Iron deficiency is a significant comorbidity of heart failure (HF), defined as the inability of the myocardium to provide sufficient blood flow. However, iron deficiency remains insufficiently detected. Iron-deficiency anemia, defined as a decrease in hemoglobin caused by iron deficiency, is a late consequence of iron deficiency, and the symptoms of iron deficiency, which are not specific, are often confused with those of HF or comorbidities. HF patients with iron deficiency are often rehospitalized and present reduced survival. The correction of iron deficiency in HF patients is associated with improved functional capacity, quality of life, and rehospitalization rates. Because of the inflammation associated with chronic HF, which complicates the picture of nutritional deficiency, only the parenteral route can bypass the tissue sequestration of iron and the inhibition of intestinal iron absorption. Given the negative impact of iron deficiency on HF progression, the frequency and financial implications of rehospitalizations due to decompensation episodes, and the efficacy of this supplementation, screening for this frequent comorbidity should be part of routine testing in all HF patients.
- iron deficiency
- heart failure
- transferrin saturation coefficient
- serum ferritin
- ferric carboxymaltose
1. Introduction
2. Iron Deficiency, the Most Common Comorbidity of Heart Failure
HF is frequently associated with comorbidities, the most common being chronic kidney disease (40%) [3], diabetes (30–40%) [4], and chronic obstructive pulmonary disease (20.5%) [5]. These comorbidities have a significant impact on hospitalizations and mortality. Iron deficiency is often associated with chronic HF [6][7], with or without anemia, as the most common nutritional deficiency. Indeed, iron deficiency is most often discovered in anemia [8]. Iron-deficiency frequencies from 37% to 61% were reported in different studies [9][10][11]. In the study by Klip et al. on European cohorts of 1500 HF patients, iron deficiency was diagnosed in 61.2% of patients with anemia and 45.6% without anemia [12]. The CARENFER study recently conducted in France on 1661 HF hospitalized patients reported that 49.6% had iron deficiency [13]. Even in the absence of anemia, iron deficiency is a poor prognostic factor in HF [14]. Iron deficiency increases the relative risk of death by 40–60%. For example, a prospective study on nearly 550 patients with NYHA class II–III chronic HF (mean LVEF of 26%) reported that the adjusted relative risk of the composite endpoint of all-cause death