Myocarditis comprises many clinical presentations ranging from asymptomatic to sudden cardiac death. The history, physical examination, cardiac biomarkers, inflammatory markers, and electrocardiogram are usually helpful in the initial assessment of suspected acute myocarditis. Echocardiography is the primary tool to detect ventricular wall motion abnormalities, pericardial effusion, valvular regurgitation, and impaired function. The advancement of cardiac magnetic resonance (CMR) imaging has been helpful in clinical practice for diagnosing myocarditis. The present review highlights the unmet clinical needs for uniform CMR criteria for diagnosing acute and chronic myocarditis in children and differentiating dilated chronic myocarditis phenotype from idiopathic dilated cardiomyopathy.
- myocarditis
- children
1. Introduction
2. CMR Findings of Myocardial Inflammation and Pathological Correlations
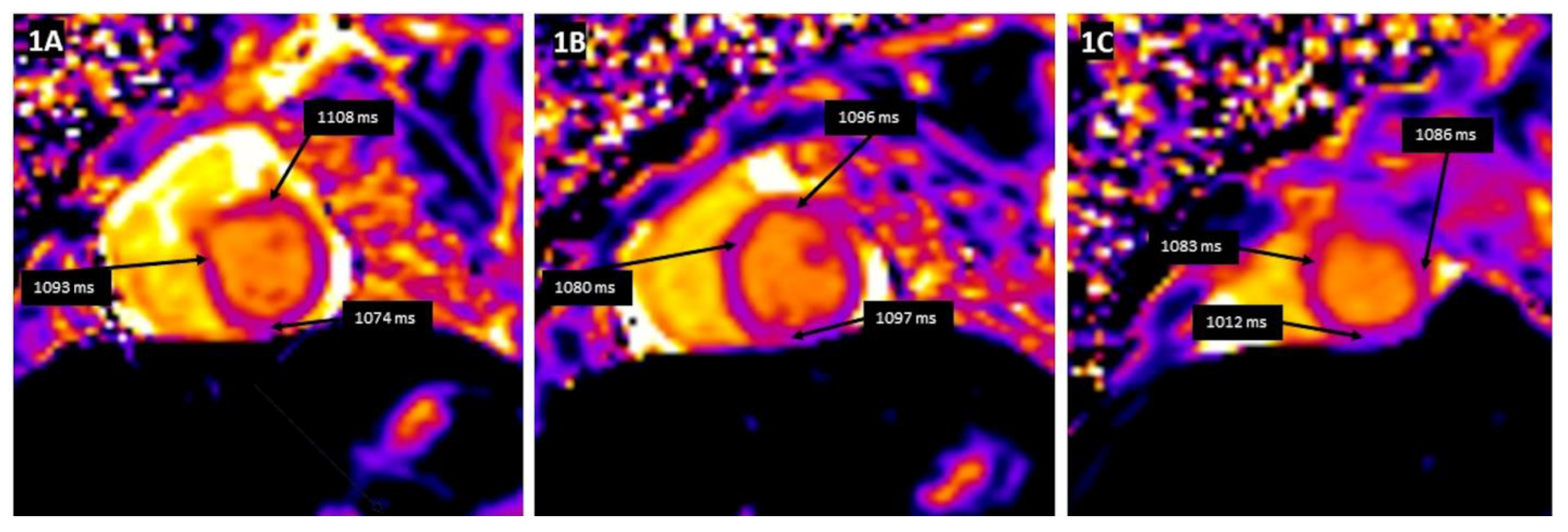
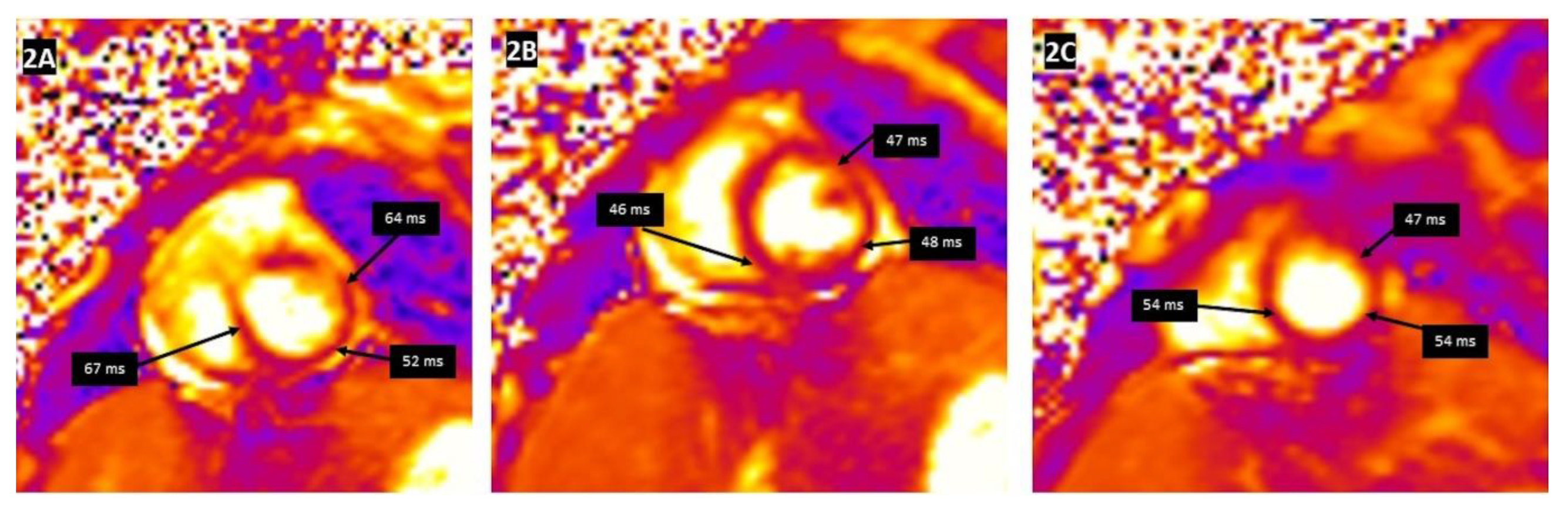
| Reference | n | Age | CMR in Days Following Acute Clinical Myocarditis | Abnormal T1 Plus T2 CMR Findings n (%) | LGE + n (%) |
|---|---|---|---|---|---|
| Gagliardi et al. [33] | 11 | 9 mo– 9 yrs |
24–48 days | Tissue characterization obtained in T1 weighed spin-echo sequences in 100% of pts | |
