM, its composition can also vary significantly, especially within urban atmospheres, which are affected by a variety of pollution sources. The chemical composition of PM2.5 is extremely complex and usually contains inorganic substances, such as mineral dust particles and sulfate, nitrate, and ammonium salts; organic matter, such as organic acids, aromatic hydrocarbons, and aerobic organic matter; and trace elements [26]. Atmospheric mineral particulate matter (AMPM) is the most important component of atmospheric aerosols, accounting for approximately 30–60% of the mass concentration of tropospheric atmospheric particles [12,20,27][12][20][27]. These particles mainly originate from ground dust in arid and semi-arid desert areas, and their annual emissions are approximately 1500–4400 Tg [19,28][19][28]. Studies on the composition and characteristics of inhalable particulate matter in Beijing, Shanghai, Zhengzhou, Wuhan, and other large cities in China have shown that mineral particles are an important component of urban atmospheric particles, and AMPM accounts for approximately 50% of the mass concentration of APM in dry areas [29]. For example, AMPM in Beijing accounts for approximately 30–70% of the total particulate matter, and while AMPM in Chengdu is lower than in Beijing and some cities in the northwest, where it is still between 34–40% [30]. Clay and diagenetic minerals are the main components of urban particulate matter in dust paths in China, with aluminosilicate particles being the most common [31]. Among them, sodium feldspar, illite, potassium feldspar, anorthosite, hornblende, and chlorite account for 61.59% of the total particulate matter, and calcite and quartz particles account for 13.59%. Dolomite, gypsum, unformed amorphous substances, and other mineral components are also present [31]. Dong et al. [32] found that quartz, clay minerals, and amorphous materials accounted for 24.1%, 28.5%, and 20% of inhalable particles in northern China during dusty weather, respectively. Wang et al. [33] studied the composition of APM during two extremely large sandstorms in Beijing in 2015 and found that AMPM accounted for 85.3% and 95.4% of APM, respectively, among which the clay mineral content was the highest, being more than 50%, followed by quartz, feldspar, and carbonate particles. In India, Spain, Italy, and North Africa, AMPM account for 30–70% of the total particulate matter, and the main mineral phase is the same as in China. However, because of the different geographical locations and pollution situations, the proportion of each mineral phase is different [34]. The inorganic salts in APM are mainly sulfate, nitrate, and ammonium salts from the homogeneous and heterogeneous reactions of SO2, NOx, and NH3 in the atmosphere [7,12,35,36][7][12][35][36]. The amount of inorganic salts in particulate matter varies depending on source variations, meteorological conditions, and varying atmospheric transformations [15]. Gao et al. [37] measured the concentration of water-soluble ions in Jinan PM2.5, among which the highest SO42− average concentration was 38.33 ± 26.20 μg/m3, accounting for 44.65 ± 11.30% of the total water-soluble ions, while the NH4+ and NO3− average concentrations were 21.16 ± 16.28 and 15.77 ± 12.06 μg/m3, accounting for 17.63 ± 7.61% and 23.07 ± 5.85% of the total ions, respectively. Lai et al. [38] measured water-soluble ions in particulate matter in Guangzhou, Shenzhen, Zhuhai, and