or heart transplantation was increased by 58% hen iron deficiency was present. In contrast, anemia was not an independent risk factor [15]. Another study in the United Kingdom included 150 patients with HF [16]. Compared with patients without anemia and iron deficiency, the relative risk of death was not significantly increased in anemic patients without iron deficiency. In contrast, it was twice as high in nonanemic patients with iron deficiency [17]. In the European-cohort analysis by Klip et al., iron deficiency was an independent mortality risk factor (relative risk increased by 42% in multivariate analyses), along with the classic risk factors (sex, age, NYHA class, diabetes, hypertension, etc.) [12]. Anemia was not an independent risk factor.3. Iron Is an Essential Element for the Correct Functioning of the Heart Muscle
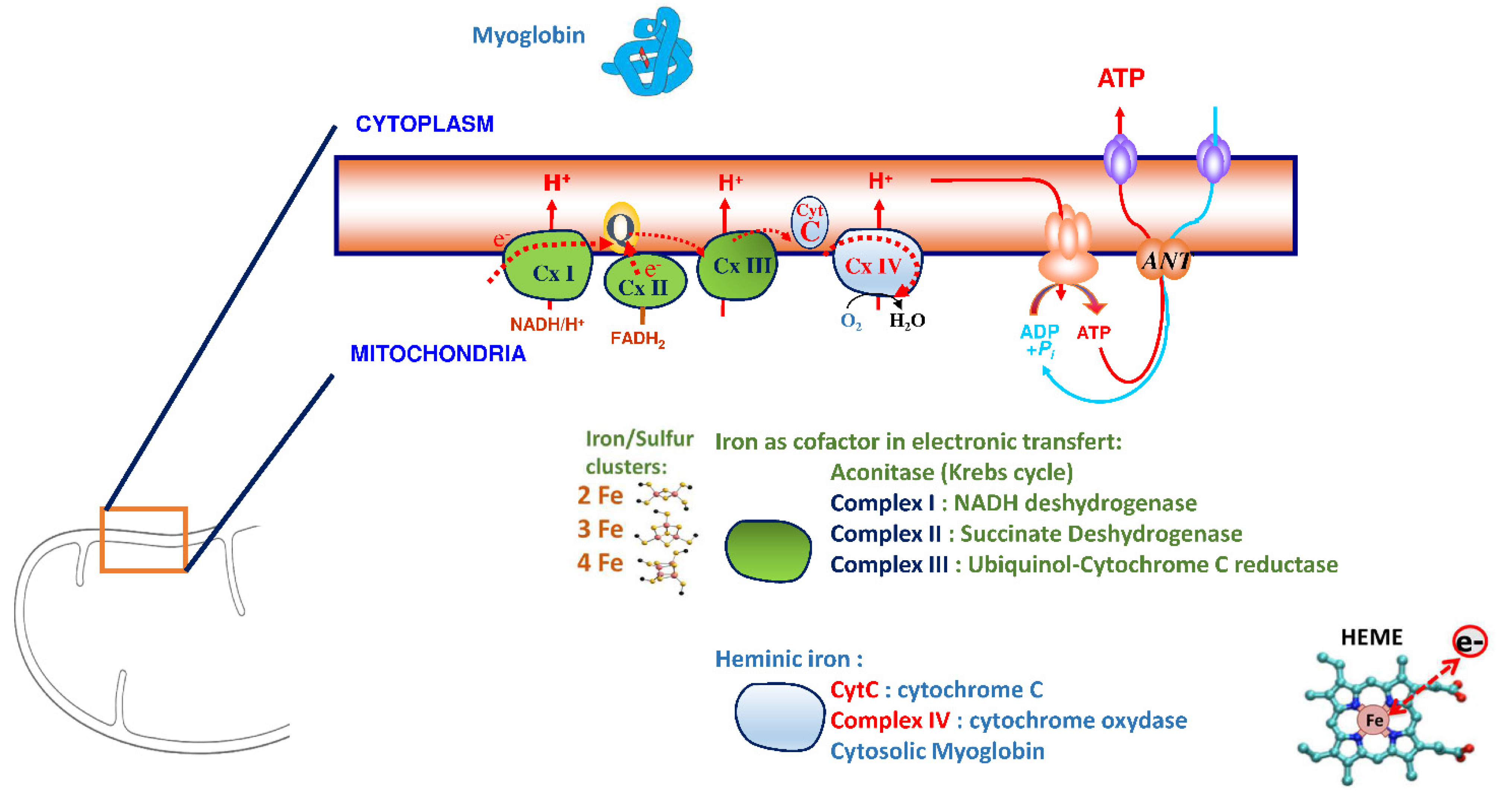
Iron plays a significant role in erythropoiesis and oxygen transport. However, it also participates in DNA replication and repair, cell growth and differentiation, brain function, dioxygen storage in myoglobin, and energy metabolism of striated muscles and heart (ATP synthesis) [18][19] (Figure 1). Cardiomyocytes are characterized by a high myoglobin concentration in the cytosol and contain numerous mitochondria, which produce the energy necessary for cardiac-muscle contractions. Iron plays a significant role in mitochondrial functions as a cofactor in iron–sulfur-cluster-containing proteins, heme-containing proteins, and iron-ion-containing proteins (Figure 2). Approximately 90% of the ATP required for the proper functioning of the heart muscle (i.e., for contraction) is produced by the mitochondrial enzymatic complexes of the respiratory chain [20]. Cellular iron deficiency was shown to result in reduced activity of Fe-S-cluster-based complexes in the mitochondria of human cardiomyocytes and to be associated with impaired mitochondrial respiration and morphology, ATP production, and contractility (Figure 1) [21][22]. Interestingly, restoring intracellular iron concentrations can reverse these effects on muscle [22][23].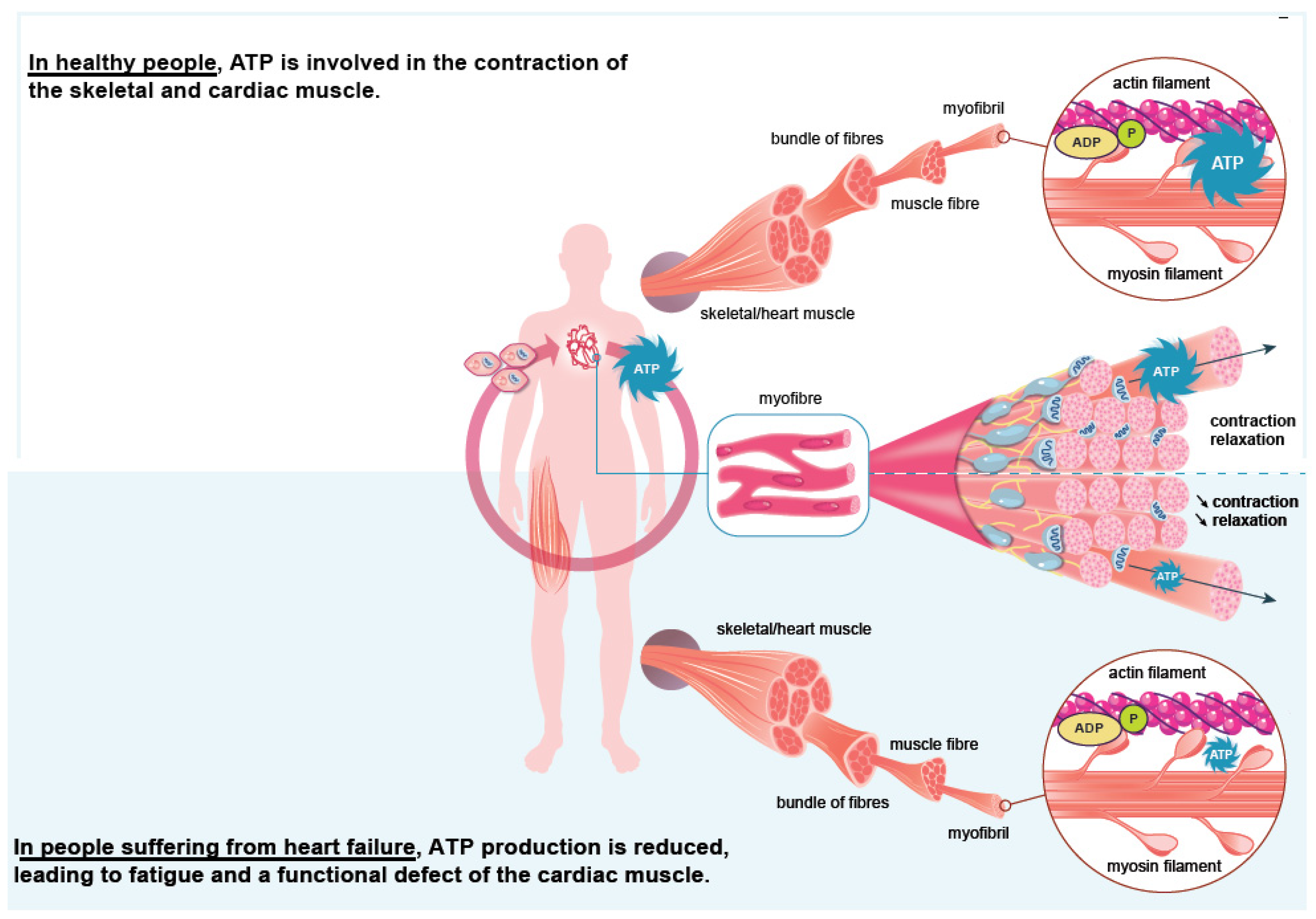
4. Iron Supplementation in Heart Failure
