Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by G.G. Flores-Rojas and Version 3 by Beatrix Zheng.
Magnetic nanoparticles (MNPs) are nanoscale particles (1–100 nm) that can be guided through an external magnetic field due to their superparamagnetic, ferrimagnetic, and ferromagnetic properties, which may provide features for biomedical applications.
- magnetic drug delivery
- hyperthermia
- superparamagnetic
- nanoparticles
- superconductors
1. Introduction
Magnetic nanoparticles (MNPs) with superparamagnetic properties are of special interest because they exhibit strong magnetic interactions under an external magnetic field, which disappear once the external magnetic field is removed. This property allows for the design of ferrofluids, since MNPs can be stabilized in solutions because they do not present magnetic interactions when the external magnetic field is switched off; and allows for in vivo performance, as in (i) cell marking [1], (ii) drug systems guided by a magnetic field [2], (iii) image contrast agents [3], and (iv) as heat generators in hyperthermia treatments [4].
MNPs generally contains two main parts: the core and the coating. The core presents a predominant quantum effect, which commonly incorporates magnetic elements such as Fe, Ni, or Co as well as their corresponding oxides, while the coating is responsible for stabilizing and protecting the core from the chemical effects of the medium [5]. The coating also plays an essential role since it provides specific properties and functions to the nucleus; for example, biocompatible natural polymers, such as chitosan or cellulose, work as cargo vectors of therapeutic agents to release in a controlled manner (Figure 1). Due to these characteristics, biomedical device synthesis has shown great interest in low toxic superparamagnetic MNPs, to avoid embolization or other secondary effects for the patient at the molecular or cellular level [6].
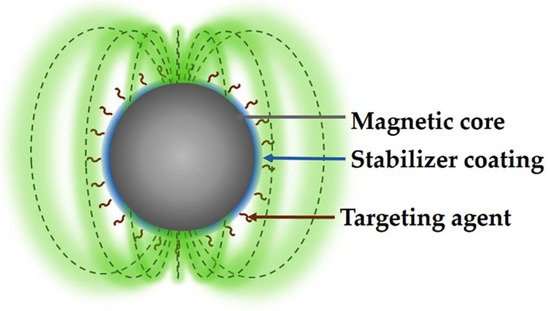
Figure 1.
Structure of functionalized MNP for medical applications.
2. Synthesis of Magnetic Nanoparticles
The synthesis of nanoparticles (NPs) can be classified into three strategies: these are chemical, physical, and biological; chemical and physical classifications are widely used due to the significant number of synthesis methods that vary from precipitation to advanced methods and complex. Some of the most used synthesis methods are microemulsions [7], hydrothermal reactions [8], hydrolysis [9], sonolysis [10], thermolysis [11], electrochemistry [12], flow injection [13][14][13,14], electrospray [15], reduction [16], micellar [17], and gamma rays [18]; each of these methods are carried out in unique synthesis conditions that modify the physicochemical properties of MNPs, and therefore produce changes in their magnetic properties [19].
Precipitation and coprecipitation methods are widely used to obtain MNPs with a varied composition, such as Fe3O4 and γ-Fe2O3, as well as other NPs using a single element in their composition, such as Fe, Co, and Ni. However, some MNPs with medical applications have varied compositions, including metal and alloys such as Fe-Pd [20], Mn-Zn-Gd-ferrite [21], and lanthanides, for example, Ln(III):Fe3O4 (Sm, Eu, Gd) [22]. This methodology has also allowed for the synthesis of bioactive ferrimagnetic glass-ceramic MNPs with a layer of apatite similar to bone, which allow for the binding of proteins and drugs, providing adequate orientation to the affected tissue [23].
2.1. Precipitation and Coprecipitation
Precipitation and coprecipitation are easy and versatile methods that are widely used in the synthesis of MNPs with magnetic metal oxide nuclei from the corresponding salts in a basic medium, inert atmosphere, and at room or higher temperature, with the method being highly reproducible once the reaction conditions are adjusted, and therefore allowing for the size dispersion to be reduced [24]. This method’s most common example of MNPs synthesized are Fe3O4 or γ-Fe2O3 NPs, obtained from Fe2+/Fe3+. The synthesis of this type of MNPs depends mainly on the sources of metal ions and factors such as temperature, pH, and ionic strength of the reaction medium. These MNPs presented low stability to environmental conditions, oxidizing or dissolving in the acid medium. Fe3O4 or γ-Fe2O3 MNPs can be oxidized as part of their synthesis by treating them with Fe3+ solution, resulting in stable MNPs in acidic and basic media.