Hong Kong and found that SO42−, NH4+, and NO3− together accounted for 59.3–77.7% and 59.3–77.1% of PM2.5 and PM10 water-soluble ions in winter, respectively. In summer, they accounted for 56.5–84.5% and 46.3–79.2% of PM2.5 and PM10, respectively. In particular, the concentration of SO42−, at 6.0–22.0 μg/m3, was higher than all other ions. The contents of sulfate and nitrate in the particulates in Chengdu were 21.55% and 11.20%, respectively, which were lower than those in Beijing, Shanghai, and Guangzhou [39]. Huang et al. [26] measured the chemical composition of PM2.5 in Beijing, Shanghai, Guangzhou, and Xi’an during high pollution events in 2013, among which SO42−, NO3−, and NH4+ accounted for approximately 8–18%, 7–14%, and 5–10% of the total mass of PM2.5, respectively. Kim et al. showed that the water-soluble ions NH4+, NO3−, and SO42− in particulate matter detected at five stations on St. Nicholas Island in the United States accounted for 8–9%, 23–26%, and 6–11% of the total mass of PM10, and 14–17%, 28–41%, and 9–18% of PM2.5, respectively [40]. Rajeev et al. [41] measured the chemical composition of PM2.5 and rainwater in India during the El Niño and Pacific Decadal Oscillation (PDO). Among them, SO42−, NH4+, and NO3− accounted for 33.6%, 23%, and 8.8% of the total amount of water-soluble ions in PM2.5, and 6.9%, 4.8%, and 7.5% in rainwater, respectively. Weagle et al. [42] interpreted the chemical composition and source of global PM2.5 through global chemical transport model (GEOS-Chem) simulation and surface particulate matter network (SPARTAN) site observation. It was found that the secondary inorganic aerosols (SIA, the sum of SO42−, NO3-, and NH4+) in each observation site accounted for 15–40% of the total mass of PM2.5, and the content of SO42− was the highest, accounting for 50–80% of the total SIA (Table 1). Moreover, they found that Beijing, Kanpur, and Dhaka all had much higher levels of PM2.5 and SIA than other cities.| Site | PM2.5 | SIA | SO42− | NH4+ | NO3− | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| obs | GC | obs | GC | obs | GC | obs | GC | obs | GC | |
| Beijing, China | 67.1 ± 9.9 | 75.0 | 19.7 ± 2.3 | 36.3 | 11.2 ± 1.4 | 13.3 | 3.6 ± 0.6 | 9.0 | 4.9 ± 1.4 | 1.4 |
| Bandung, Indonesia | 30.8 ± 4.5 | 20.0 | 7.6 ± 0.8 | 9.9 | 5.6 ± 0.7 | 7.2 | 1.4 ± 0.3 | 2.6 | 0.6 ± 0.2 | 0.1 |
| Manila, Philippines | 19.2 ± 2.8 | 24.0 | 3.0 ± 0.3 | 12.0 | 2.1 ± 0.3 | 9.1 | 0.5 ± 0.1 | 2.9 | 0.4 ± 0. | 0.0 |
| Rehovot, Israel | 17.5 ± 2.6 | 23.0 | 6.4 ± 0.7 | 7.7 | 4.7 ± 0.6 | 5.6 | 0.9 ± 0.1 | 2.0 | 0.8 ± 0.2 | 0.1 |
| Dhaka, Bangladesh | 49.9 ± 7.3 | 79.0 | 11.3 ± 1.2 | 28.0 | 7.1 ± 0.9 | 15.1 | 2.2 ± 0.4 | 7.2 | 2.0 ± 0.6 | 5.7 |
| Buenos Aires, Argentina | 10.7 ± 1.6 | 15.0 | 2.5 ± 0.3 | 6.2 | 1.3 ± 0.2 | 4.4 | 0.4 ± 0.1 | 1.5 | 0.8 ± 0.2 | 0.3 |
| Ilorin, Nigeria | 15.8 ± 2.3 | 17.5 | 2.4 ± 0.2 | 1.9 | 1.7 ± 0.2 | 1.3 | 0.5 ± 0.1 | 0.5 | 0.2 ± 0.1 | 0.1 |
| Singapore, Vietnam | 15.8 ± 2.4 | 15.6 | 4.0 ± 0.4 | 3.5 | 3.2 ± 0.4 | 2.2 | 0.6 ± 0.1 | 0.9 | 0.2 ± 0.1 | 0.4 |
| Kanpur, India | 71.9 ± 10.6 | 94.0 | 18.6 ± 1.9 | 29.2 | 10.2 ± 1.3 | 16.6 | 4.6 ± 0.1 | 7.6 | 3.8 ± 1.1 | 5.0 |
| Hanoi, Vietnam | 50.9 ± 7.5 | 45.0 | 17.2 ± 1.8 | 17.1 | 10.1 ± 1.3 | 10.0 | 3.4 ± 0.6 | 4.5 | 3.7 ± 1.1 | 2.6 |
| Pretoria, South Africa | 17.5 ± 2.6 | 30.6 | 7.3 ± 0.7 | 15.7 | 5.3 ± 0.7 | 11.3 | 1.4 ± 0.2 | 3.7 | 0.6 ± 0.2 | 0.7 |
3. Surface Heterogeneous Reactions of AMPM