In case of iron deficiency, iron can be provided either via the oral or parenteral route. Although cheap and readily available, the oral route is frequently (up to 70%) associated with poor gastrointestinal tolerance and various levels of efficacy and compliance [27][28]. The IV route of new-generation iron preparations [29] might be associated with limited gastrointestinal side effects, injection-site reactions, and infrequent anaphylactic reactions; the safety is considered quite satisfactory [30]. Published in 2017, the double-blind IRONOUT-HF study on 225 patients with chronic stable HF (NYHA classes II–IV) and iron deficiency showed that oral iron administration (150 mg × 2/day) for 16 weeks was comparable to placebo in terms of functional capacity as assessed via peak oxygen consumption and walking [31]. In contrast, the FAIR-HF, CONFIRM-HF, FERRIC-HF, and EFFECT-HF studies demonstrated the efficacy of IV iron on HF symptoms [32][33][34][35]. This difference in the effectiveness of the oral and IV routes is explained by a high concentration of hepcidin in chronic HF [31]. In the AFFIRM-HF study, the effect of IV iron supplementation on mortality was evaluated in chronic HF patients (LVEF < 50%) with iron deficiency (according to the 2016 ESC criteria) hospitalized for an acute episode of HF [33]. At discharge, stabilized patients were randomized to IV iron carboxymaltose or placebo. After a 52-week follow-up (1108 evaluable patients), the relative risk of hospitalization for a new decompensation was reduced by 26%, without significant effects on cardiovascular mortality. Jankowska et al.’s [16] meta-analysis included five randomized studies totaling 851 patients with systolic HF and iron deficiency. Patients were treated with IV iron or a comparator (oral or IV placebo, oral iron). In all patients (with or without anemia), the relative risk of composite endpoint “all-cause death or hospitalization for cardiovascular reasons” was reduced by 56%, and the relative risk of composite endpoint “cardiovascular death or hospitalization for progression of chronic heart disease” was reduced by 61%. Another meta-analysis by Anker et al. [36] included four randomized [33][36][37][38], double-blind studies (FER-CARS-01, FAIR-HF, EFFICACY-HF, and CONFIRM-HF) with a total of 839 patients with chronic systolic HF and iron deficiency to compare IV iron carboxymaltose and placebo. The relative risk of cardiovascular hospitalization or death was reduced by 41% in the iron-supplemented group. IV iron supplementation, therefore, has a demonstrated clinical benefit in iron-deficient chronic HF patients, anemic or not. Iron deficiency should be considered an independent therapeutic goal in this population [9]. According to 2017 US recommendations, IV iron supplementation for iron deficiency has to be considered in NYHA II–III patients [39]. The iron status should be reassessed during routine visits (once or twice a year) and