However, despite the challenge of promoting uniform nucleation of the MNPs, the real task consists of controlling the particle size to obtain a narrow size distribution in a slow nuclei growth. One of the most important factors controlling nuclei growth is temperature since this can lead to the formation of a wide range of NP sizes since this synthesis method tends to produce quite polydisperse NPs. However, obtaining NPs with low dispersity is possible once the synthesis conditions are standardized and adequately controlled.
Currently, precipitation and coprecipitation methods include several additives in the NPs synthesis process, which have the main function of stabilizing them, and in addition, can act as a reducing agent promoting nucleation. Some compounds used as stabilizing or reducing agents are polyvinyl alcohol (PVA), poloxamers, poloxamines, oleic acid, and derivatives of poly(ethylene glycol) (PEG) or even liposomes [25]. For example, the concentration of PVA and citric acid in the synthesis of MNPs can modify the size and shape of the NPs [26]. However, chelation of metal ions can affect nucleation, promoting the formation of larger particles due to the low availability of nuclei in the reaction system and resulting in a massive growth of the available nuclei, with the synthesis system being dominated by the particle growth. Moreover, the adsorption of additives on the nuclei as coatings can decrease the growth rate, favoring the formation of smaller NPs.
2.2. Thermal Decomposition
The synthesis of NPs by thermal decomposition consists of the heating decomposition of organometallic compounds in organic solvents (with a high boiling point) and in the presence of stabilizing agents (i.e., surfactants) [27][28][27,28]. Some of the organometallic precursors of NPs are, for example, those synthesized with acetylketonates, N-nitrosophenylhydroxylamine (cupferronates), carbonyls, among others having as metallic centers Fe2+,3+, Mn2+,3+, Co2+,3+, Ni2+,3+, and using fatty acids, oleic acids, and hexadecylamine as stabilizing agents are some examples [29]. The type of NPs that can be obtained will depend on the organometallic compound used as a source; for example, if the organometallic compound contains metallic centers with a zero-oxidation state (i.e., Fe(CO)5), it will take place the formation of metallic NPs. On the other hand, organometallic compounds with cationic metal centers will form metal oxide NPs such as Fe3O4, from [Fe(acac)3] used as a precursor [30][31][30,31]. Furthermore, metal precursors can be combined in the reaction system, resulting in NPs with magnetic alloys such as CoPt3 [32] and FePt [33]. These types of MNPs present a high anisotropy and magnetic susceptibility as well as large coercivities [34].
This synthesis method allows for the size and shape of the NPs to be controlled, mainly by the ratios of the reagents; that is, the source of metal ions, stabilizing agent, solvent, reaction time, and temperature, which modify the reaction rate, morphology, and size of the NPs. Another variable to consider is the type of stabilizer used. For example, the chain length of fatty acids as stabilizers modifies the reactivity of metal precursors; that is, by reducing the alkyl chain of fatty acids, the reaction rate increases. Some of these variables were used by Jana et al., who synthesized different types of metal oxide MNPs such as Fe3O4, Cr2O3, MnO, Co3O4, and NiO, controlling the size and shape through concentration and reactivity. For example, iron oxide MNPs were synthesized from metal fatty acid salts, obtaining almost monodispersed NPs with an adjustable size between 6–50 nm and shapes that included points and cubes with increasing reaction time [35].
On the other hand, water-soluble magnetite NPs were also synthesized using a straightforward synthesis consisting of FeCl3·6H2O as an iron source and 2-pyrrolidone as a coordination solvent [36]. The size of the NPs was controlled by the reaction time, giving the following sizes 4, 12, and 60 nm for the times of 1, 10, and 24 h, respectively. The reaction time also modified the morphology of the NPs, changing from spherical at an early stage to cubic over longer times. The same group recently developed a one-step synthesis of water-soluble MNPs using similar reaction conditions by adding a dicarboxyl-terminated PEG as a surface-protecting agent [37]. MNPs showed potential application as magnetic resonance imaging (MRI) contrast agents for diagnosing cancerous tissue.