| Banka et al. [24] | 143 | 16 yrs (mean) |
2 days (mean) |
LLC + ve in 117 (82%), negative in 18 (13%), And equivocal in 7 (5%) yielding a sensitivity of 82% |
115 (81%) |
| Martinez-Villar et al. [7] | 26 | 0–16 yrs (median) 14 yrs |
11–53 days | Total of 2 of 3 LLC in all 26 patients (100%) | 26 (100%) |
| Cornicelli et al. [22] | 23 | 15.1 yrs (mean) |
4.5 days (mean) |
LLC: diagnostic yield 57% Revised LLC increased diagnostic yield to77% |
86% |
| Chinali et al. [25] | 40 | 2–17 yrs (median) 13 yrs |
At admission 10/40 had FU CMR |
Myocardial edema in 33 (82.5%) 6 recovered, 4(40%) had persistent fibrosis |
19 (47.5%) 4(40%) had persistent LGE |
| Dubey et al. [34] | 34 (Follow-up CMR in 12 who had LGE at baseline) |
10–17 yrs (median 16 yrs) |
After discharge | Persistence LGE in 10/12 (83%) | |
| Martin et al. [28] | 125 | Average 15.1 yrs | Average 8 days | Revised LLC in 94 (76%) 79 had FU: 16 pts had disappearance of LLC |
93 (74.4%) 35(28%) had persistent LGE |
| Isaak et al. [21] | 43 (Follow-up CMR in 27/43 pts But only 17 had parametric mapping available) |
8–21 yrs (mean) 17 yrs |
1–9 days of initial diagnosis 53 days from the initial CMR (median) |
Focal edema in 32 (74.4%) Persistent focal edema in 12/27 (44.4%) |
36 (83.7%) LGE persistent in 20/27 (74%) |