Atmospheric heterogeneous reactions refer to gas–solid–liquid three-phase reactions occurring on the surfaces/interfaces of micro-nano atmospheric particles [15]. These surface/interface reactions are involved in all aspects of atmospheric chemical processes and are of great significance for climate change, human health, and ecological balance [62][63]. In the troposphere, aerosol particles and gas composition are complicated by human activities and natural emissions [23]. Heterogeneous chemical reactions can easily occur between pollutant gases and particulate matter, which are coupled with environmental conditions, such as temperature, relative humidity (RH), illumination, and pressure, making the atmospheric environment more complex and affecting a wider area of this environment [7]. Mineral particles usually manifest with a heteromorphic and porous structure, along with a large surface area, strong adsorption properties, and high reactivity, features which enable them to provide a carrier for the adsorption, catalysis, oxidation, and hydrolysis of a variety of gaseous pollutants [12]. Field observation and laboratory simulation studies have confirmed that the heterogeneous reactions on the surfaces of mineral particles have an important impact on the removal of common gas pollutants in the atmosphere [36][50]. Mineral particles are an important source and sink of various gas pollutants, and also produce new secondary components, thus changing the chemical composition of atmospheric particles [44][47][49]. To understand the contribution of heterogeneous reactions involving mineral particulate matter and polluting gases to haze, a large number of laboratory simulations and external field observations have been conducted to reveal the heterogeneous reaction mechanisms, kinetic parameters, and other information related to haze formation. Therefore, the heterogeneous reaction of AMPM surfaces has become an increasingly important research field in atmospheric and environmental science [64].
Research on atmospheric heterogeneous reactions usually adopts the combination technology of the reaction device (Knudsen cell [65][66][67][68] or flow tube [69][70]) and the in situ detection equipment [71][72][73][74][75][76]; diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS); time of flight mass spectrometer (TOF); gas chromatograph–mass spectrometer (GC-MS); micro-Raman spectroscopy) to explore the kinetic parameters (uptake coefficient (γ)) of the reactions of atmospheric particles and atmospheric trace gases that change with humidity, temperature, and time.
References
- Zheng, B.; Tong, D.; Li, M.; Liu, F.; Hong, C.; Geng, G.; Li, H.; Li, X.; Peng, L.; Qi, J.; et al. Trends in China’s anthropogenic emissions since 2010 as the consequence of clean air actions. Atmos. Chem. Phys. 2018, 18, 14095–14111.
- Ministry of Ecology and Environment of the People’s Republic of China. China Ecological and Environment Bulletin. Available online: https://www.mee.gov.cn/hjzl/ (accessed on 2 February 2022).
- Xing, J.; Wang, J.; Mathu, R.; Wang, S.; Sarwar, G.; Pleim, J.; Hogrefe, C.; Zhang, Y.; Jiang, J.; Wong, D.C.; et al. Impacts of aerosol direct effects on tropospheric ozone through changes in atmospheric dynamics and photolysis rates. Atmos. Chem. Phys. 2017, 17, 9869–9883.
- Xu, J.; Zhang, Y.; Zheng, S.; He, Y. Aerosol effects on ozone concentrations in Beijing: A model sensitivity study. J. Environ. Sci. 2014, 24, 645–656.
