Atrazine, an herbicide used to control grassy and broadleaf weed, has become an essential part of agricultural crop protection tools. It is widely sprayed on corn, sorghum and sugar cane, with the attendant problems of its residues in agri-food and washing water. If ingested into humans, this residual atrazine can cause reproductive harm, developmental toxicity and carcinogenicity. It is therefore important to find clean and economical degradation processes for atrazine.
- atrazine
- degradation
- residue
- agri-food
- water
1. Introduction
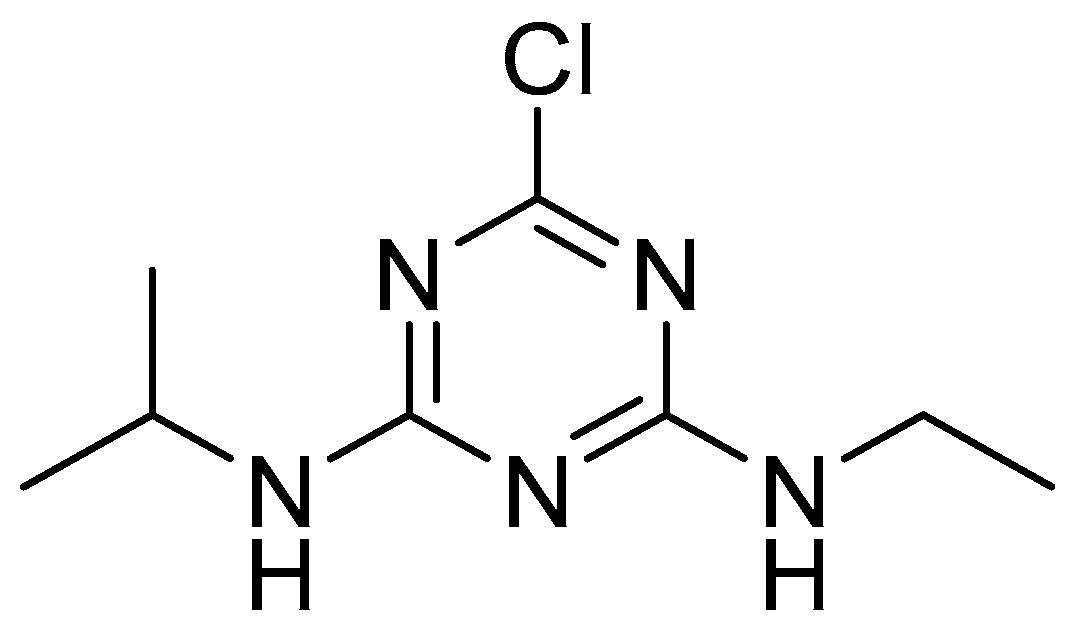
Figure 1.
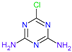
Atrazine (2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine).
2. Biodegradation
2.1. Microbial Degradation
Table 51.
Microbial degradation of aqueous atrazine.
| Strain | Origin | Removal Effect |
|---|---|---|
| Arthrobacter sp. DNS10 | Black soil [54][35] | The removal rate of 100 mg/L atrazine reached 95% and 86% in 0.05 mM Zn2+ and 1.0 mM Zn2+, respectively at 48 h [55][36]. |
| Bacillus badius ABP6 | Maize fields | Response-surface-methodology (RSM) was used to optimize environmental factors such as pH, temperature, agitation speed and atrazine-concentration on atrazine degradation by utilizing Bacillus badius ABP6 strain. In the optimum conditions (pH 7.05, temperature 30.4 °C, agitation speed 145.7 rpm, and atrazine-concentration 200.9 ppm), the degradation rate of atrazine reaches a maximum value of 90% [56][37]. |
| Bjerkanderaadusta | Rotten wood surfaces | In the optimum conditions (pH 4, temperature 28 °C, biomass 2 g, and atrazine-concentration 50 ppm), the removal rate of atrazine was up to 92% in 5 days [57][38]. |
| Agrobacterium sp. WL-1, |
| Plant | Gene/Enzymes | Result |
|---|---|---|
