Facing greenhouse effects and the rapid exhaustion of fossil fuel, CO2 electrochemical reduction presents a promising method of environmental protection and energy transformation. Low onset potential, large current density, high faradaic efficiency (FE), and long-time stability are required for industrial production, due to economic costs and energy consumption. Copper is one of the few metals that can reduce CO2 to hydrocarbons and alcohols with decent efficiency, and copper-based catalysts have received much attention. The uniqueness of Cu as a CO2RR electrocatalyst is explained by the fact that it is the only metal that has negative adsorption energy for *CO and positive adsorption energy for *H.
- CO2
- electroreduction
- faradaic efficiency
- flow cell
1. Introduction
2. Electroreduction Pathways
CO2RR activity is carried out through multiple proton and electron transfer steps involving many possible intermediates. The catalytic performance can be tuned and determined largely by key reaction intermediates. Advances in operando spectroscopy and computational techniques provide significant scope for exploring the evolution of surface-bound species and rationalizing a pathway to the desired product. The reaction pathways of CO2 reduction with key intermediates on copper-based catalysts are summarized (Figure 1).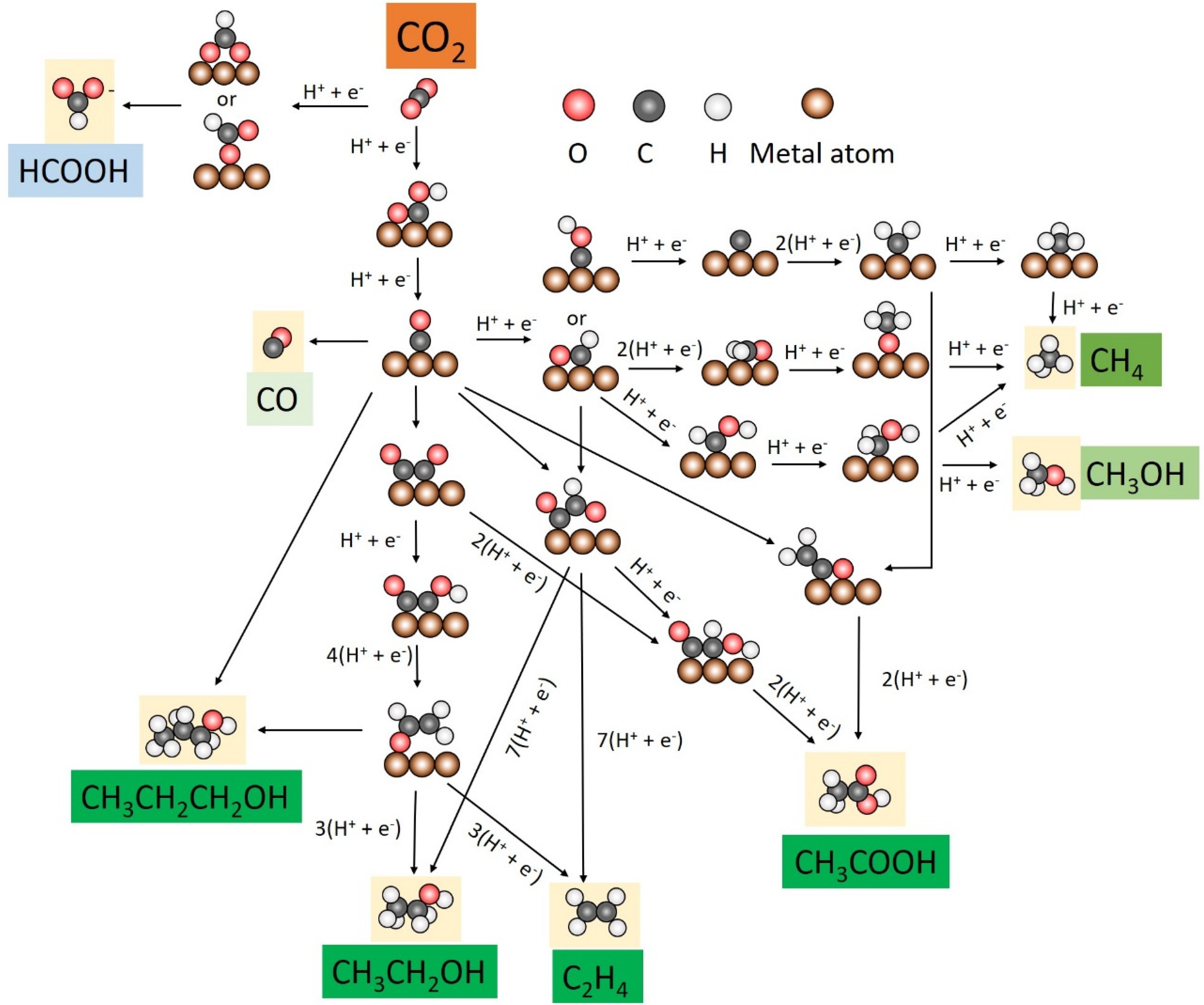
3. Advances in CO2 Electroreduction
The recent research into CO2 electroreduction focuses on reducing overpotential, enhancing the FE of the target product, enlarging current density, and maintaining stability. Different synthesis methods of the catalysts and catalysis engineering have been introduced to promote catalytic performance.3.1. Low Overpotential
Overpotential is defined as the additional potential (above the thermodynamic requirement) to drive a reaction at a specific current density [48]. It can be simulated by theoretical calculations such as density functional theory (DFT). The adsorption energies of the evolving intermediates were calculated to build up the thermodynamic energy diagram for CO2RR, and the overpotential was attributed to the most unfavorable step in the electroreduction pathway [27[27][39],40], which was called the rate-determining step (RDS). Although some methods of calculation correction have been developed to assess the kinetic barrier [49[49][50],50], the difference in adsorption energy of intermediates was found to influence the overpotential, and other effects such as local CO concentration and charge transfer resistance also played a vital role. Recent research on reducing the onset potential for CO2RR products is shown in Table 1. The potential for the target product is quite different. Low overpotential is required for CO and formate, while high overpotential is needed for deep electroreduction products of methane, ethanol, and ethylene.| Major Product | Catalyst | Onset Potential (V vs. RHE) |
Reference |
|---|---|---|---|
| CO | Cu-N2/CN | −0.33 | [51] |
| FeN5 | −0.2 * | [52] | |