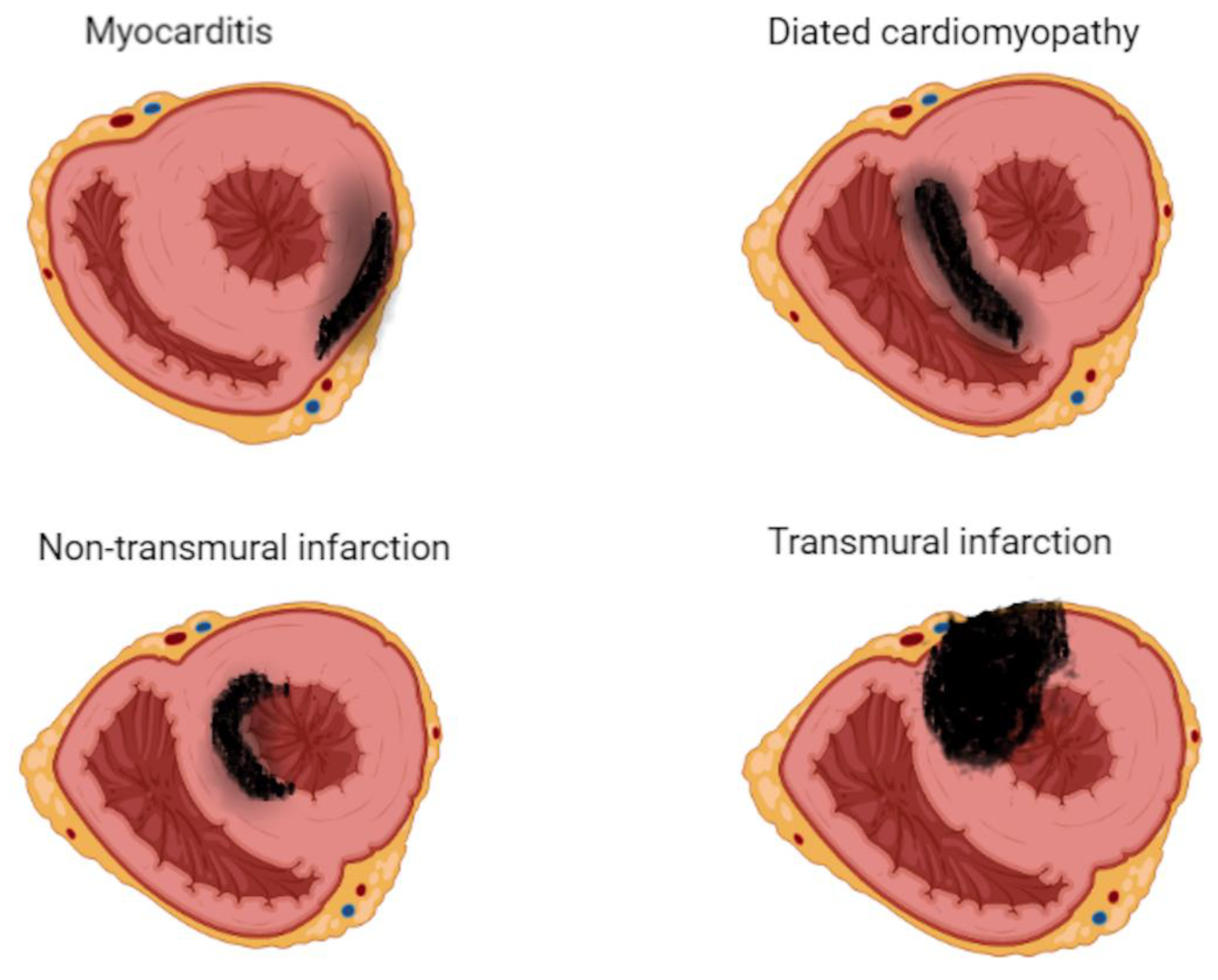
It is important to differentiate between inflammatory cardiomyopathy (subacute/chronic myocarditis with abnormal ventricular function) from idiopathic dilated cardiomyopathy as there is a significant difference in prognosis and outcomes between the two. In chronic myocarditis, the sensitivity of CMR drops significantly as edema becomes progressively less prominent than in acute myocarditis and even disappears entirely in up to 84% of cases. Furthermore, long-term studies are necessary to determine whether CMR can assist in predicting long-term outcomes.
References
- Klugman, D.; Berger, J.T.; Sable, C.A.; He, J.; Khandelwal, S.G.; Slonim, A.D. Pediatric patients hospitalized with myocarditis: A multi-institutional analysis. Pediatric Cardiol. 2010, 31, 222–228.
- Ghelani Sunil, J.; Spaeder Michael, C.; Pastor, W.; Spurney Christopher, F.; Klugman, D. Demographics, Trends, and Outcomes in Pediatric Acute Myocarditis in the United States, 2006 to 2011. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 622–627.
- Rodriguez-Gonzalez, M.; Sanchez-Codez, M.I.; Lubian-Gutierrez, M.; Castellano-Martinez, A. Clinical presentation and early predictors for poor outcomes in pediatric myocarditis: A retrospective study. World J. Clin. Cases 2019, 7, 548–561.
