You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 1 by Omojola Awogbemi.
The need to arrest the continued environmental contamination and degradation associated with the consumption of fossil-based fuels has continued to serve as an impetus for the increased utilization of renewable fuels. The demand for biodiesel has continued to escalate in the past few decades due to urbanization, industrialization, and stringent government policies in favor of renewable fuels for diverse applications. One of the strategies for ensuring the intensification, commercialization, and increased utilization of biodiesel is the adaptation of reactor technologies, especially tubular reactors.
- tubular reactor
- transesterification
- reactor technologies
1. Modes of Biodiesel Production in Reactor Technologies
Transesterification is the most widely used method for biodiesel production. Basically, there are four steps involved in biodiesel production via transesterification. The first step is the collection of the feedstock, reagents, and other materials needed for the process. In this stage, the production reaction parameters and conditions are also determined and implemented in the reacting vessel. When the process in the reacting vessel is completed, the second step, which involves separating the slurry comprising the crude biodiesel, glycerol, catalyst, excess methanol, and other water is activated. This involves the use of the difference in densities to achieve phase separation among the resultant slurry. During this process, one of the major and predominantly low-cost gravity separation techniques including filtration, centrifugation, floatation, decantation, or sedimentation is deployed [48,49][1][2]. The heterogeneous catalyst is recovered in this stage for reuse.
In the third step, crude biodiesel is subjected to gentle heating with stirring to remove unreacted alcohol and excess moisture trapped in the biodiesel. In the fourth and final step, the biodiesel is purified to further remove any undesirable compounds such as the catalyst, soap, unconverted triglyceride, and moisture. The purification can involve the use of wet or dry washing methods, membrane filtration, and evaporation to obtain clean biodiesel. The biodiesel produced at this stage must meet the ASTM D6751 and EN 14214 standards. The wet washing purification process, though most frequently used, extends production duration, requires a large volume of clean water, and generates lots of wastewater. The treatment and disposal of wastewater and the drying of the water-washed biodiesel are energy-intensive and expensive. Drying washing is more ecofriendly, does not require water, and produces fuel of better quality when compared with wet washing. However, the cost of adsorbents and other additional apparatus makes the process uneconomical. The membrane separation technique, though still largely undeveloped and not commonly used, is environmentally benign, consumes less energy, requires no chemicals, and generates high quality products [50,51,52][3][4][5]. The biodiesel generation processes can be intensified by the use of reactor technologies. The deployment of reactor technologies contributes significantly to ensuring the mass production of biodiesel.
A reactor is a device or vessel with compartments where chemical reactions take place for the transformation of raw materials into desired products under specific and predetermined conditions. A reactor can also be an enclosed volume, an apparatus, or a specialized container where specific chemical reactions take place under a controlled atmosphere. A good reactor must contain mechanisms or facilities for the injection of the raw materials and other reagents, provide enough residence time for the chemical reaction to take place, and discharge the products. There must be facilities for heat addition and heat removal, safe operation and maintenance, and effective control to ensure operational safety, effectiveness, and an acceptable level of productivity. To achieve an optimum reactor operation, effective performance, and high product yield, the design stage must consider the configuration, construction materials, cost, reaction kinetics, heat and mass transfer, reaction parameters, and the environmental sustainability of the reactor [53,54][6][7]. A reactor can be operated either as a batch or a continuous process. In recent years, some researchers have reported an amalgamation of the batch and continuous process, which they dubbed the semi-batch/semi-continuous process to overcome some technical and operational associated with both batch and continuous production of biodiesel by transesterification.
1.1. Batch-Mode Reactors
The batch-mode reactors are the oldest, most convenient, and most popular method of biodiesel synthesis. The batch-mode reactor of biodiesel production was developed from a laboratory-scale production process by the optimization of the production parameters [16,55][8][9]. It involved the upgrading of the laboratory-scale production into commercial and industrial production scale to meet the increasing demand for biodiesel. The main feature of a batch production method is the intermittency of the process. There is no continuous flow of materials into and out of the reactor during the production period. Rather, a known quantity of raw materials is injected into the reaction and allowed to be converted into the desired product in a specified period. At the end of the process, the resultant slurry is allowed to exit the reactor and transmitted for separation, purification, and further processing [56][10]. When operating under the batch production mode, there is control of the inflow, adequate mixing of the reactants, and monitoring of the outflow of the materials. Despite the simplicity in the design and operation of batch reactors, the major drawbacks of the process include a longer residence time, a high operation cost, higher energy consumption, and large space requirements [57][11].