- Ding, A.; Fu, C.; Yang, X.; Sun, J.; Zheng, F.; Xie, Y.; Herrmann, E.; Nie, W.; Petaja, T.; Kerminen, V.; et al. Ozone and fine particle in the western Yangtze River Delta: An overview of 1 yr data at the SORPES station. Atmos. Chem. Phys. 2013, 13, 5813–5830.
- Hallquist, M.; Wenger, J.C.; Baltensperger, U.; Rudich, Y.; Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N.M.; George, C.; Goldstein, A.H.; et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155–5236.
- George, I.J.; Abbatt, J.P. Heterogeneous oxidation of atmospheric aerosol particles by gas-phase radicals. Nat. Chem. 2010, 2, 713–722.
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C.; et al. Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 2004, 109, 2655–2671.
- Ramanathan, V.; Crutzen, P.J.; Kiehl, J.T.; Rosenfeld, D. Aerosols, Climate, and the Hydrological Cycle. Science 2001, 294, 2119–2124.
- Tang, M.; Huang, X.; Lu, K.; Ge, M.; Li, Y.; Cheng, P.; Zhu, T.; Ding, A.; Zhang, Y.; Gligorovski, S.; et al. Heterogeneous reactions of mineral dust aerosol: Implications for tropospheric oxidation capacity. Atmos. Chem. Phys. 2017, 17, 11727–11777.
- George, C.; Ammann, M.; D’Anna, B.; Donaldson, D.J.; Nizkorodov, S.A. Heterogeneous Photochemistry in the Atmosphere. Chem. Rev. 2015, 115, 4218–4258.
- Dong, F.; Shao, L.; Feng, C.; Ju, Y.; Li, J.; Huo, T.; Zhao, Y. Interfacial Reacyion of Atmospheric Micro/Nano Particles and Significance of Mineral Coevolution. Earth Sci. 2018, 43, 1709–1724, (In Chinese with English abstract).
- Ciere, R.; Querol, X. Solid Particulate Matter in the Atmosphere. Elements 2010, 6, 215–222.
- Novak, G.A.; Bertram, T.H. Reactive VOC production from photochemical and heterogeneous reactions occurring at the air–ocean interface. Acc. Chem. Res. 2020, 53, 1014–1023.
- Kolb, C.E.; Cox, R.A.; Abbatt, J.P.; Ammann, M.; Davis, E.J.; Donaldson, D.J.; Garrett, B.C.; George, C.; Griffiths, P.T.; Hanson, D.R.; et al. An overview of current issues in the uptake of atmospheric trace gases by aerosols and clouds. Atmos. Chem. Phys. 2010, 10, 10561–10605.
- Shi, B.; Wang, W.; Zhou, L.; Li, J.; Wang, J.; Chen, Y.; Zhang, W.; Ge, M. Kinetics and mechanisms of the gas-phase reactions of OH radicals with three C15 alkanes. Atmos. Environ. 2019, 207, 75–81.
- Lv, S.; Liu, Q.; Zhao, Y.; Zhang, M.; Jiang, L.; He, S. Formaldehyde Generation in Photooxidation of Isoprene on Iron Oxide Nanoclusters. J. Phys. Chem. C 2019, 123, 5120–5127.
- Ji, Y.; Wang, H.; Li, G.; An, T. Theoretical investigation on the role of mineral dust aerosol in atmospheric reaction: A case of the heterogeneous reaction of formaldehyde with NO2 onto SiO2 dust surface. Atmos. Environ. 2015, 103, 207–214.
- Zhang, W.; Guo, Z.; Zhang, W.; Ji, Y.; Li, G.; An, T. Contribution of reaction of atmospheric amine with sulfuric acid to mixing particle formation from clay mineral. Sci. Total Environ. 2022, 821, 153336.