after each hospitalization for HF. The recently updated European Society of Cardiology guidelines (2021) indicate that IV iron carboxymaltose therapy should be considered in patients with symptomatic chronic HF with LVEF ≤ 45% and iron deficiency defined as serum ferritin < 100 µg/L or serum ferritin at 100–299 µg/L with TSAT < 20% to alleviate symptoms, and improve function and quality of life [40]. Iron supplementation with IV iron carboxymaltose should also be considered in patients recently hospitalized for HF with LVEF < 50% and iron deficiency according to the exact definition to reduce the risk of HF-related rehospitalization.References
- Gibelin, P. Insuffisance Cardiaque: Aspects Épidémiologiques, Cliniques et Pronostiques. EMC Cardiol. 2018, 13, 11-024-A-10.
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.G.F.; Kozhuharov, N.; Coats, A.J.S.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology Practical Guidance on the Use of Natriuretic Peptide Concentrations. Eur. J. Heart Fail. 2019, 21, 715–731.
- Shiba, N.; Shimokawa, H. Chronic Kidney Disease and Heart Failure--Bidirectional Close Link and Common Therapeutic Goal. J. Cardiol. 2011, 57, 8–17.
- Komajda, M. Diabète et Insuffisance Cardiaque: Données Épidémiologiques et Implications Thérapeutiques. Bull Acad. Natl. Med. 2018, 202, 909–916.
- Rutten, F.H.; Cramer, M.J.; Grobbee, D.E.; Sachs, A.P.; Kirkels, J.H.; Lammers, J.W.; Hoes, A.W. Unrecognized Heart Failure in Elderly Patients with Stable Chronic Obstructive Pulmonary Disease. Eur. Heart J. 2005, 26, 1887–1894.
- Anand, I.S.; Gupta, P. Anemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation 2018, 138, 80–98.
- Chopra, V.K.; Anker, S.D. Anaemia, Iron Deficiency and Heart Failure in 2020: Facts and Numbers. ESC Heart Fail. 2020, 7, 2007–2011.
- Haute Autorité de Santé. Guide Du Parcours de Soins. Insuffisance Cardiaque. Available online: https://www.has-sante.fr/jcms/c_1242988/fr/guide-parcours-de-soins-insuffisance-cardiaque (accessed on 24 July 2014).
- Cappellini, M.D.; Comin-Colet, J.; de Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R.; et al. Iron Deficiency across Chronic Inflammatory Conditions: International Expert Opinion on Definition, Diagnosis, and Management. Am. J. Hematol. 2017, 92, 1068–1078.
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron Deficiency Anaemia. Lancet 2016, 387, 907–916.
- Peyrin-Biroulet, L.; Williet, N.; Cacoub, P. Guidelines on the Diagnosis and Treatment of Iron Deficiency across Indications: A Systematic Review. Am. J. Clin. Nutr. 2015, 102, 1585–1594.