1.2. Semi Batch-Mode Reactors
In the semi-batch/semi continuous mode reactor, there is the intermittent addition or removal of one more reagent or product during the process. There can also be a variation of the reaction parameters as the reaction proceeds. For example, more feedstock or methanol can be introduced into the reactor during the process to improve the reaction rate or product yield. In this way, the reaction equilibrium is altered in support of biodiesel formation by the gradual removal of the product during the process. Similar to the batch process, the semi-batch mode is characterized by a high operation cost, low production rate, and high energy consumption. There is a high rate of human intervention during the process leading to a highly strenuous and labor-intensive process [60,61,62][12][13][14].
1.3. Continuous-Mode Reactors
The continuous mode reactor allows for the continuous inflow of reactants into the reactor and the simultaneous outflow of the products from the system throughout the operation period. After the initial loading of the reactor with feedstock, catalyst, and methanol, the process is initiated and agitated at the required speed to ensure a homogeneous mixing of the reactants, adequate mass, and heat transfer. At the expiration of the set residence time, the reactants are converted into products and allowed to flow out of the reactor. The process continues almost seamlessly with little or no human intervention [54,64][7][15]. The process is inbuilt with mechanisms set up to control the inflow of feedstock, catalyst, and methanol, monitoring agitation speed, residence time, and discharge of the resultant slurry.
It must be noted that the biodiesel production industry is moving towards a continuous mode of production and the use of automation and other innovative technologies to ensure large scale and industrial biodiesel production processes. When compared with the batch production process, the continuous production of biodiesel is achieved at lower operating costs, a reduced energy consumption, and with a less labor-intensive process [65][16]. The deployment of a continuous flow reactor for biodiesel synthesis increases the mass interfacial transport between methanol and oil leading to the synthesis of quality products at the lowest cost per unit volume of fuel [66][17].
2. Tubular Reactor Technologies for Biodiesel Production
Reactor technologies for the conversion of feedstocks into biodiesel by transesterification are classified by various factors. Some of these factors include the mode of operation, operating conditions, phase numbers, mixing systems, nature of reactants and products, operating temperature and pressure, production size, residence time, mass transfer, heat exchange, and control system.
The tubular or plug-flow reactors are the simplest form of reactor technology for biodiesel production. In this type of reactor technology, reactants and reagents are fed into the reactor through the inlet and are allowed to spend some time in the reactor before being allowed to flow out from the outlet at a constant velocity. The mixing of the reactants and reagents takes place in the tubes or pipe fittings. At a constant velocity, the longer the length of the pipes, the longer the mixing time and the longer the residence time. However, the length of the mixing device and the residence time can be adjusted by altering the system pressure. Moreover, an increase in the viscosity of the mixture of the reactants and reagents will lead to laminar flow in the tubular reactors. The improvement in the reaction, length of the mixing device, and reactor size can be achieved by deploying various mixers such as in-line mechanical mixers, static mixers, and other injection devices. Moreover, the application of static mixers ensures effective radial mixing of multiple immiscible flowing liquids. Figure 81 shows the different configurations of static mixers. The suitability of a typical static mixer is determined by the type of reaction, reaction temperature, reactor configuration, Reynolds number, and viscosity of the fluids. These configurations facilitate the efficient transesterification of the different oils used as feedstock.