- Canter, C.E.; Simpson, K.E. Diagnosis and treatment of myocarditis in children in the current era. Circulation 2014, 129, 115–128.
- Dasgupta, S.; Iannucci, G.; Mao, C.; Clabby, M.; Oster, M.E. Myocarditis in the pediatric population: A review. Cong Heart Dis. 2019, 14, 868–877.
- Bejiqi, R.; Retkoceri, R.; Maloku, A.; Mustafa, A.; Bejiqi, H.; Bejiqi, R. The diagnostic and clinical approach to pediatric myocarditis: A review of the current literature. Open Access Maced. J. Med. Sci. 2019, 7, 162–173.
- Martinez-Villar, M.; Gran, F.; Sabaté-Rotés, A.; Tello-Montoliu, A.; Castellote, A.; Figueras-Coll, M.; Ferrer, Q.; Roses-Noguer, F. Acute myocarditis with infarct-like presentation in a pediatric population: Role of cardiovascular magnetic resonance. Pediatric Cardiol. 2018, 39, 51–56.
- Abrar, S.; Ansari, M.J.; Mittal, M.; Kushwaha, K.P. Predictors of mortality in pediatric myocarditis. J. Clin. Diagn. Res. 2016, 10, Sc12–Sc16.
- Kociol, R.D.; Cooper, L.T.; Fang, J.C. recognition and initial management of fulminant myocarditis: A scientific statement from the American Heart Association. Circulation 2020, 141, e69–e92.
- Law, Y.M.; Lal, A.K.; Chen, S.; Cihakova, D.; Cooper, L.T.; Deshpande, S.; Godown, J.; Grosse-Wortmann, L.; Robinson, J.D.; Towbin, J.A.; et al. American Heart Association Pediatric Heart Failure and Transplantation Committee of the Council on Lifelong Congenital Heart Disease and Heart Health in the Young and Stroke Council. Diagnosis and management of myocarditis in children: A scientific statement from the American Heart Association. Circulation 2021, 144, e123–e135.
- Ammirati, E.; Frigerio, M.; Adler, E.; Basso, C.; Birnie, D.H.; Brambatti, M.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hubner, N.; et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: An expert consensus document. Circ. Heart Fail. 2020, 13, e007405.
- McNamara, D.M.; Starling, R.C.; Cooper, L.T.; Boehmer, J.P.; Mather, P.J.; Janosko, K.M.; Gorscan, J., 3rd; Kip, K.E.; Dec, G.W. Clinical and demographic predictors of outcomes in recent-onset dilated cardiomyopathy. Results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)-2 study. J. Am. Coll. Cardiol. 2011, 58, 1112–1118.
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–4814.
- Tschope, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193.
- Klein, A.L.; Abbara, S.; Agler, D.A.; Appleton, C.P.; Asher, C.R.; Hoit, B.; Hung, J.; Garcia, M.J.; Kronzon, I.; Oh, J.K.; et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease. J. Am. Soc. Echocardiogr. 2013, 26, 965–1012.
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; Liu, P.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; et al. Cardiovascular magnetic resonance in myocarditis: A JACC white paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487.
- Wei, S.; Fu, J.; Chen, L.; Yu, S. Performance of cardiac magnetic resonance imaging for diagnosis of myocarditis compared with endomyocardial biopsy. Med. Sci. Monit. 2017, 23, 3687–3696.
- Pan, J.A.; Lee, Y.J.; Salerno, M. Diagnostic performance of extracellular volume, native T1, and T2 mapping versus lake Louis criteria by cardiac magnetic resonance for detection of acute myocarditis: A meta-analysis. Circ. Cardiovasc. Imaging 2018, 11, e007598.
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kinderman, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: Expert recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176.
- Luetkens, J.A.; Faron, A.; Isaak, A.; Kuetting, D.; Gliem, C.; Dabir, D.; Kornblum, C.; Thomas, D. Comparison of original and 2018 Lake Louise criteria for diagnosis of acute myocarditis: Results of a validation cohort. Radiol. Cardiothorac. Imaging 2019, 1, e190010.
- Isaak, A.; Bischoff, L.; Faron, A.; Endler, C.; Mesropyan, N.; Sprinkart, A.; Peiper, C.C.; Kuetting, D.; Dabir, D.; Attenberger, U.; et al. Multiparametric cardiac magnetic resonance imaging in pediatric and adolescent patients with acute myocarditis. Pediatric Radiol. 2021, 51, 2470–2480.