- Klip, I.T.; Comin-Colet, J.; Voors, A.A.; Ponikowski, P.; Enjuanes, C.; Banasiak, W.; Lok, D.J.; Rosentryt, P.; Torrens, A.; Polonski, L.; et al. Iron Deficiency in Chronic Heart Failure: An International Pooled Analysis. Am. Heart J. 2013, 165, 575–582.e3.
- Cohen, L.A.; Gutierrez, L.; Weiss, A.; Leichtmann-Bardoogo, Y.; Zhang, D.; Crooks, D.R.; Sougrat, R.; Morgenstern, A.; Galy, B.; Hentze, M.W.; et al. Serum Ferritin Is Derived Primarily from Macrophages through a Nonclassical Secretory Pathway. Blood 2010, 116, 1574–1584.
- Nikolaou, M.; Chrysohoou, C.; Georgilas, T.A.; Giamouzis, G.; Giannakoulas, G.; Karavidas, A.; Papadopoulos, C.; Patsilinakos, S.; Tziakas, D.; Parissis, J. Management of Iron Deficiency in Chronic Heart Failure: Practical Considerations for Clinical Use and Future Directions. Eur. J. Intern. Med. 2019, 65, 17–25.
- Martens, P.; Dupont, M.; Dauw, J.; Nijst, P.; Herbots, L.; Dendale, P.; Vandervoort, P.; Bruckers, L.; Tang, W.H.W.; Mullens, W. The Effect of Intravenous Ferric Carboxymaltose on Cardiac Reverse Remodelling Following Cardiac Resynchronization Therapy-the IRON-CRT Trial. Eur. Heart J. 2021, 42, 4905–4914.
- Jankowska, E.A.; Rozentryt, P.; Witkowska, A.; Nowak, J.; Hartmann, O.; Ponikowska, B.; Borodulin-Nadzieja, L.; Banasiak, W.; Polonski, L.; Filippatos, G.; et al. Iron Deficiency: An Ominous Sign in Patients with Systolic Chronic Heart Failure. Eur. Heart J. 2010, 31, 1872–1880.
- Okonko, D.O.; Mandal, A.K.; Missouris, C.G.; Poole-Wilson, P.A. Disordered Iron Homeostasis in Chronic Heart Failure: Prevalence, Predictors, and Relation to Anemia, Exercise Capacity, and Survival. J. Am. Coll Cardiol. 2011, 58, 1241–1251.
- Cairo, G.; Bernuzzi, F.; Recalcati, S. A Precious Metal: Iron, an Essential Nutrient for All Cells. Genes Nutr. 2006, 1, 25–39.
- Loncar, G.; Obradovic, D.; Thiele, H.; Haehling, S.; Lainscak, M. Iron Deficiency in Heart Failure. ESC Heart Fail. 2021, 8, 2368–2379.
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Expert Consensus Document: Mitochondrial Function as a Therapeutic Target in Heart Failure. Nat. Rev. Cardiol. 2017, 14, 238–250.
- Xu, W.; Barrientos, T.; Mao, L.; Rockman, H.A.; Sauve, A.A.; Andrews, N.C. Lethal Cardiomyopathy in Mice Lacking Transferrin Receptor in the Heart. Cell Rep. 2015, 13, 533–545.
- Hoes, M.F.; Grote Beverborg, N.; Kijlstra, J.D.; Kuipers, J.; Swinkels, D.W.; Giepmans, B.N.G.; Rodenburg, R.J.; van Veldhuisen, D.J.; de Boer, R.A.; van der Meer, P. Iron Deficiency Impairs Contractility of Human Cardiomyocytes through Decreased Mitochondrial Function. Eur. J. Heart Fail. 2018, 20, 910–919.
- Rineau, E.; Gaillard, T.; Gueguen, N.; Procaccio, V.; Henrion, D.; Prunier, F.; Lasocki, S. Iron Deficiency without Anemia Is Responsible for Decreased Left Ventricular Function and Reduced Mitochondrial Complex I Activity in a Mouse Model. Int. J. Cardiol. 2018, 266, 206–212.
- Lacour, P.; Dang, P.L.; Morris, D.A.; Parwani, A.S.; Doehner, W.; Schuessler, F.; Hohendanner, F.; Heinzel, F.R.; Stroux, A.; Tschoepe, C.; et al. The Effect of Iron Deficiency on Cardiac Resynchronization Therapy: Results from the RIDE-CRT Study. ESC Heart Fail. 2020, 7, 1072–1084.