| Pennisetum cladestinum | Soil dehydrogenase | Within 80 days, nearly 45% of atrazine was degraded [59][42]. |
| Rice | Two novel methyltransferases LOC_Os04g09604, LOC_Os11g15040 | Atrazine degradation and detoxification are regulated [62][45] |
| Alfalfa (Medicago sativa) | Genes encoding glycosyltransferases, glutathione S-transferases or ABC transporters | Atrazine in alfalfa can be detoxified through different pathways [63][46]. |
| Arthrobacter sp. ZXY-2 | Jilin Pesticide Plant | After adding biochar ZXY-2 pellets, the removal rate of atrazine reached 61% within 1 h, higher than that treated by ZXY-2 pellets without biochar. The addition of biochar could enhance the connection between ZXY-2 and pellets-based carrier, and the favorable biodegradation pH of ZXY-2 changed to 6 and 10 [58][34]. |
| Chlorella sp. | The Freshwater Algae Culture Collection at the Institute of Hydrobiology, China | Atrazine with initial concentration of 5 mg/L was photocatalytic degraded for 60 min with degradation ratio of 31%. After an 8 d exposure of the microalga Chlorella sp., 83% and 64% of the atrazine were removed from the degraded solutions containing 40 μg/L and 80 μg/L of atrazine, respectively [124][39]. |
| Myriophyllum spicatum | Wuhan Botanical Garden | Myriophyllum spicatum absorbed more than 18-fold the amount of atrazine in sediments and degraded atrazine to hydroxyatrazine (HA), deelthylatrazine (DEA), didealkylatrazine (DDA), cyanuric acid (CYA) and biuret. The formation of biuret suggested for the first time, the ring opening of atrazine in an aquatic plant. The residual rate of atrazine was 6.5 ± 2.0% in M. spicatum-grown sediment on day 60 [125][40]. |
2.2. Phytodegradation
3. Degradation Pathways, Atrazine Mineralization and Metabolites Toxicity