Although metal oxide NPs are the most synthesized by thermal decomposition, it is also possible to obtain metal NPs using suitable metal carbonyl sources. The metallic NPs, in comparison with the NPs of their corresponding oxides, show a higher magnetization, being of particular interest not only in the medical area but also in the technological area, mainly in data storage. Some of the MNPs synthesized using carbonyl complexes are, for example, Fe MNPs, which were synthesized using Fe(CO)5 as an iron source in the presence of polyisobutene in decalin under an inert nitrogen atmosphere at 170 °C [38], giving a particle size range of 2–10 nm and a polydispersity of 10%, depending on the Fe(CO)5/polyisobutene ratio. In the case of cobalt MNPs, [Co2(CO)8] was used as the cobalt source in the presence of aluminum alkyl compounds (AlR3); the Co MNPs ranged from 3–11 nm by controlling the size of the alkyl (R) chain length. In addition, these particles showed strong oxidation under air atmosphere, requiring a subsequent treatment to inhibit oxidation; such treatments were coatings with polymers, resulting in stable MNPs in air, being more attractive due to their easy handling, storage, and applications in oxidizing media.
2.3. Microemulsion
Microemulsions are produced by dispersing one immiscible liquid in another (commonly water), forming droplets between 1 and 50 nm, which are stabilized by a surfactant [39]. Microemulsions are used as a nanoreactor capable of forming NPs due to the formation of microdroplets that contain the desired reagents. These droplets collide and merge in the reaction system, mixing the reagents and carrying out the formation of NPs. However, this method’s drawbacks are polydispersity, low yield of NPs, large volumes of solvents required, and poor versatility in metal precursors compared to coprecipitation and thermal decomposition synthesis methods. Therefore, microemulsion would be a complicated method of synthesizing NPs.
Despite the drawbacks of the microemulsion method, various NPs have been synthesized, such as Fe3O4 NPs or MFe2O4 alloys with Co, Cu, Ni, Cd, etc., modifying their magnetic properties by incorporating these elements. For example, MnFe2O4-type alloyed NPs with sizes between 4–15 nm were synthesized by reverse micelles using sodium dodecylbenzenesulfonate as a surfactant [40], and the results showed that the size of the MNPs was related to the ratio between the water and solvent. Similarly, Fe3O4 nanorods were synthesized using reverse micelles as sol–gel method and FeCl3·6H2O as metal source. The studies in the synthesis showed that the phase of nanorods can be controlled through the reaction conditions such as temperature, atmosphere, and hydration degree of the gels. On the other hand, MNPs alloyed with CoFe2O4 were obtained by mixing FeCl3 and Co(AcO)2, in the presence of sodium dodecyl sulfate. The size of the MNPs was controlled by the concentrations of the metal sources and the surfactant. The average size of the MNPs varied from 2 to 5 nm, with a high polydispersity from 30 to 35% [41].
2.4. Coatings of MNPs
In the last decade, the synthesis of MNPs has made significant progress [42]; however, some problems remain, such as maintaining the stability of the NPs for long periods of storage, since uncoated MNPs are not stable in aqueous media, forming aggregates that later precipitate, a behavior that has also been studied in blood. In addition, once the MNPs enter the body they begin to be absorbed by proteins to finally be phagocytosed by macrophages [43]. Therefore, one approach to overcome these drawbacks in MNPs is a necessary coating to eliminate or minimize their aggregation in a physiological environment and phagocytosis, which can be generated in situ or as a post-synthesis [44][45][44,45].
Amphiphilic polymers are generally used to coat the surface of MNPs; some examples are poloxamers, poloxamines, and PEG derivatives or even liposomes, which have also given encouraging results in the assemble of magnetoliposomes [25]. One of the most widely used polymers as coatings is PEG, which produces biocompatible coatings that confer several other properties to NPs, such as high stability and dispersion in aqueous solutions and a prolonged circulation time in the blood. On the other hand, the adequate functionalization of PEG chains can allow bioconjugation with different ligands or therapeutic agents, expanding its possible applications in the biomedical area [46][47][48][46,47,48]. However, one of the drawbacks of the coatings is the modification of the final diameter of the MNPs and their thickness, which can significantly affect the relaxation and distribution capacity in vivo [49]. Consequently, it is critical to provide NPs with an amphiphilic polymer coating of appropriate molecular weight and ratio when designing MNP-bearing probes for imaging and targeted therapy.
Despite significant advances in developing MNPs, some obstacles remain, mainly in developing coatings that can stabilize MNPs and provide a chemically functional surface for bioconjugation with "probe" ligands. In addition, several of the ligands and polymers used to stabilize MNPs show weak interactions, allowing for their easy separation under physiological and storage conditions (Table 1). On the other hand, the degree of success of the MNPs depends mainly on the magnetic properties of the MNPs, which are entirely linked to their morphology, structure, and crystal uniformity, as well as the orientation of the MNPs given by conjugation with biomolecules. Another factor to consider is the size distribution, being preferable to a size less than 100 nm, allowing for the decrease in phagocytosis [50][51][50,51].
Table 1.