- Martens, P.; Nijst, P.; Verbrugge, F.H.; Smeets, K.; Dupont, M.; Mullens, W. Impact of Iron Deficiency on Exercise Capacity and Outcome in Heart Failure with Reduced, Mid-Range and Preserved Ejection Fraction. Acta Cardiol. 2018, 73, 115–123.
- van Veldhuisen, D.J.; Anker, S.D.; Ponikowski, P.; Macdougall, I.C. Anemia and Iron Deficiency in Heart Failure: Mechanisms and Therapeutic Approaches. Nat. Rev. Cardiol. 2011, 8, 485–493.
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.A.; Powell, J.J. Ferrous Sulfate Supplementation Causes Significant Gastrointestinal Side-Effects in Adults: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0117383.
- DeLoughery, T.G. Safety of Oral and Intravenous Iron. Acta Haematol. 2019, 142, 8–12.
- Auerbach, M.; Rodgers, G.M. Intravenous Iron. N. Engl. J. Med. 2007, 357, 93–94.
- Avni, T.; Bieber, A.; Grossman, A.; Green, H.; Leibovici, L.; Gafter-Gvili, A. The Safety of Intravenous Iron Preparations. Mayo Clin. Proc. 2015, 90, 12–23.
- Lewis, G.D.; Malhotra, R.; Hernandez, A.F.; McNulty, S.E.; Smith, A.; Felker, G.M.; Tang, W.H.W.; LaRue, S.J.; Redfield, M.M.; Semigran, M.J.; et al. Effect of Oral Iron Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF Randomized Clinical Trial. JAMA 2017, 317, 1958–1966.
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Luscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency. N. Engl. J. Med. 2009, 361, 2436–2448.
- Ponikowski, P.; van Veldhuisen, D.J.; Comin-Colet, J.; Ertl, G.; Komajda, M.; Mareev, V.; McDonagh, T.; Parkhomenko, A.; Tavazzi, L.; Levesque, V.; et al. Beneficial Effects of Long-Term Intravenous Iron Therapy with Ferric Carboxymaltose in Patients with Symptomatic Heart Failure and Iron Deficiency. Eur. Heart J. 2015, 36, 657–668.
- Okonko, D.O.; Grzeslo, A.; Witkowski, T.; Mandal, A.K.; Slater, R.M.; Roughton, M.; Foldes, G.; Thum, T.; Majda, J.; Banasiak, W.; et al. Effect of Intravenous Iron Sucrose on Exercise Tolerance in Anemic and Nonanemic Patients with Symptomatic Chronic Heart Failure and Iron Deficiency FERRIC-HF: A Randomized, Controlled, Observer-Blinded Trial. J. Am. Coll Cardiol. 2008, 51, 103–112.
- van Veldhuisen, D.J.; Ponikowski, P.; van der Meer, P.; Metra, M.; Bohm, M.; Doletsky, A.; Voors, A.A.; Macdougall, I.C.; Anker, S.D.; Roubert, B.; et al. Effect of Ferric Carboxymaltose on Exercise Capacity in Patients With Chronic Heart Failure and Iron Deficiency. Circulation 2017, 136, 1374–1383.
- Anker, S.D.; Kirwan, B.A.; van Veldhuisen, D.J.; Filippatos, G.; Comin-Colet, J.; Ruschitzka, F.; Luscher, T.F.; Arutyunov, G.P.; Motro, M.; Mori, C.; et al. Effects of Ferric Carboxymaltose on Hospitalisations and Mortality Rates in Iron-Deficient Heart Failure Patients: An Individual Patient Data Meta-Analysis. Eur. J. Heart Fail. 2018, 20, 125–133.
- Anker, S.D.; Colet, J.C.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Luscher, T.F.; Mori, C.; von Eisenhart Rothe, B.; Pocock, S.; et al. Rationale and Design of Ferinject Assessment in Patients with IRon Deficiency and Chronic Heart Failure (FAIR-HF) Study: A Randomized, Placebo-Controlled Study of Intravenous Iron Supplementation in Patients with and without Anaemia. Eur. J. Heart Fail. 2009, 11, 1084–1091.
- Arutyunov, G.; Bylova, N.; Ivleva, A.; Kobalava, Z. The Safety of Intravenous (IV) Ferric Carboxymaltose versus IV Iron Sucrose on Patients with Chronic Heart Failure (CHF) and Chronic Kidney Disease (CKD) with Iron Deficincy (ID). Eur. J. Heart Fail. 2009, 8, ii71.
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Card. Fail. 2017, 23, 628–651.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726.