The degradation of atrazine is a complex process with different pathways through different biotic or abiotic water treatment processes. Regarding the biotic degradation processes, there are two stages [146][49] (Figure 32). In the first stage, hydrolytic dichlorination and N-dealkylation of atrazine generate cyanuric acid in the role of the enzymes that have broad substrate specificity [147][50]. For hydrolytic dichlorination of atrazine, enzyme atrazine chlorohydrolase (AtzA) [148][51] or hydrolase triazine (TrzN) [149][52] catalyzes hydrolytic dichlorination of atrazine, but they display substantial differences in their substrate ranges: AtzA is restricted to atrazine analogs with a chlorine substituent at carbon 2 and N-alkyl groups, ranging in size from methyl to t-butyl [150][53], and TrzN hydrolyzes a range of leaving groups (e.g., OCH3, –SCH3, –Cl, –F, –CN) from both triazines and pyrimidines [149][52]. For N-dealkylation of atrazine, hydroxyatrazine N-ethylaminohydrolase (AtzB) [151][54] catalyzes the hydrolytic conversion of hydroxyatrazine to N-isopropylammelide, and N-isopropylammelide isopropylaminohydrolas (AtzC) [152][55] catalyzes the hydrolysis of N-isopropylammelide to cyanuric acid. In the second stage, cyanuric acid is converted to ammonium and carbon dioxide by a set of enzymes AtzDEF [153,154][56][57] and TrzD [153,155][56][58].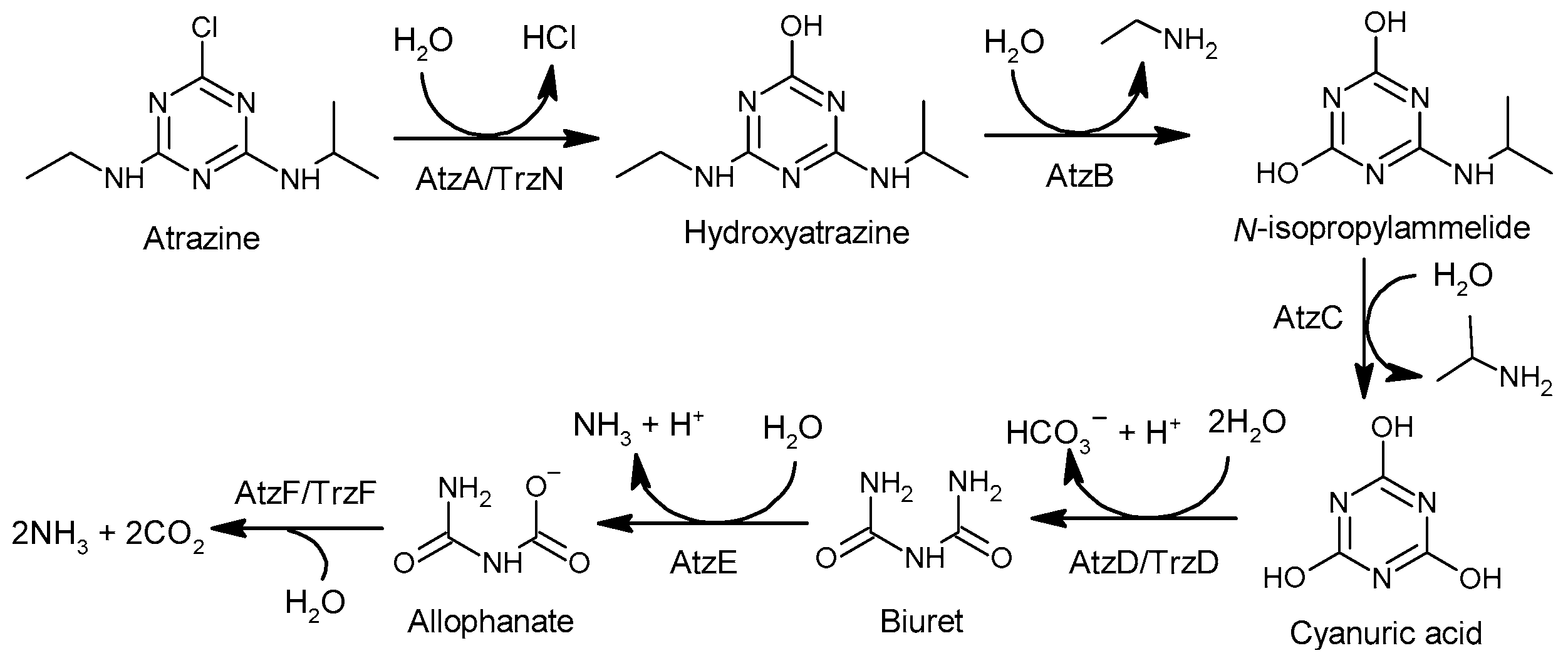
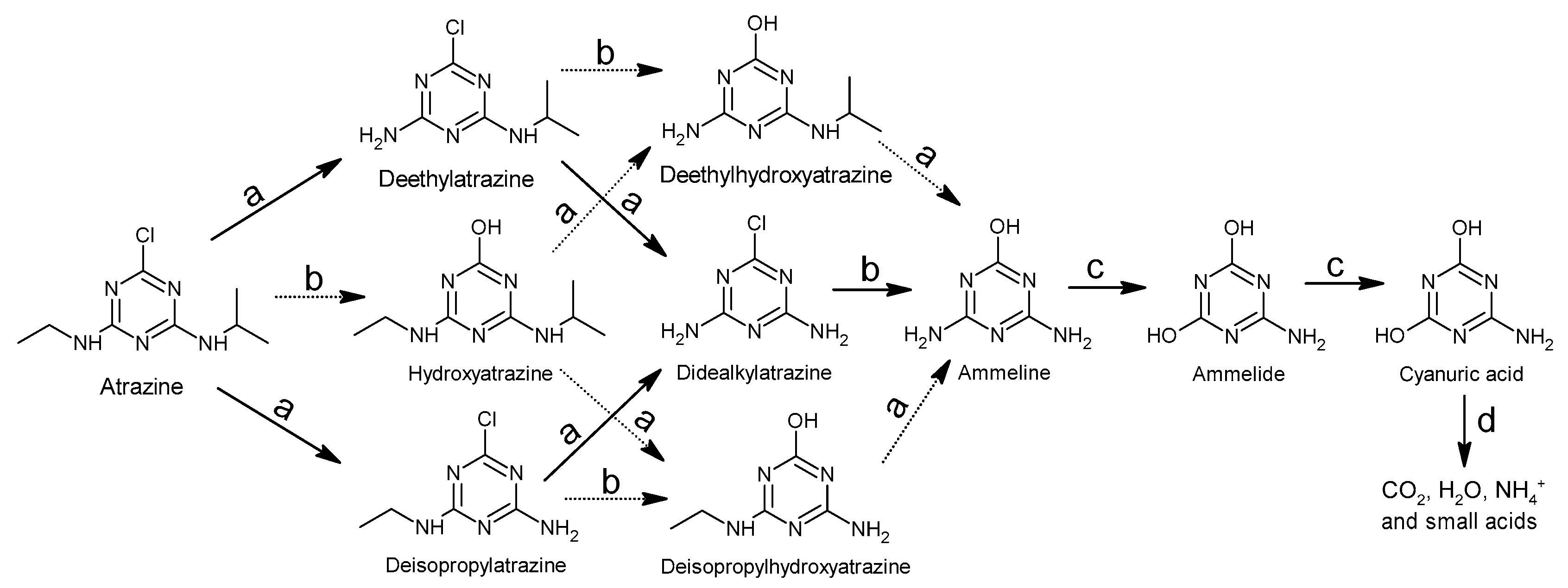
Figure 43. General involved degradation mechanisms of atrazine: (a) dealkylation of the amino groups; (b) dechlorination and hydroxylation of the s-triazine ring; (c) oxidation of the amino groups and deamination; (d) the opening of the s-triazine ring.
Table 83.
Chemical structures and toxicity tests data of atrazine and its metabolites.