| Ni–N3S | −0.17 | [53] | |
| Fe3+-N-C | −0.2 | [54] | |
| Co–N–Ni/NPCNSs | −0.2 | [55] | |
| CoPc©Fe-N-C | −0.13 | [56] | |
| Formate | single-atom Snδ+ on N-doped graphene | −0.18 | [57] |
| BiN4/C | −0.51 | [58] | |
| Methane | AuAgPtPdCu | −0.3 * | [59] |
| Ethylene | Organosuperbases modified Cu-NC | −0.43 | [60] |
| F-Cu | ~−0.2 | [61] | |
| Ethanol | Cun (n = 3 and 4) cluster | −0.3~−0.4 | [62] |
| Au/Cu | −0.7 * | [63] | |
| FeTPP[Cl]/Cu | −0.42 | [64] | |
| Acetate | Cu–Cu2O/Cu | ~−0.2 * | [65] |
3.1.1. Two-Electron Electroreduction Products
Generally, two-electron electroreduction products with a relatively simple process require lower overpotential, compared with other reduction products such as methane and ethylene. Since the properties of active metal centers in SACS can be finely tuned through changing the near-range coordination environment and long-range interactions, SACs could effectively lower the overpotential of CO2RR products. Considering the promotion effect of unsaturated coordination on catalytic activity, Zheng et al. fabricated coordinatively unsaturated single-atom with nitrogen sites anchored on graphene (Cu-N2/CN) [51]. Aberration corrected high-angle annular dark field scanning transmission electron microscopy (AC HAADF-STEM) and X-ray absorption spectroscopy (XAS) demonstrated that the single-atom Cu species were uniformly distributed and coordinated with two N atoms, and inductively coupled plasma mass spectrometry (ICP-MS) determined the Cu content of 1.45 wt% in Cu–N2/GN nanosheets. The coordinatively unsaturated Cu not only promoted the adsorption of CO2 on the catalyst surface, but also accelerated the electron transfer from Cu–N2 sites to *CO2. The Cu–N2/CN catalyst produced CO at a low overpotential, with a maximum FE of 81% at −0.50 V vs. RHE, and onset potential −0.33 V vs. RHE (Table 1). They found that the electronegative N atoms near the coordinatively unsaturated metal center further reduced the energy barrier, lowering the onset potential to −0.30 V vs. RHE [66]. Though wresearchers focus on Cu-based catalysts in this reviewentry, their effect on reducing the overpotential of CO2RR, especially for two-electron products, is inferior to catalysts with other metals. Zhang et al. prepared singly dispersed FeN5 sites supported on N-doped graphene with an additional axial ligand coordinated to FeN4 through thermal pyrolysis [52]. The AC HAADF-STEM images showed the atomically dispersed Fe and an absence of larger clusters in as-prepared catalysts. In electrochemical tests, the over-coordinated catalyst exhibited a high FE of 97% for CO production at low overpotential. DFT calculation disclosed that the additional coordination number weakened the *CO binding strength of FeN5, and facilitated the desorption of *CO, which changed the RDS and lowered the overpotential. The coordinated adjustment was also an effective way to modify the electronic structures of the metal center and enhance the catalytic performance. Yang et al. synthesized an