- Yue, Y.; Cheng, J.; Lee, K.; Stocker, R.; He, X.; Yao, M.; Wang, J. Effects of relative humidity on heterogeneous reaction of SO2 with CaCO3 particles and formation of CaSO4·2H2O crystal as secondary aerosol. Atmos. Environ. 2021, 268, 118776.
- Kameda, T.; Azumi, E.; Fukushima, A.; Tang, N.; Matsuki, A.; Kamiya, Y.; Toriba, A.; Hayakawa, K. Mineral dust aerosols promote the formation of toxic nitropolycyclic aromatic compounds. Sci. Rep. 2016, 6, 24427.
- Zhu, T. Heterogeneous reactions on the surface of atmospheric particulate matter. Sci. Sin. Chim. 2010, 40, 1729–1730. (In Chinese)
- Gentner, D.R.; Jathar, S.H.; Gordon, T.D.; Bahreini, R.; Day, D.A.; Haddad, I.E.; Hayes, P.L.; Pieber, S.M.; Platt, S.M.; Gouw, J.A.; et al. A review of urban secondary organic aerosol formation from gasoline and diesel motor vehicle emissions. Environ. Sci. Technol. 2017, 51, 1074–1093.
- Huneeus, N.; Schulz, M.; Balkanski, Y.; Griesfeller, J.; Prospero, J.; Kinne, S.; Bauer, S.; Boucher, O.; Chin, M.; Dentener, F.; et al. Global dust model intercomparison in AeroCom phase I. Atmos. Chem. Phys. 2011, 11, 7781–7816.
- Ning, C.; Gao, Y.; Zhang, H.; Wang, L.; Yu, H.; Zou, L.; Cao, R.; Chen, J. Molecular chemodiversity of water-soluble organic matter in atmospheric particulate matter and their associations with atmospheric conditions. Sci. Total Environ. 2021, 809, 151171.
- Huang, R.; Zhang, Y.; Bozzetti, C.; Ho, K.; Cao, J.; Han, Y.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F.; et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218–222.
- Liu, W.; He, X.; Pang, S.; Zhang, Y. Effect of relative humidity on O3 and NO2 oxidation of SO2 on α-Al2O3 particles. Atmos. Environ. 2017, 167, 245–253.
- Yuan, S.; Liu, S.; Wang, X.; Zhang, H.; Yuan, S. Atomistic insights into heterogeneous reaction of hydrogen peroxide on mineral oxide particles. Appl. Surf. Sci. 2021, 556, 149707.
- He, K.; Yang, F.; Ma, Y.; Zhang, Q.; Yao, X.; Chan, C.; Cadle, S.; Chan, T.; Mulawa, P. The characteristics of PM2.5 in Beijing, China. Atmos. Environ. 2001, 35, 4959–4970.
- Zhang, X.; Zhang, Y.; Cao, G. Aerosol Chemical Compositions of Beijing PM1 and Its Control Countermeasures. J. Appl. Meteorol. Sci. 2012, 23, 257–264, (In Chinese with English abstract).
- Shao, L.; Li, W.; Yang, S.; Shi, Z.; Lv, S. Mineralogical characteristics of airborne particles collected in Beijing during a severe Asian dust storm period in spring 2002. Sci. China Ser. D Earth Sci. 2007, 6, 953–959.
- Dong, F.; He, X.; Li, G. Study on the basic characteristics of several atmospheric dusts in the norther China. Miner. Pet. 2005, 25, 114–117, (In Chinese with English abstract).
- Wang, W.; Shao, L.; Zhang, D.; Li, Y.; Li, W.; Liu, P.; Xing, J. Mineralogical similarities and differences of dust storm particles at beijing from deserts in the north and northwest. Sci. Total Environ. 2022, 803, 149980.