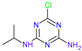
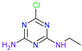
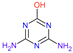
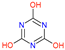
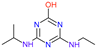
| Name | Atrazine (ATZ) | Deeth-Ylatrazine (DEA) | Deisoprop-Ylatrazine (DIA) | Ammeline (AM) | Cyanuric Acid | Dideal-Kylatrazine (DDA) |
Hydroxy-Atrazine (HA) |
|---|---|---|---|---|---|---|---|
| Chemical structure | 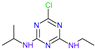 |
 |
 |
 |
 |
 |
 |
| Acute oral toxicity in male rats (LD50) [169][83] | 1870–3090 mg/kg | 1890 mg/kg | 2290 mg/kg | 3690 mg/kg | >5050 mg/kg | ||
| Median lethal concentrations (LC50) for Pseudokirchneriella subcapitata in 96 h of exposure [170][84] | 1600 μg/L | 2000 μg/L | >3000 μg/L | ||||
| Concentration for 50% of maximal effect (EC50) on algal photosynthesis for A. variabilis [171][85] | 0.1 ppm | 0.7 ppm | 4.7 ppm | 100 ppm | >100 ppm | ||
| Acute oral toxicity in rats (LD50) [171][85] | >5000 mg/kg | ||||||
| Adverse effects in sheep [172][86] | An average daily intake of ammeline 296 mg/kg body weight per day for 42 days for sheep caused half death. | No adverse effects at doses from 198 to 600 mg/kg body weight per day for 77 days. |
References
- Udiković-Kolić, N.; Scott, C.; Martin-Laurent, F. Evolution of atrazine-degrading capabilities in the environment. Appl. Microbiol. Biotechnol. 2012, 96, 1175–1189.
- Fan, X.; Song, F. Bioremediation of atrazine: Recent advances and promises. J. Soils Sediments 2014, 14, 1727–1737.
- He, H.; Liu, Y.; You, S.; Liu, J.; Xiao, H.; Tu, Z. A Review on Recent Treatment Technology for Herbicide Atrazine in Contaminated Environment. Int. J. Environ. Res. Public Health 2019, 16, 5129.
- Komang Ralebitso, T.; Senior, E.; van Verseveld, H.W. Microbial aspects of atrazine degradation in natural environments. Biodegradation 2002, 13, 11–19.
- Chandra, P.N.; Usha, K. Removal of atrazine herbicide from water by polyelectrolyte multilayer membranes. Mater. Today Proc. 2021, 41, 622–627.
- European Food Safety, A. Reasoned opinion on the setting of a new maximum residue level for atrazine in cereals. EFSA J. 2015, 13, 4126.
- Wirbisky, S.E.; Freeman, J.L. Atrazine Exposure and Reproductive Dysfunction through the Hypothalamus-Pituitary-Gonadal (HPG) Axis. Toxics 2015, 3, 414.
- Hayes, T.B.; Khoury, V.; Narayan, A.; Nazir, M.; Park, A.; Brown, T.; Adame, L.; Chan, E.; Buchholz, D.; Stueve, T.; et al. Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis). Proc. Natl. Acad. Sci. USA 2010, 107, 4612.
- Pathak, R.K.; Dikshit, A.K. Atrazine and Human Health. Int. J. Ecosyst. 2011, 1, 14–23.
- Farruggia, F.T.; Rossmeisl, C.M.; Hetrick, J.A.; Biscoe, M.; Branch, M., III. Refined Ecological Risk Assessment for Atrazine; US Environmental Protection Agency, Office of Pesticide Programs: Washington, DC, USA, 2016.
- Esteves, R.C.; do Amaral Vendramini, A.L.; Accioly, F. A qualitative meta-synthesis study of the convergence between organic crop regulations in the United States, Brazil, and Europe. Trends Food Sci. Technol. 2021, 107, 343–357.
- Megiato, E.I.; Massuquetti, A.; de Azevedo, A.F.Z. Impacts of integration of Brazil with the European Union through a general equilibrium model. EconomiA 2016, 17, 126–140.
- Wu, S.; Li, H.; Li, X.; He, H.; Yang, C. Performances and mechanisms of efficient degradation of atrazine using peroxymonosulfate and ferrate as oxidants. Chem. Eng. J. 2018, 353, 533–541.
- Khan, S.U.; Saidak, W.J. Residues of atrazine and its metabolites after prolonged usage. Weed Res. 1981, 21, 9–12.
- James, T.K.; Ghanizadeh, H.; Harrington, K.C.; Bolan, N.S. Degradation of atrazine and bromacil in two forestry waste products. Sci. Rep. 2021, 11, 3284.
- Wauchope, R.D.; Buttler, T.M.; Hornsby, A.G.; Augustijn-Beckers, P.W.M.; Burt, J.P. The SCS/ARS/CES Pesticide Properties Database for Environmental Decision-Making. In Reviews of Environmental Contamination and Toxicology: Continuation of Residue Reviews; Ware, G.W., Ed.; Springer: New York, NY, USA, 1992; pp. 1–155.