S-doped Ni-SAC (Ni–N3S) by pyrolysis treatment [53]. The single-Ni-atom catalyst was prepared by pyrolysing a mixture of the amino acid (l-alanine or l-cysteine), melamine, and nickel acetate in argon, with the addition of a sulfur precursor (l-cysteine)The catalytic results showed that the S doping reduced the onset overpotential at only 70 mV, 100 mV lower compared to the Ni–N4 catalyst without S doping. Ni K-edge X-ray absorption near-edge structure (XANES) spectra indicated that the non-centrosymmetric ligand strength of Ni–N3S highly distorted the geometry, which was considered to promote the adsorption of reactants and intermediates, and reduce the overpotential. Cl and N dual-coordinated Mn-SAC ((Cl, N)-Mn/G) synthesized by Zhang et al. improved CO2RR, and the catalytic activity was obtained at low overpotential [67]. The d-band center of (Cl, N)-Mn/G was lower than that of MnN4, thus weakening the strong adsorption for *CO. DFT calculation proved that the energy barrier of the RDS (desorption of *CO) decreased from 1.64 eV for MnN4 to 0.65 eV for (Cl, N)-Mn/G. As there were four types of N atoms existing in SACs, different types of N atoms coordinated with the metal center and resulted in the variation of catalytic performance. Gu et al. prepared Fe3+-N-C coordinated with pyrrolic N, which displayed a CO partial current density of 94 mA cm−2 at an overpotential of 340 mV [54]. In this catalyst, the pyrrolic N coordination rendered the Fe3+/2+ reduction potential more negative than the Fermi level of the carbon support, leading to the stabilization of the Fe3+ ions in CO2RR conditions. Then, the stabilized Fe3+ induced faster CO2 adsorption and weaker CO absorption, which contributed to the high activity with low overpotential.
References
- Olah, G.A.; Prakash, G.K.S.; Goeppert, A. Anthropogenic Chemical Carbon Cycle for a Sustainable Future. J. Am. Chem. Soc. 2011, 133, 12881–12898.
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672.
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059.
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the Development of Novel Cobalt Fischer−Tropsch Catalysts for Synthesis of Long-Chain Hydrocarbons and Clean Fuels. Chem. Rev. 2007, 107, 1692–1744.
- Zheng, T.; Zhang, M.; Wu, L.; Guo, S.; Liu, X.; Zhao, J.; Xue, W.; Li, J.; Liu, C.; Li, X.; et al. Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering. Nat. Catal. 2022, 5, 388–396.
- Glockler, G. Carbon–Oxygen Bond Energies and Bond Distances. J. Phys. Chem. 1958, 62, 1049–1054.
- Siewert, I. Proton-Coupled Electron Transfer Reactions Catalysed by 3 d Metal Complexes. Chem. A Eur. J. 2015, 21, 15078–15091.
- Ross, M.B.; De Luna, P.; Li, Y.; Dinh, C.-T.; Kim, D.; Yang, P.; Sargent, E.H. Designing materials for electrochemical carbon dioxide recycling. Nat. Catal. 2019, 2, 648–658.
- Burdyny, T.; Smith, W.A. CO2 reduction on gas-diffusion electrodes and why catalytic performance must be assessed at commercially-relevant conditions. Energy Environ. Sci. 2019, 12, 1442–1453.