- Tang, M.; Cziczo, D.J.; Grassian, V.H. Interactions of Water with Mineral Dust Aerosol: Water Adsorption, Hygroscopicity, Cloud Condensation, and Ice Nucleation. Chem. Rev. 2016, 116, 4205–4259.
- Shang, D.; Peng, J.; Guo, S.; Hu, M. Secondary aerosol formation in winter haze over the BeijingTianjin-Hebei Region, China. Front. Environ. Sci. Eng. 2021, 15, 34.
- Yan, G.; Zhang, P.; Yang, J.; Zhang, J.; Zhu, G.; Cao, Z.; Fan, J.; Liu, Z.; Wang, Y. Chemical characteristics and source apportionment of PM2.5 in a petrochemical city:implications for primary and secondary carbonaceous component. J. Environ. Sci. 2021, 103, 322–335.
- Gao, X.; Yang, L.; Cheng, S.; Gao, R.; Zhou, Y.; Xue, L.; Shou, Y.; Wang, J.; Wang, X.; Nie, W.; et al. Semi-continuous measurement of water-soluble ions in PM2.5 in Jinan, China: Temporal variations and source apportionments. Atmos. Environ. 2011, 45, 6048–6056.
- Lai, S.; Zou, S.; Cao, J.; Lee, S.; Ho, K. Characterizing ionic species in PM2.5 and PM10 in four Pearl River Delta cities, South China. J. Environ. Sci. 2007, 19, 939–947.
- Wang, W.; Fu, G. The Research of Source and Composition of Dust Haze in Chengdu and its Control Countermeasures. Adv. Mater. Res. 2014, 726–731, 2066–2073.
- Kim, B.M.; Teffera, S.; Zeldin, M.D. Characterization of PM 25 and PM 10 in the South Coast Air Basin of Southern California: Part 1—Spatial Variations. J. Air Waste Manag. Assoc. 2000, 50, 2034–2044.
- Rajeev, P.; Rajput, P.; Gupta, T. Chemical characteristics of aerosol and rain water during an el-nio and pdo influenced indian summer monsoon. Atmos. Environ. 2016, 145, 192–200.
- Weagle, C.L.; Snider, G.; Li, C.; Donkelaar, A.V.; Philip, S.; Bissonnette, P.; Burke, J.; Jackson, J.; Latimer, R. Global Sources of Fine Particulate Matter: Interpretation of PM2.5 Chemical Composition Observed by SPARTAN using a Global Chemical Transport Model. Environ. Sci. Technol. 2018, 52, 11670–11681.
- Li, X.; Lin, D.; TSONA, N.; GE, M. Anthropogenic Effects on Biogenic Secondary Organic Aerosol Formation. Adv. Atmos. Sci. 2021, 38, 1053–1084.
- Wang, J.; Zhao, B.; Wang, S.; Yang, S.; Xing, J.; Morawska, L.D.; Ding, A.; Kulmala, M.; Kerminen, V.M.; Kujansuu, J.; et al. Particulate matter pollution over China and the effects of control policies. Sci. Total Environ. 2017, 584–585, 426–447.
- Cheng, M.; Tang, G.; Lv, B.; Li, X.; Wang, Y. Source apportionment of PM2.5 and visibility in Jinan, China. J. Environ. Sci. 2021, 102, 207–215.
- Atkinson, R.; Arey, J. Atmospheric degradation of volatile organic compounds. Chem. Rev. 2003, 103, 4605–4638.
- Hu, J.; Cheng, Z.; Qin, X.; Dong, P. Reversible and irreversible gas–particle partitioning of dicarbonyl compounds observed in the real atmosphere. Atmos. Chem. Phys. 2022, 22, 6971–6987.
- Shi, B.; Wang, W.; Zhou, L.; Sun, Z.; Fan, C.; Chen, Y.; Zhang, W.; Qiao, Y.; Qiao, Y.; Ge, M. Atmospheric oxidation of C10~14 n-alkanes initiated by Cl atoms: Kinetics and mechanism. Atmos. Environ. 2020, 222, 117166.