- Balci, B.; Oturan, N.; Cherrier, R.; Oturan, M.A. Degradation of atrazine in aqueous medium by electrocatalytically generated hydroxyl radicals. A kinetic and mechanistic study. Water Res. 2009, 43, 1924–1934.
- Belluck, D.A.; Benjamin, S.L.; Dawson, T. Groundwater Contamination by Atrazine and Its Metabolites. In Pesticide Transformation Products; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1991; Volume 459, pp. 254–273.
- Mahía, J.; Martín, A.; Carballas, T.; Díaz-Raviña, M. Atrazine degradation and enzyme activities in an agricultural soil under two tillage systems. Sci. Total Environ. 2007, 378, 187–194.
- Bohn, T.; Cocco, E.; Gourdol, L.; Guignard, C.; Hoffmann, L. Determination of atrazine and degradation products in Luxembourgish drinking water: Origin and fate of potential endocrine-disrupting pesticides. Food Addit. Contam. Part A 2011, 28, 1041–1054.
- Pereira, W.E.; Rostad, C.E. Occurrence, distributions, and transport of herbicides and their degradation products in the Lower Mississippi River and its tributaries. Environ. Sci. Technol. 1990, 24, 1400–1406.
- Yan, D.-h.; He, Y.; Wang, H. Environmental characteristics of the atrazine in the waters in East Liaohe River Basin. Huan Jing Ke Xue 2005, 26, 203–208. (In Chinese)
- Antić, N.; Radišić, M.; Radović, T.; Vasiljević, T.; Grujić, S.; Petković, A.; Dimkić, M.; Laušević, M. Pesticide Residues in the Danube River Basin in Serbia—A Survey during 2009–2011. CLEAN Soil Air Water 2015, 43, 197–204.
- Steffens, C.; Ballen, S.C.; Scapin, E.; da Silva, D.M.; Steffens, J.; Jacques, R.A. Advances of nanobiosensors and its application in atrazine detection in water: A review. Sens. Actuators Rep. 2022, 4, 100096.
- Salahshoor, Z.; Ho, K.-V.; Hsu, S.-Y.; Lin, C.-H.; Fidalgo de Cortalezzi, M. Detection of Atrazine and its metabolites by photonic molecularly imprinted polymers in aqueous solutions. Chem. Eng. J. Adv. 2022, 12, 100368.
- Farooq, S.; Wu, H.; Nie, J.; Ahmad, S.; Muhammad, I.; Zeeshan, M.; Khan, R.; Asim, M. Application, advancement and green aspects of magnetic molecularly imprinted polymers in pesticide residue detection. Sci. Total Environ. 2022, 804, 150293.
- Farooq, S.; Nie, J.; Cheng, Y.; Yan, Z.; Li, J.; Bacha, S.A.S.; Mushtaq, A.; Zhang, H. Molecularly imprinted polymers’ application in pesticide residue detection. Analyst 2018, 143, 3971–3989.
- Gouma, S.; Fragoeiro, S.; Bastos, A.C.; Magan, N. 13—Bacterial and Fungal Bioremediation Strategies. In Microbial Biodegradation and Bioremediation; Das, S., Ed.; Elsevier: Oxford, UK, 2014; pp. 301–323.
- Lasserre, J.-P.; Fack, F.; Revets, D.; Planchon, S.; Renaut, J.; Hoffmann, L.; Gutleb, A.C.; Muller, C.P.; Bohn, T. Effects of the Endocrine Disruptors Atrazine and PCB 153 on the Protein Expression of MCF-7 Human Cells. J. Proteome Res. 2009, 8, 5485–5496.
- Yanze-Kontchou, C.; Gschwind, N. Mineralization of the herbicide atrazine as a carbon source by a Pseudomonas strain. Appl. Environ. Microbiol. 1994, 60, 4297–4302.
- Struthers, J.K.; Jayachandran, K.; Moorman, T.B. Biodegradation of Atrazine by Agrobacterium radiobacter J14a and Use of This Strain in Bioremediation of Contaminated Soil. Appl. Environ. Microbiol. 1998, 64, 3368–3375.
- Bhatt, P.; Pathak, V.M.; Joshi, S.; Bisht, T.S.; Singh, K.; Chandra, D. Chapter 12—Major metabolites after degradation of xenobiotics and enzymes involved in these pathways. In Smart Bioremediation Technologies; Bhatt, P., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 205–215.
- Vidali, M. Bioremediation. An overview. Pure Appl. Chem. 2001, 73, 1163–1172.
- Yu, T.; Wang, L.; Ma, F.; Wang, Y.; Bai, S. A bio-functions integration microcosm: Self-immobilized biochar-pellets combined with two strains of bacteria to remove atrazine in water and mechanisms. J. Hazard. Mater. 2020, 384, 121326.