- Cornicelli, M.D.; Rigsby, C.K.; Rychlik, K.; Pahl, E.; Robinson, J.D. Diagnostic performance of cardiovascular magnetic resonance native T1 and T2 mapping in pediatric patients with acute myocarditis. J. Cardiovasc. Magn. Reson. 2019, 21, 40.
- Hales-Kharazmi, A.; Hirsch, N.; Slesnik, T.; Deshpande, S.R. Utility of cardiac MRI in pediatric myocarditis. Cardiol. Young 2018, 3, 377–385.
- Banka, P.; Robinson, J.D.; Uppu, S.C.; Harris, M.A.; Hasbani, K.; Lai, W.W.; Richmond, M.; Fratzz, S.; Jain, S.; Johnson, T.R.; et al. Cardiovascular magnetic resonance techniques and findings in children with myocarditis: A multicenter retrospective study. J. Cardiovasc. Magn. Reson. 2015, 17, 96.
- Chinali, M.; Franceschini, A.; Ciancarella, P.; Lisignoli, V.; Curione, D.; Ciliberti, P.; Esposito, C.; Pasqua, A.D.; Rinelli, G.; Secinaro, A. Echocardiographic two-dimensional speckle tracking identifies acute regional myocardial edema and sub-acute fibrosis in pediatric focal myocarditis with normal ejection fraction: Comparison with cardiac magnetic resonance. Sci. Rep. 2020, 10, 11321.
- Francone, M.; Chimenti, C.; Galea, N.; Scopelliti, F.; Verardo, R.; Galea, R.; Carbone, I.; Catalano, C.; Fedele, F.; Frustaci, A. CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsy-proven acute myocarditis. JACC Cardiovasc. Imaging 2014, 7, 254–263.
- Yuan, W.-F.; Zhao, X.-X.; Sun, W.-J.; Shao-Ping, W.; Ya-Bin, L.; Tang, X. LGE-MRI in the assessment of left ventricular remodeling in myocarditis. Curr. Med. Imaging 2019, 15, 900–905.
- Martins, D.S.; Ait-Ali, L.; Khraiche, D.; Festa, P.; Barison, A.; Martini, N.; Benadjaoud, Y.; Anjos, R.; Boddaert, N.; Bonnet, D.; et al. Evolution of acute myocarditis in a pediatric population: An MRI based study. Int. J. Cardiol. 2021, 329, 226–233.
- Grigoratus, C.; Bella, G.D.; Aquaro, G.D. Diagnostic and prognostic role of cardiac magnetic resonance in acute myocarditis. Heart Fail. Rev. 2019, 24, 81–90.
- Dusenbery, S.M.; Newburger, J.W.; Colan, S.D.; Gauvreau, K.; Baker, A.; Powell, A.J. Myocardial fibrosis in patients with a history of Kawasaki disease. IJC Heart Vasc. 2021, 32, 100713.
- Secinaro, A.; Ntsinjana, H.; Tann, O.; Schuler, P.K.; Muthurangu, V.; Hughes, M.; Tsang, V.; Taylor, A.M. Cardiovascular magnetic resonance findings in repaired anomalous left coronary artery to pulmonary artery connection (ALCAPA). J. Cardiovasc. Magn. Reson. 2011, 1391, 27.
- Mahrholdt, H.; Wagner, A.; Deluigi, C.C.; Kispert, E.; Hager, S.; Meinhardt, G.; Vogelseberg, H.; Fritz, P.; Dippon, J.; C-Thomas, B.; et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 2006, 114, 1581–1590.
- Gagliardi, M.G.; Bevilacqua, M.; Di Renzi, P.; Picardo, S.; Passariello, R.; Marcelletti, C. Usefulness of magnetic resonance imaging for diagnosis of acute myocarditis in infants and children, and comparison with endomyocardial biopsy. Am. J. Cardiol. 1991, 68, 1089–1091.
- Dubey, S.; Agarwal, A.; Nguyen, S.; Adebo, D. Persistence of late gadolinium enhancement on follow-up CMR imaging in children with acute myocarditis. Pediatric Cardiol. 2020, 41, 1777–1782.
- Saeed, M.; Hetts, S.W.; Jablonowski, R.; Wilson, M.W. Magnetic resonance imaging and multi-detector computed tomography assessment of extracellular compartment in ischemic and nonischemic myocardial pathologies. World J. Cardiol. 2014, 6, 1192–1218.
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75.