- Zeineddine, M.N.; Romanias, M.N.; Gaudion, V.; Riffault, V.; Thévenet, F. Heterogeneous interaction of isoprene with natural gobi dust. ACS Earth Space Chem. 2017, 1, 236–243.
- Shen, X.; Zhao, Y.; Chen, Z.; Huang, D. Heterogeneous reactions of volatile organic compounds in the atmosphere. Atmos. Environ. 2013, 68, 297–314.
- Tillmann, R.; Hallquist, M.; Jonsson, Å.M.; Kiendler-Scharr, A.; Saathoff, H.; Iinuma, Y.; Mentel, T.F. Influence of relative humidity and temperature on the production of pinonaldehyde and OH radicals from the ozonolysis of α-pinene. Atmos. Chem. Phys. 2010, 10, 7057–7072.
- Mila, A.; Ningbo, G.; Rong, C.; Zhang, H.; Xiuhua, Z.; Jiping, C. Research progress of analytical methods and pollution characteristics of typical organic pollutant in atmospheric particulate matter. Environ. Chem. 2021, 40, 3774–3786, (In Chinese with English abstract).
- Kyllnen, K.; Vestenius, M.; Anttila, P.; Makkonen, U.; Aurela, M.; Wangberg, I.; Mastromonaco, M.N.; Hakola, H. Trends and source apportionment of atmospheric heavy metals at a subarctic site during 1996–2018. Atmos. Environ. 2020, 236, 117644.
- He, A.; Xie, J.; Yuan, C. Heavy Metal Speciation Analysis and Distribution Characteristics in Atmospheric Particulate Matters. Prog. Chem. 2021, 33, 1627–1647, (In Chinese with English abstract).
- Xu, J.W.; Martin, R.V.; Henderson, B.H.; Meng, J.; Öztanere, Y.B.; Handf, J.L.; Hakamie, A.; Strum, M.; Phillips, S.B. Simulation of airborne trace metals in fine particulate matter over north america. Atmos. Environ. 2019, 214, 116883.
- Rajput, J.S.; Trivedi, M.K. Determination and assessment of elemental concentration in the atmospheric particulate matter: A comprehensive review. Environ. Monit. Assess. 2022, 194, 243.
- Maia, P.D.; Vieira-Filho, M.; Prado, L.F.; Silva, L.C.; Sodré, F.F.; Ribeiro, H.D.; Ventura, R.S. Assessment of atmospheric particulate matter (PM10) in Central Brazil: Chemical and morphological aspects. Atmos. Pollut. Res. 2022, 13, 101362.
- Zhi, M.; Zhang, K.; Lv, W. A review of research advances in the distributions and risk assessments of metal elements in atmospheric particles with different particle sizes. J. Environ. Eng. 2022, 12, 998–1006, (In Chinese with English abstract).
- Zou, T.; Kang, W.; Zhang, J.; Wang, M.; Fang, X.; Xue, S.; Zhong, B. Concentrations and Distribution Characteristics of Atmospheric Heavy Metals in Urban Areas of China. Res. Environ. Sci. 2015, 28, 1053–1061, (In Chinese with English abstract).
- Tan, J.; Duan, J. Heavy metals in aerosol in China: Pollution, sources, and control strategies. J. Univ. Chin. Acad. Sci. 2013, 30, 145–155, (In Chinese with English abstract).
- Wang, M.; Deng, Y.Q.; Dong, F.Q.; He, X.C.; Dai, Q.W.; Sun, S.Y.; Huang, Y.B.; Tang, J.; Chen, W.; Liu, L.Z.; et al. Speciation analysis of cadmium in dust fall about northern China towns. Asian J. Chem. 2013, 25, 7522–7526.