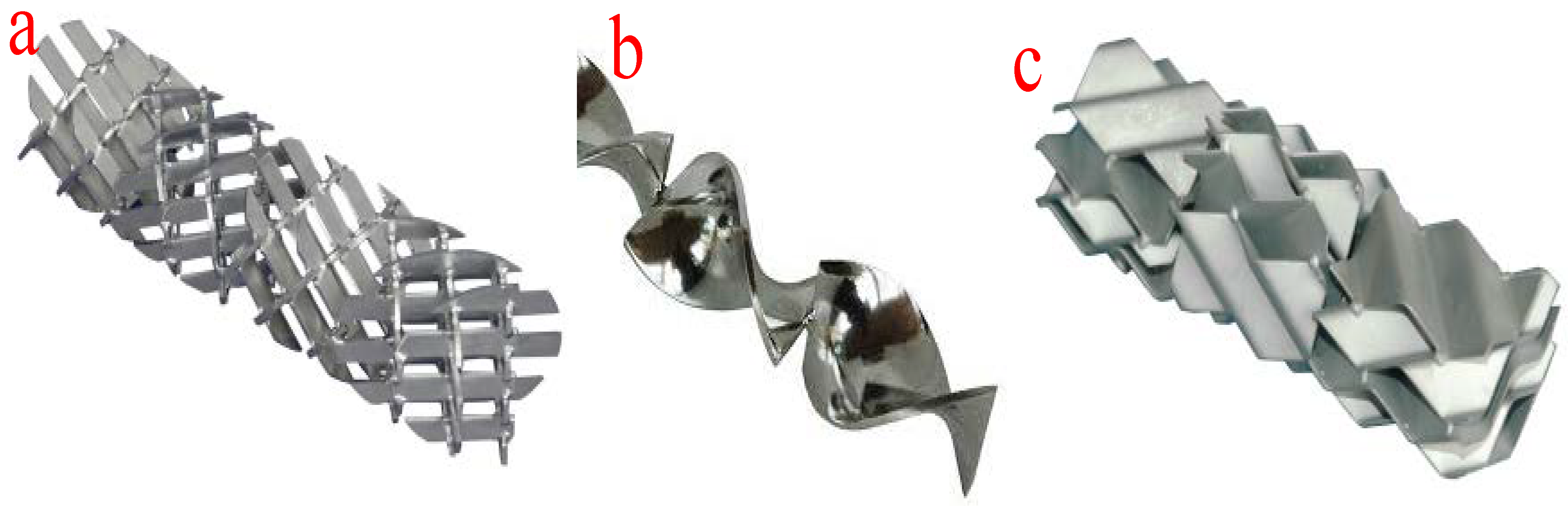
Figure 81.
Different configurations of static mixers. (
a
) X-grid static mixer; (
b
) helical twist static mixer; (
c
) corrugated plate static mixer.
When compared with other reactor technologies, tubular reactors are more efficient, require minimum maintenance, and ensure the fast and homogeneous mixing of the fluids. They are not capital intensive and require less space for the construction. Reactors operating on the tubular technology can be used at high pressure and under steady-state conditions. The reactor technology allows continuous operation over a long period and easy product separation. This ensures adequate product separation and recovery of excess methanol and unreacted oils for recycling. Moreover, there is a short residence time when using tubular reactors due to the reduced length of the reactor [68,69][18][19]. However, there is a noticeable temperature and pressure drop during the reaction and between the inlet and the outlet points. Moreover, these reactors experience significant temperature changes at different points between the inlet and outlet. Moreover, the reactor requires a large length-to-diameter ratio and a limitation for the Reynolds number. In most cases, tubular reactors require a slow mixing process which often leads to large hold-ups and clogging [21,70][20][21]. Notable examples of tubular/plug flow reactors include packed bed reactors, fluidized bed reactors, trickle bed reactors, oscillatory flow reactors, and micro-channel reactors.
2.1. Packed Bed Reactors
Packed bed reactors, also known as fixed bed reactors, are one of the most used tubular reactors in chemical industries, especially for biodiesel production using heterogeneous catalysts. They can also work in the supercritical mode for improved biodiesel production. During the transesterification process for biodiesel production, the packed bed reactor provides a substrate for enzyme immobilization to improve production. Much more than the size or volume of the reactor, the amount of the solid catalyst in the tube influences the conversion of the feedstock into biodiesel [21][20]. The reactors are in tubular forms and the tubes are filled with packing materials including heterogeneous catalysts and activated carbon. The performance of a packed bed reactor is greatly affected by the catalyst particle size, bed structure, and the spaces between catalyst particles. The arrangement of the packing materials is governed by factors such as (i) physical attributes of the tube, (ii) the shape, size, and the surface structure of the catalyst, and (iii) the intensity, method, and speed of deposition [71][22].