Coatings of NPs with possible medical applications.
| Coating | Advantages | Medical Application | |||
|---|---|---|---|---|---|
| PEG [52][53] | PEG [52,53] | Enhanced water solubility, reduced phagocytosis, and increased blood circulation time | MRI, tumor diagnosis, and treatment | ||
| Polyethylenimine [54][55][56] | Polyethylenimine [54,55,56] | Good biocompatibility | Gene and vectors | ||
| PVA [57][58][59] | PVA [57,58,59] | Elevated stability, reducing the particle aggregation | MRI, vectors, and bioseparation | ||
| Glucan [60][61][62][63][64] | Glucan [60,61,62,63,64] | Excellent stability and extended blood circulation time | Vectors, MRI | ||
| Liposome [65][66][67][68] | Liposome [65,66,67,68] | Good biocompatibility | Tumor treatment, thermotherapy, and MRI | ||
| Chitosan [69][70][] | Chitosan [69,70 | 71 | ,71] | Good biocompatibility, essential small-molecule vitamin for the human body | Vector, thermotherapy, and radiotherapy |
| White blood cells [72][73] | White blood cells [72,73] | Biomimetic properties, excellent biocompatibility | Vector, nanovaccines, and treatment |
3. Magnetic Drug-Delivery System (MDDS)
Currently, the conventional way of administering drugs to patients is partially inefficient because only part of the drug reaches the site of interest. Therefore, the conventional ways of consuming drugs may be ineffective and even harm the human body caused by their poor selectivity to the diseased area. The benefit of MNPs combined with superconductors is their application in magnetic drug-delivery systems (MDDS), which have effectively transported and delivered drugs with greater precision in the required area. In general terms, the development and research of MDDS began in the 1970s, when there was a need for magnets capable of generating an appropriate magnetic field to systematically guide drug-loaded superparamagnetic NPs to a specific region to treat or give therapy to a disease. Recently, MDDS has become a fundamental method of therapy that uses a superconducting magnet with a magnetic field capable of guiding MNPs to a specific organ or tissue and subsequently administering a drug with a high concentration, allowing the high levels to be eradicated or reduce toxicity within normal tissue, providing promising advances in drug-delivery systems (DDS) [74]. Therefore, the need for drug vectors with superparamagnetic properties at the nanometric level has become essential for carrying out these treatments.
Another essential characteristic of MNPs, in addition to the magnetic properties of the nucleus, is the chemical functionalization of the surface, binding molecules of biological interest on their surface and allowing them to interact with cellular and subcellular structures, as well as with molecules, helping to increase their selectivity with sick tissues [75]. In this sense, MDDS can have various potential applications, highlighting the treatment of cancerous tissues because the drug is concentrated only in the affected tissue, increasing its efficacy and minimizing its side effects. The first clinical tests in treatments against cancer cells using MDDS showed effective results when tested in the affected areas [76].
The MDDS process initially involves introducing the MNPs into the bloodstream and then directed to the affected area through an external magnetic field, for finally concentrate the MNPs near to a magnet placed on the body surface. In this process, the blood flow plays a fundamental role since it distributes the MNPs throughout the body and then concentrates them using a magnetic field, as shown in Figure 2 [77]. Therefore, MNPs must meet a specific size to reach diseased tissue because they must pass through the pulmonary capillaries and avoid phagocytosis. It is recommended that the MNPs have a size of less than 100 nm to achieve this goal, reducing pain in the surrounding tissue when concentrated. However, if the MNPs are too small, they will not be able to show the proper magnetic behavior to be directed adequately with an external magnetic field [78]. Ideally, MNPs should present a high magnetization at body temperature, and once the magnetic field is removed, they should not retain the magnetization, avoiding the formation of aggregates capable of forming emboli and facilitating their elimination from the body.
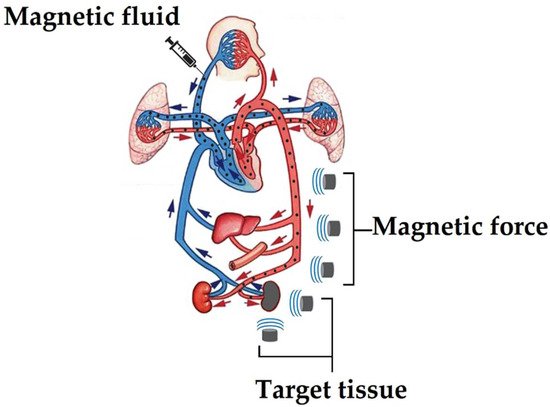
Figure 2.
Pathway for a drug to reach a diseased part using MDDS.