- Iuga, C.; Sainz-Díaz, C.I.; Vivier-Bunge, A. Interaction energies and spectroscopic effects in the adsorption of formic acid on mineral aerosol surface models. J. Phys. Chem. C 2012, 116, 2904–2914.
- Usher, C.R.; Michel, A.E.; Grassian, V.H. Reactions on mineral dust. Chemical reviews. Chem. Rev. 2003, 103, 4883–4939.
- Wang, T.; Liu, Y.; Deng, Y.; Fu, H.; Zhang, L.; Chen, J. Adsorption of SO2 on mineral dust particles influenced by atmospheric moisture. Atmos. Environ. 2018, 191, 153–161.
- Goodman, A.L.; Li, P.; Usher, C.R.; Grassian, V.H. Heterogeneous uptake of sulfur dioxide on aluminum and magnesium oxide particles. J. Phys. Chem. A 2001, 105, 6109–6120.
- Usher, C.R.; Al-Hosney, H.; Carlos-Cuellar, S.; Grassian, V.H. A laboratory study of the heterogeneous uptake and oxidation of sulfur dioxide on mineral dust particles. J. Geophys. Res. Atmos. 2002, 107, ACH 16-1–ACH 16-9.
- Ullerstam, M.; Johnson, M.S.; Vogt, R.; Ljungström, E. DRIFTS and Knudsen cell study of the heterogeneous reactivity of SO2 and NO2 on mineral dust. Atmos. Chem. Phys. 2003, 3, 2043–2051.
- Wu, L.; Liu, Q.; Tong, S.; Jing, B.; Wang, W.; Guo, Y.; Ge, M. Mechanism and Kinetics of Heterogeneous Reactions of Unsaturated Organic Acids on α-Al2O3 and CaCO3. ChemPhysChem 2016, 17, 3515–3523.
- Huang, L.; Zhao, Y.; Li, H.; Chen, Z. Kinetics of heterogeneous reaction of sulfur dioxide on authentic mineral dust: Effects of relative humidity and hydrogen peroxide. Environ. Sci. Technol. 2015, 49, 10797–10805.
- Kebede, M.A.; Varner, M.E.; Scharko, N.K.; Gerber, R.B.; Raff, J.D. Photooxidation of Ammonia on TiO2 as a Source of NO and NO2 under Atmospheric Conditions. J. Am. Chem. Soc. 2013, 135, 8606–8615.
- Fu, H.; Wang, X.; Wu, H.; Yin, Y.; Chen, J. Heterogeneous uptake and oxidation of SO2 on iron oxides. J. Phys. Chem. C 2007, 111, 6077–6085.
- Wang, R.; Yang, N.; Li, J.; Xu, L.; Tsona, N.T.; Du, L.; Wang, W. Heterogeneous reaction of SO2 on CaCO3 particles: Different impacts of NO2 and acetic acid on the sulfite and sulfate formation. J. Environ. Sci. 2022, 114, 149–159.
- Ullerstam, M.; Vogt, R.; Langer, S.; Ljungström, E. The kinetics and mechanism of SO2 oxidation by O3 on mineral dust. Phys. Chem. Chem. Phys. 2002, 4, 4694–4699.
- Li, L.; Chen, Z.; Zhang, Y.; Zhu, T.; Li, J.; Ding, J. Kinetics and mechanism of heterogeneous oxidation of sulfur dioxide by ozone on surface of calcium carbonate. Atmos. Chem. Phys. 2006, 6, 2453–2464.
- Ma, Q.; Liu, Y.; He, H. Synergistic Effect between NO2 and SO2 in Their Adsorption and Reaction on γ-Alumina. J. Phys. Chem. A 2008, 112, 6630–6635.
- Liu, C.; Ma, Q.; Liu, Y.; Ma, J.; He, H. Synergistic reaction between SO2 and NO2 on mineral oxides: A potential formation pathway of sulfate aerosol. Phys. Chem. Chem. Phys. 2012, 14, 1668–1676.