With packed bed reactors, the higher conversion efficiency of oils per unit of solid catalysts is feasible and shortens the residence time. Another major benefit of using packed bed reactors is the downstream elimination of catalysts from the product since the catalysts are packed in the tube. According to Sakdasri et al. [73][23], the greatest advantage of the deployment of packed bed reactors is their high conversion efficiency and ability to use heterogeneous catalysts. Despite these advantages, the reactor suffers from acute and sudden pressure drops, increased cost of operation, and high energy consumption. The pressure drops can be attributed to fluid friction, fluid viscosity, and reactor tube length. Because of these advantages, several researchers have utilized packed bed reactors for biodiesel production.
2.2. Fluidized Bed Reactors
Fluidized bed reactors, also known as expanded bed reactors, are the most popular configurations employed for the conversion of oils into biodiesel on a laboratory or commercial scale. The basic principle of the operation of a fluidized bed reactor involves a fluidization medium (gas or liquid) made to flow through the bed of solid reactants at a velocity high enough to suspend the solid and make it behave as a fluid. The reactor consists of a reservoir and a column. The reservoir is for the housing and preparation of the liquid feedstock while the column consists of a calming section, distributor, fluidized bed, and freeboard. The calming section helps to equalize the liquid feedstock flow while the distributor creates enough pressure difference across the fluidized bed. At a low fluid velocity, the particle in the vessel is stagnant, similar to the packed bed reactors. However, as the fluid velocity increases, the drag force will overcome the weight of the fluid and propel the particles into an upward movement which signifies the start of the fluidization process. At a higher fluid velocity, the particles expand and swirl around and upward in the fluidized bed. The freeboard disallows the catalyst from flowing out of the column [21,74][20][24].
Fluidized bed reactors have become popular for the transesterification of oil into biodiesel due to their ability to ensure uniform particle mixing, uniform temperature gradients, and the ability to be operated effectively on a continuous scale [76][25]. However, the sudden pressure loss in the column creates a pressure loss scenario and the possible erosion of internal components. Moreover, due to the likely expansion of the bed materials in the reactor, there is a need for an increment in the reactor size and consequently, the cost of the reactor construction. Other disadvantages of fluidized bed reactors include a high operating cost, reactor wall erosion, the likelihood of particle entrainment, and high catalyst attrition [77][26]. The practical application of a fluidized bed reactor by Kutálek et al. [78][27], Fidalgo et al. [75][28], and Wang et al. [79][29] yielded a biodiesel conversion efficiency of 77%, 98.1%, and 91.5% respectively.
2.3. Trickle Bed Reactors
Trickle bed reactors are some of the most used industrial reactors in chemical and related industries including the electrochemical, petroleum, petrochemical, coal, pharmaceutical, oil and gas, waste treatment, and biochemical processes. A notable application of trickle bed reactors includes the conversion of vegetable oil into biodiesel, hydrogenation of biooils, polymerization of monomers, purification of feedstocks, and manufacturing of pharmaceuticals [80][30]. It is a continuous system where liquids are made to flow through a packed bed containing a packing medium. There is a platform for the solid, liquid, and gas based on gravity or pressure forces.
The trickle bed reactors for biodiesel production consist of a tubular tank and structure for solid catalysts at the base of the reactor [81][31]. The feedstock is introduced from the top of the column while the alcohol can be fed either top or bottom. The heating jacket mounted at the reactor wall helps to maintain the reaction temperature. The continuous heating ensures that the alcohol is vaporized while the unreacted alcohol can be separated from the product. The outlet at the top of the reactor allows for alcohol gas recycling while the outlet at the bottom of the reactor is for the products and unreacted oil to flow out [82][32].