- Zhang, Y.; Jiang, Z.; Cao, B.; Hu, M.; Wang, Z.; Dong, X. Metabolic ability and gene characteristics of Arthrobacter sp. strain DNS10, the sole atrazine-degrading strain in a consortium isolated from black soil. Int. Biodeterior. Biodegrad. 2011, 65, 1140–1144.
- Jiang, Z.; Chen, J.; Li, J.; Cao, B.; Chen, Y.; Liu, D.; Wang, X.; Zhang, Y. Exogenous Zn2+ enhance the biodegradation of atrazine by regulating the chlorohydrolase gene trzN transcription and membrane permeability of the degrader Arthrobacter sp. DNS10. Chemosphere 2020, 238, 124594.
- Khatoon, H.; Rai, J.P.N. Optimization studies on biodegradation of atrazine by Bacillus badius ABP6 strain using response surface methodology. Biotechnol. Rep. 2020, 26, e00459.
- Dhiman, N.; Jasrotia, T.; Sharma, P.; Negi, S.; Chaudhary, S.; Kumar, R.; Mahnashi, M.H.; Umar, A.; Kumar, R. Immobilization interaction between xenobiotic and Bjerkandera adusta for the biodegradation of atrazine. Chemosphere 2020, 257, 127060.
- Hu, N.; Xu, Y.; Sun, C.; Zhu, L.; Sun, S.; Zhao, Y.; Hu, C. Removal of atrazine in catalytic degradation solutions by microalgae Chlorella sp. and evaluation of toxicity of degradation products via algal growth and photosynthetic activity. Ecotoxicol. Environ. Saf. 2021, 207, 111546.
- Qu, M.; Li, N.; Li, H.; Yang, T.; Liu, W.; Yan, Y.; Feng, X.; Zhu, D. Phytoextraction and biodegradation of atrazine by Myriophyllum spicatum and evaluation of bacterial communities involved in atrazine degradation in lake sediment. Chemosphere 2018, 209, 439–448.
- Newman, L.A.; Reynolds, C.M. Phytodegradation of organic compounds. Curr. Opin. Biotechnol. 2004, 15, 225–230.
- Singh, N.; Megharaj, M.; Kookana, R.S.; Naidu, R.; Sethunathan, N. Atrazine and simazine degradation in Pennisetum rhizosphere. Chemosphere 2004, 56, 257–263.
- Zhang, J.J.; Lu, Y.C.; Yang, H. Chemical Modification and Degradation of Atrazine in Medicago sativa through Multiple Pathways. J. Agric. Food Chem. 2014, 62, 9657–9668.
- Lu, Y.C.; Feng, S.J.; Zhang, J.J.; Luo, F.; Zhang, S.; Yang, H. Genome-wide identification of DNA methylation provides insights into the association of gene expression in rice exposed to pesticide atrazine. Sci. Rep. 2016, 6, 18985.
- Lu, Y.C.; Luo, F.; Pu, Z.J.; Zhang, S.; Huang, M.T.; Yang, H. Enhanced detoxification and degradation of herbicide atrazine by a group of O-methyltransferases in rice. Chemosphere 2016, 165, 487–496.
- Zhang, J.J.; Lu, Y.C.; Zhang, S.H.; Lu, F.F.; Yang, H. Identification of transcriptome involved in atrazine detoxification and degradation in alfalfa (Medicago sativa) exposed to realistic environmental contamination. Ecotoxicol. Environ. Saf. 2016, 130, 103–112.
- Murphy, I.J.; Coats, J.R. The capacity of switchgrass (Panicum virgatum) to degrade atrazine in a phytoremediation setting. Environ. Toxicol. Chem. 2011, 30, 715–722.
- Pérez, D.J.; Doucette, W.J.; Moore, M.T. Atrazine uptake, translocation, bioaccumulation and biodegradation in cattail (Typha latifolia) as a function of exposure time. Chemosphere 2022, 287, 132104.
- Govantes, F.; Porrúa, O.; García-González, V.; Santero, E. Atrazine biodegradation in the lab and in the field: Enzymatic activities and gene regulation. Microb. Biotechnol. 2009, 2, 178–185.
- Shapir, N.; Mongodin, E.F.; Sadowsky, M.J.; Daugherty, S.C.; Nelson, K.E.; Wackett, L.P. Evolution of Catabolic Pathways: Genomic Insights into Microbial s-Triazine Metabolism. J. Bacteriol. 2007, 189, 674–682.
- de Souza, M.L.; Sadowsky, M.J.; Wackett, L.P. Atrazine chlorohydrolase from Pseudomonas sp. strain ADP: Gene sequence, enzyme purification, and protein characterization. J. Bacteriol. 1996, 178, 4894–4900.
- Shapir, N.; Pedersen, C.; Gil, O.; Strong, L.; Seffernick, J.; Sadowsky Michael, J.; Wackett Lawrence, P. TrzN from Arthrobacter aurescens TC1 Is a Zinc Amidohydrolase. J. Bacteriol. 2006, 188, 5859–5864.