Major advantages of using trickle bed reactors include simplicity in operation even at a high temperature and pressure, high catalyst loading per unit volume, and low capital and operating costs. Moreover, trickle bed reactors can be used for diverse applications and can accommodate a large volume of production. When used for biodiesel production, trickle bed reactors ensure a higher feedstock conversion rate and improve product productivity [81][31]. Despite these benefits, trickle bed reactors suffer from poor heat transfer rate, limited diffusion among particles, and unequal fluid distribution. Trickle bed reactors are difficult to scale up and controlling vessel parameters might pose a huge challenge [84][33]. In research, Muharam et al. [80][30] reported a 78.22% conversion efficiency while Jindapon et al. [83][34] reported a biodiesel yield of 92.3% and a product purity of 93.6%.
2.4. Oscillatory Bed Reactors
This type of tubular reactor contains equally spaced tubes with orifice plate constrictions arranged to generate oscillatory flow with intermittent changes in the flow direction using a piston drive. This unique oscillation motion produces vortex mixing that results in the filling of the entire cross-section of the baffles cavity due to fluid obstruction. The configuration of the baffles, rather than the Reynolds number of the fluid, plays an important role in the effectiveness of the reactor. Typically, baffles can be of helical, axial, integral, or wire wool configurations with a tube diameter of less than 15 mm to ensure vigorous mixing and to minimize frictional loss. For the purpose of biodiesel production from vegetable oil using heterogeneous catalysts, a tube diameter of about 5 mm is recommended to minimize the overbearing construction and feedstock costs. In the same vein, an oscillatory Reynolds number of 10 is adequate to ensure turbulent flow in the tube [85][35].
The use of oscillatory flow reactors ameliorates the challenges associated with the deployment of conventional flow reactors by ensuring vigorous mixing, superb heat transfer, and an excellent plug flow experience. The flow generated by the oscillatory motion is not affected by the net flow rate, the residence time, and the hydrodynamic properties of the slurry. Similarly, the moderation of the net flow rate ensures a smaller reactor volume, a compact setup, minimizes space requirement, and guarantees quality mixing [86][36]. To achieve efficient and economically viable biodiesel production, oscillatory flow reactors should have a short length/diameter ratio [87][37].
When compared with conventional reactors, oscillatory flow reactors consume less energy and generate less waste. They also offer improved mixing efficiency and better mass and heat transfer. The reactor can be operated on both baths and continuous modes offer process flexibility and can easily be scaled up to accommodate increased production [89][38]. However, the oscillatory flow reactor suffers acute pressure drops as a result of persistent frictional loss. The generation of gas bubbles during operation dampens the oscillations and upsets the plug flow [90][39].
2.5. Micro-Channel Reactors
Micro-channel reactors are another type of tubular reactor using micro-channel technology for processing chemicals and other diverse applications. They are made up of narrow channels or tubes, in the millimeter range, which allow a high surface/volume ratio, minimize diffusion length, and improve mass and heat transfer. The flow of fluid in this type of reactor is orderly, predictable, and measurable, which also requires a lengthy pipe to ensure thorough mixing [91][40]. The requirement of a lengthy mixing path is a challenge that is addressed by the application of passive micromixers. The deployment of micromixers of diverse configurations and arrangements achieves an improved contact surface through the mixing of two or more liquids. For example, serial lamination micromixers split the inlet flow and merge them first horizontally and then vertically. For injection micromixers, the oil is allowed to split into substreams before the injection of methanol through a collection of nozzles. Droplet micromixers employ an internal flow field to ensure the mixing of the liquids and transport them by capillary effects, pressure gradient, and flow instability of two or more fluids [92,93][41][42].
When compared with conventional reactors, micro-channel reactors demonstrate a high surface/volume ratio, better heat and mass transfer, and improved homogeneous fluid mixing. This type of reactor also allows a shorter reaction duration, less degradation, better scalability, and easier optimization and monitoring. With micro-channel reactors, there is an opportunity for more precision reaction control, selectivities, better conversion efficiency, faster reaction speed, and improved product yield. Moreover, temperature control is easier and more precise, safer, and allows for prompt phase separation [94,95][43][44]. However, the micro-channel reactors can handle a limited volume of feedstock at a time due to the small volume of the tubes. They are also prone to intermittent clogging, fouling, tube blockage, and corrosion. Other drawbacks of this type of reactor include a high rate of leakages between channels and the prohibitive cost of building the reactor. Because the micro-channel reactors are small, they are not usually applied at an industrial scale [64,96][15][45].