- Seffernick Jennifer, L.; Johnson, G.; Sadowsky Michael, J.; Wackett Lawrence, P. Substrate Specificity of Atrazine Chlorohydrolase and Atrazine-Catabolizing Bacteria. Appl. Environ. Microbiol. 2000, 66, 4247–4252.
- Seffernick Jennifer, L.; Aleem, A.; Osborne Jeffrey, P.; Johnson, G.; Sadowsky Michael, J.; Wackett Lawrence, P. Hydroxyatrazine N-Ethylaminohydrolase (AtzB): An Amidohydrolase Superfamily Enzyme Catalyzing Deamination and Dechlorination. J. Bacteriol. 2007, 189, 6989–6997.
- Shapir, N.; Osborne Jeffrey, P.; Johnson, G.; Sadowsky Michael, J.; Wackett Lawrence, P. Purification, Substrate Range, and Metal Center of AtzC: The N-Isopropylammelide Aminohydrolase Involved in Bacterial Atrazine Metabolism. J. Bacteriol. 2002, 184, 5376–5384.
- Rousseaux, S.; Hartmann, A.; Soulas, G. Isolation and characterisation of new Gram-negative and Gram-positive atrazine degrading bacteria from different French soils. FEMS Microbiol. Ecol. 2001, 36, 211–222.
- García-González, V.; Govantes, F.; Porrúa, O.; Santero, E. Regulation of the Pseudomonas sp. Strain ADP Cyanuric Acid Degradation Operon. J. Bacteriol. 2005, 187, 155–167.
- Karns Jeffrey, S. Gene Sequence and Properties of ans-Triazine Ring-Cleavage Enzyme from Pseudomonassp. Strain NRRLB-12227. Appl. Environ. Microbiol. 1999, 65, 3512–3517.
- Getenga, Z.; Dörfler, U.; Iwobi, A.; Schmid, M.; Schroll, R. Atrazine and terbuthylazine mineralization by an Arthrobacter sp. isolated from a sugarcane-cultivated soil in Kenya. Chemosphere 2009, 77, 534–539.
- Xu, L.J.; Chu, W.; Graham, N. Atrazine degradation using chemical-free process of USUV: Analysis of the micro-heterogeneous environments and the degradation mechanisms. J. Hazard. Mater. 2014, 275, 166–174.
- Bahena, C.L.; Martínez, S.S.; Guzmán, D.M.; del Refugio Trejo Hernández, M. Sonophotocatalytic degradation of alazine and gesaprim commercial herbicides in TiO2 slurry. Chemosphere 2008, 71, 982–989.
- Bianchi, C.L.; Pirola, C.; Ragaini, V.; Selli, E. Mechanism and efficiency of atrazine degradation under combined oxidation processes. Appl. Catal. B Environ. 2006, 64, 131–138.
- Wen, D.; Chen, B.; Liu, B. An ultrasound/O3 and UV/O3 process for atrazine manufacturing wastewater treatment: A multiple scale experimental study. Water Sci. Technol. 2021, 85, 229–243.
- Zhou, S.; Bu, L.; Shi, Z.; Bi, C.; Yi, Q. A novel advanced oxidation process using iron electrodes and ozone in atrazine degradation: Performance and mechanism. Chem. Eng. J. 2016, 306, 719–725.
- Sarmento, S.M.; Miranda, J.T.G. Kinetics of the atrazine degradation process using H2O2-UVC. Water Sci. Technol. 2014, 69, 2279–2286.
- Popova, S.A.; Matafonova, G.G.; Batoev, V.B. Removal of organic micropollutants from water by sonophotolytic-activated persulfate process. IOP Conf. Ser. Mater. Sci. Eng. 2019, 687, 066051.
- Moreira, A.J.; Borges, A.C.; Gouvea, L.F.C.; MacLeod, T.C.O.; Freschi, G.P.G. The process of atrazine degradation, its mechanism, and the formation of metabolites using UV and UV/MW photolysis. J. Photochem. Photobiol. A Chem. 2017, 347, 160–167.
- Moreira, A.J.; Pinheiro, B.S.; Araújo, A.F.; Freschi, G.P.G. Evaluation of atrazine degradation applied to different energy systems. Environ. Sci. Pollut. Res. 2016, 23, 18502–18511.
- Fareed, A.; Hussain, A.; Nawaz, M.; Imran, M.; Ali, Z.; Haq, S.U. The impact of prolonged use and oxidative degradation of Atrazine by Fenton and photo-Fenton processes. Environ. Technol. Innov. 2021, 24, 101840.