Generally, tubular reactors are some of the simplest and easy to construct and operate chemical reactors for biodiesel production. They are cost effective, environmentally friendly, and safe to operate. They can be operated both on batch and continuous modes, are easy to clean, and ensure a high product yield. The quality of the product generated by tubular reactors meets international standards. Though some of them suffer from sudden pressure drops, and high catalyst attrition, they nonetheless find practical industrial applications.
References
- De Paola, M.G.; Mazza, I.; Paletta, R.; Lopresto, C.G.; Calabrò, V. Small-scale biodiesel production plants—An overview. Energies 2021, 14, 1901.
- Ghazvini, M.; Kavosi, M.; Sharma, R.; Kim, M. A review on mechanical-based microalgae harvesting methods for biofuel production. Biomass Bioenergy 2020, 158, 106348.
- Arenas, E.; Villafán-Cáceres, S.M.; Rodríguez-Mejía, Y.; García-Loyola, J.A.; Masera, O.; Sandoval, G. Biodiesel dry purification using unconventional bioadsorbents. Processes 2021, 9, 194.
- Jariah, N.F.; Hassan, M.A.; Taufiq-Yap, Y.H.; Roslan, A.M. Technological advancement for efficiency enhancement of biodiesel and residual glycerol refining: A mini review. Processes 2021, 9, 1198.
- Hayyan, A.; Ng, Y.S.; Hadj-Kali, M.K.; Junaidi, M.U.M.; Ali, E.; Aldeehani, A.K.; Alkandari, K.H.; Alajmi, F.D.H.; Yeow, A.T.H.; Zulkifli, M.Y. Natural and low-cost deep eutectic solvent for soap removal from crude biodiesel using low stirring extraction system. Biomass Convers. Biorefin. 2022, 12, 113–121.
- Athar, M.; Zaidi, S. A review of the feedstocks, catalysts, and intensification techniques for sustainable biodiesel production. J. Environ. Chem. Eng. 2020, 8, 104523.
- Akubude, V.; Jaiyeoba, K.; Oyewusi, T.; Abbah, E.; Oyedokun, J.; Okafor, V. Overview on Different Reactors for Biodiesel Production. In Biodiesel Technology and Applications; Inamuddin, M.I., Ahamed, R.B., Mashallah, R., Eds.; Scrivener Publishing: Beverly, MA, USA, 2021; pp. 341–359.
- Bashir, M.A.; Wu, S.; Zhu, J.; Krosuri, A.; Khan, M.U.; Aka, R.J.N. Recent development of advanced processing technologies for biodiesel production: A critical review. Fuel Process. Technol. 2022, 227, 107120.
- Topare, N.S.; Patil, K.D.; Khedkar, S.V.; Inamdar, N. Lab Scale Batch Reactor Design, Fabrication and Its Application for Biodiesel Production. In Techno-Societal 2020; Pawar, P.M., Balasubramaniam, R., Ronge, B.P., Salunkhe, S.B., Vibhute, A.S., Melinamath, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 819–828.
- Mehboob, A.; Nisar, S.; Rashid, U.; Choong, T.S.Y.; Khalid, T.; Qadeer, H.A. Reactor designs for the production of biodiesel. Int. J. Chem. Biochem. Sci. 2016, 10, 87–94.
- Zahan, K.A.; Kano, M. Technological Progress in biodiesel production: An overview on different types of reactors. Energy Procedia 2019, 156, 452–457.
- Costa, W.A.; Bezerra, F.W.F.; Oliveira, M.S.; Silva, M.P.; Cunha, V.M.B.; Andrade, E.H.A.; Carvalho, R.N. Appliance of a high pressure semi-batch reactor: Supercritical transesterification of soybean oil using methanol. Food Sci. Technol. 2019, 39, 754–773.
- Silva, M.G.; Oliveira, G.S.; Carvalho, J.C.R.; Nobre, L.R.P.; Deus, M.S.; Jesus, A.A.; Oliveira, J.A.; Souza, D.F.S. Esterification of oleic acid in a semi-batch bubble reactor for biodiesel production. Braz. J. Chem. Eng. 2019, 36, 299–308.
- Silva, M.G.; Nobre, L.R.P.; Santiago, L.F.P.; Deus, M.S.; Jesus, A.A.; Oliveira, J.A.; Souza, D.F.S. Mathematical modeling and simulation of biodiesel production in a semibatch bubble reactor. Energy Fuels 2018, 32, 9614–9623.
- Tran-Nguyen, P.L.; Ong, L.K.; Go, A.W.; Ju, Y.H.; Angkawijaya, A.E. Non-catalytic and heterogeneous acid/base-catalyzed biodiesel production: Recent and future developments. Asia-Pac. J. Chem. Eng. 2020, 15, e2490.
- Qiao, B.Q.; Zhou, D.; Li, G.; Yin, J.Z.; Xue, S.; Liu, J. Process enhancement of supercritical methanol biodiesel production by packing beds. Bioresour. Technol. 2017, 228, 298–304.
- Tran, N.N.; Gelonch, M.E.; Liang, S.; Xiao, Z.; Sarafraz, M.M.; Tišma, M.; Federsel, H.J.; Ley, S.V.; Hessel, V. Enzymatic pretreatment of recycled grease trap waste in batch and continuous-flow reactors for biodiesel production. Chem. Eng. J. 2021, 426, 131703.
- Bogatykh, I.; Osterland, T. Characterization of residence time distribution in a plug flow reactor. Chem. Ing. Tech. 2019, 91, 668–672.
- Sungwornpatansakul, P.; Hiroi, J.; Nigahara, Y.; Jayasinghe, T.K.; Yoshikawa, K. Enhancement of biodiesel production reaction employing the static mixing. Fuel Process. Technol. 2013, 116, 1–8.
- Tabatabaei, M.; Aghbashlo, M.; Dehhaghi, M.; Panahi, H.K.S.; Mollahosseini, A.; Hosseini, M.; Soufiyan, M.M. Reactor technologies for biodiesel production and processing: A review. Prog. Energy Combust. Sci. 2019, 74, 239–303.
- Farobie, O.; Sasanami, K.; Matsumura, Y. A novel spiral reactor for biodiesel production in supercritical ethanol. Appl. Energy 2015, 147, 20–29.
- Jakobsen, H.A. Packed bed reactors. In Chemical Reactor Modeling; Jakobsen, H.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 953–984.
- Sakdasri, W.; Ngamprasertsith, S.; Daengsanun, S.; Sawangkeaw, R. Lipid-based biofuel synthesized from palm-olein oil by supercritical ethyl acetate in fixed-bed reactor. Energy Convers. Manag. 2019, 182, 215–223.
- Antunes, F.; Machado, P.; Rocha, T.; Melo, Y.; Santos, J.; da Silva, S. Column reactors in fluidized bed configuration as intensification system for xylitol and ethanol production from napier grass (Pennisetum Purpureum). Chem. Eng. Process. Process Intensif. 2021, 164, 108399.
- Borges, M.; Díaz, L. Catalytic packed-bed reactor configuration for biodiesel production using waste oil as feedstock. Bioenergy Res. 2013, 6, 222–228.
- Wang, K.; Brown, R.C.; Homsy, S.; Martinez, L.; Sidhu, S.S. Fast pyrolysis of microalgae remnants in a fluidized bed reactor for bio-oil and biochar production. Bioresour. Technol. 2013, 127, 494–499.
- Kutálek, K.; Čapek, L.; Smoláková, L.; Kubička, D. Aspects of Mg–Al mixed oxide activity in transesterification of rapeseed oil in a fixed-bed reactor. Fuel Process. Technol. 2014, 122, 176–181.
- Fidalgo, W.R.R.; Ceron, A.; Freitas, L.; Santos, J.C.; de Castro, H.F. A fluidized bed reactor as an approach to enzymatic biodiesel production in a process with simultaneous glycerol removal. J. Ind. Eng. Chem. 2016, 38, 217–223.
- Wang, Y.; Yang, G.; He, J.; Sun, G.; Sun, Z.; Sun, Y. Preparation of biochar catalyst from black liquor by spray drying and fluidized bed carbonation for biodiesel synthesis. Process Saf. Environ. Prot. 2020, 141, 333–343.