- Khan, J.A.; Shah, N.S.; Nawaz, S.; Ismail, M.; Rehman, F.; Khan, H.M. Role of eaq−, ·OH and H· in radiolytic degradation of atrazine: A kinetic and mechanistic approach. J. Hazard. Mater. 2015, 288, 147–157.
- Tauber, A.; von Sonntag, C. Products and Kinetics of the OH-radical-induced Dealkylation of Atrazine. Acta Hydrochim. Hydrobiol. 2000, 28, 15–23.
- Song, W.; Li, J.; Fu, C.; Wang, Z.; Zhang, X.; Yang, J.; Hogland, W.; Gao, L. Kinetics and pathway of atrazine degradation by a novel method: Persulfate coupled with dithionite. Chem. Eng. J. 2019, 373, 803–813.
- Khan, J.A.; He, X.; Shah, N.S.; Sayed, M.; Khan, H.M.; Dionysiou, D.D. Degradation kinetics and mechanism of desethyl-atrazine and desisopropyl-atrazine in water with •OH and SO4− based-AOPs. Chem. Eng. J. 2017, 325, 485–494.
- Aggelopoulos, C.A.; Tataraki, D.; Rassias, G. Degradation of atrazine in soil by dielectric barrier discharge plasma—Potential singlet oxygen mediation. Chem. Eng. J. 2018, 347, 682–694.
- Wang, X.; Wang, Y.; Chen, N.; Shi, Y.; Zhang, L. Pyrite enables persulfate activation for efficient atrazine degradation. Chemosphere 2020, 244, 125568.
- Zhang, R.; Wan, Y.; Peng, J.; Yao, G.; Zhang, Y.; Lai, B. Efficient degradation of atrazine by LaCoO3/Al2O3 catalyzed peroxymonosulfate: Performance, degradation intermediates and mechanism. Chem. Eng. J. 2019, 372, 796–808.
- Yanagisawa, I.; Oyama, T.; Serpone, N.; Hidaka, H. Successful Scission of a Recalcitrant Triazinic Ring. The Photoassisted Total Breakup of Cyanuric Acid in Ozonized TiO2 Aqueous Dispersions in the Presence of an Electron Acceptor (H2O2). J. Phys. Chem. C 2008, 112, 18125–18133.
- Teng, X.; Li, J.; Wang, J.; Liu, J.; Ge, X.; Gu, T. Effective degradation of atrazine in wastewater by three-dimensional electrochemical system using fly ash-red mud particle electrode: Mechanism and pathway. Sep. Purif. Technol. 2021, 267, 118661.
- Zhanqi, G.; Shaogui, Y.; Na, T.; Cheng, S. Microwave assisted rapid and complete degradation of atrazine using TiO2 nanotube photocatalyst suspensions. J. Hazard. Mater. 2007, 145, 424–430.
- Yang, N.; Liu, Y.; Zhu, J.; Wang, Z.; Li, J. Study on the efficacy and mechanism of Fe-TiO2 visible heterogeneous Fenton catalytic degradation of atrazine. Chemosphere 2020, 252, 126333.
- Ding, X.; Wang, S.; Shen, W.; Mu, Y.; Wang, L.; Chen, H.; Zhang, L. 2O3 promoted electrochemical mineralization of atrazine via a triazinon ring opening mechanism. Water Res. 2017, 112, 9–18.
- Borràs, N.; Oliver, R.; Arias, C.; Brillas, E. Degradation of Atrazine by Electrochemical Advanced Oxidation Processes Using a Boron-Doped Diamond Anode. J. Phys. Chem. A 2010, 114, 6613–6621.
- World Health Organization. Pesticide Residues in Food: 2007, Toxicological Evaluations, Sponsored Jointly by FAO and WHO, with the support of the International Programme on Chemical Safety, Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group; World Health Organization: Geneva, Switzerland, 2007.
- Ralston-Hooper, K.; Hardy, J.; Hahn, L.; Ochoa-Acuña, H.; Lee, L.S.; Mollenhauer, R.; Sepúlveda, M.S. Acute and chronic toxicity of atrazine and its metabolites deethylatrazine and deisopropylatrazine on aquatic organisms. Ecotoxicology 2009, 18, 899–905.
- Stratton, G.W. Effects of the herbicide atrazine and its degradation products, alone and in combination, on phototrophic microorganisms. Arch. Environ. Contam. Toxicol. 1984, 13, 35–42.
- EFSA Panel on Contaminants in the Food Chain (CONTAM); EFSA Panel on Food Contact Materials; Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on Melamine in Food and Feed. EFSA J. 2010, 8, 1573.
- Liu, B.; Guo, W.; Wang, H.; Si, Q.; Zhao, Q.; Luo, H.; Ren, N. Activation of peroxymonosulfate by cobalt-impregnated biochar for atrazine degradation: The pivotal roles of persistent free radicals and ecotoxicity assessment. J. Hazard. Mater. 2020, 398, 122768.