- Muharam, Y.; Aufa, T.; Santoso, T.B. Modeling of partial hydrogenation of polyunsaturated fatty acid methyl esters in a trickle bed reactor. Eng. J. 2020, 24, 195–204.
- Restrepo, J.B.; Bustillo, J.A.; Bula, A.J.; Paternina, C.D. Selection, Sizing, and Modeling of a Trickle Bed Reactor to Produce 1, 2 Propanediol from Biodiesel Glycerol Residue. Processes 2021, 9, 479.
- Azarpour, A.; Rezaei, N.; Zendehboudi, S. Performance analysis and modeling of catalytic trickle-bed reactors: A comprehensive review. J. Ind. Eng. Chem. 2021, 103, 1–41.
- Degirmenci, V.; Rebrov, E.V. Design of catalytic micro trickle bed reactors. Phys. Sci. Rev. 2016, 1, 1–29.
- Jindapon, W.; Ruengyoo, S.; Kuchonthara, P.; Ngamcharussrivichai, C.; Vitidsant, T. Continuous production of fatty acid methyl esters and high-purity glycerol over a dolomite-derived extrudate catalyst in a countercurrent-flow trickle-bed reactor. Renew. Energy 2020, 157, 626–636.
- McDonough, J.R.; Phan, A.N.; Harvey, A.P. Rapid process development using oscillatory baffled mesoreactors—A state-of-the-art review. Chem. Eng. J. 2015, 265, 110–121.
- Bianchi, P.; Williams, J.D.; Kappe, C.O. Oscillatory flow reactors for synthetic chemistry applications. J. Flow Chem. 2020, 10, 475–490.
- Thangarasu, G.R.V.; Vinayakaselvi, M.; Ramanathan, A. A critical review of recent advancements in continuous flow reactors and prominent integrated microreactors for biodiesel production. Renew. Sust. Energ. Rev. 2022, 154, 111869.
- Oliva, J.A.; Pal, K.; Barton, A.; Firth, P.; Nagy, Z.K. Experimental investigation of the effect of scale-up on mixing efficiency in oscillatory flow baffled reactors (OFBR) using principal component based image analysis as a novel noninvasive residence time distribution measurement approach. Chem. Eng. J. 2018, 351, 498–505.
- Abbott, M.; Harvey, A.; Perez, G.V.; Theodorou, M. Biological processing in oscillatory baffled reactors: Operation, advantages and potential. Interface Focus 2013, 3, 20120036.
- Natarajan, Y.; Nabera, A.; Salike, S.; Tamilkkuricil, V.D.; Pandian, S.; Karuppan, M.; Appusamy, A. An overview on the process intensification of microchannel reactors for biodiesel production. Chem. Eng. Process. Process Intensif. 2019, 136, 163–176.
- Mohd Laziz, A.; KuShaari, K.; Azeem, B.; Yusup, S.; Chin, J.; Denecke, J. Rapid production of biodiesel in a microchannel reactor at room temperature by enhancement of mixing behaviour in methanol phase using volume of fluid model. Chem. Eng. Sci. 2020, 219, 115532.
- Kumar, Y.; Das, L.; Biswas, K.G. Biodiesel: Features, Potential Hurdles, and Future Direction. In Status and Future Challenges for Non-Conventional Energy Sources; Joshi, J., Sen, R., Sharma, A., Salam, P.A., Eds.; Springer: Singapore, 2022; Volume 2, pp. 99–122.
- Tiwari, A.; Rajesh, V.M.; Yadav, S. Biodiesel production in micro-reactors: A review. Energy Sustain. Dev. 2018, 43, 143–161.
- Kiani, M.R.; Meshksar, M.; Makarem, M.A.; Rahimpour, E. Catalytic membrane micro-reactors for fuel and biofuel processing: A mini review. Top. Catal. 2021.
- Gupta, J.; Agarwal, M.; Dalai, A.K. An overview on the recent advancements of sustainable heterogeneous catalysts and prominent continuous reactor for biodiesel production. J. Ind. Eng. Chem. 2020, 88, 58–77.
More