- Choi, W.; Won, D.H.; Hwang, Y.J. Catalyst design strategies for stable electrochemical CO2 reduction reaction. J. Mater. Chem. A 2020, 8, 15341–15357.
- Jin, S.; Hao, Z.; Zhang, K.; Yan, Z.; Chen, J. Advances and Challenges for the Electrochemical Reduction of CO2 to CO: From Fundamentals to Industrialization. Angew. Chem. Int. Ed. 2021, 60, 20627–20648.
- Park, S.; Wijaya, D.T.; Na, J.; Lee, C.W. Towards the Large-Scale Electrochemical Reduction of Carbon Dioxide. Catalysts 2021, 11, 253.
- Yoshio, H.; Katsuhei, K.; Shin, S. Production of CO and CH4 in electrochemical reduction of CO2 at metal electrodes in aqueous hydrogencarbonate solution. Chem. Lett. 1985, 14, 1695–1698.
- Díaz-Sainz, G.; Alvarez-Guerra, M.; Irabien, A. Continuous electroreduction of CO2 towards formate in gas-phase operation at high current densities with an anion exchange membrane. J. CO2 Util. 2022, 56, 101822.
- Cheng, D.; Zhao, Z.-J.; Zhang, G.; Yang, P.; Li, L.; Gao, H.; Liu, S.; Chang, X.; Chen, S.; Wang, T.; et al. The nature of active sites for carbon dioxide electroreduction over oxide-derived copper catalysts. Nat. Commun. 2021, 12, 395.
- Kuo, L.; Dinh, C.-T. Toward efficient catalysts for electrochemical CO2 conversion to C2 products. Curr. Opin. Electrochem. 2021, 30, 100807.
- Xue, L.; Zhang, C.; Wu, J.; Fan, Q.-Y.; Liu, Y.; Wu, Y.; Li, J.; Zhang, H.; Liu, F.; Zeng, S. Unveiling the reaction pathway on Cu/CeO2 catalyst for electrocatalytic CO2 reduction to CH4. Appl. Catal. B 2022, 304, 120951.
- Garza, A.J.; Bell, A.T.; Head-Gordon, M. Mechanism of CO2 Reduction at Copper Surfaces: Pathways to C2 Products. ACS Catal. 2018, 8, 1490–1499.
- Feaster, J.T.; Shi, C.; Cave, E.R.; Hatsukade, T.; Abram, D.N.; Kuhl, K.P.; Hahn, C.; Nørskov, J.K.; Jaramillo, T.F. Understanding Selectivity for the Electrochemical Reduction of Carbon Dioxide to Formic Acid and Carbon Monoxide on Metal Electrodes. ACS Catal. 2017, 7, 4822–4827.
- Vijay, S.; Ju, W.; Brückner, S.; Tsang, S.-C.; Strasser, P.; Chan, K. Unified mechanistic understanding of CO2 reduction to CO on transition metal and single atom catalysts. Nat. Catal. 2021, 4, 1024–1031.
- Li, S.; Dong, X.; Chen, W.; Song, Y.; Li, G.; Wei, W.; Sun, Y. Efficient CO2 Electroreduction over Silver Hollow Fiber Electrode. Catalysts 2022, 12, 453.
- Wang, Z.; Li, T.; Wang, Q.; Guan, A.; Cao, N.; Al-Enizi, A.M.; Zhang, L.; Qian, L.; Zheng, G. Hydrophobically made Ag nanoclusters with enhanced performance for CO2 aqueous electroreduction. J. Power. Sources 2020, 476, 228705.
- Kim, J.; Choi, W.; Park, J.W.; Kim, C.; Kim, M.; Song, H. Branched Copper Oxide Nanoparticles Induce Highly Selective Ethylene Production by Electrochemical Carbon Dioxide Reduction. J. Am. Chem. Soc. 2019, 141, 6986–6994.
- Gao, Y.; Wu, Q.; Liang, X.; Wang, Z.; Zheng, Z.; Wang, P.; Liu, Y.; Dai, Y.; Whangbo, M.-H.; Huang, B. Cu2O Nanoparticles with Both and Facets for Enhancing the Selectivity and Activity of CO2 Electroreduction to Ethylene. Adv. Sci. 2020, 7, 1902820.
- Zhong, D.; Zhao, Z.-J.; Zhao, Q.; Cheng, D.; Liu, B.; Zhang, G.; Deng, W.; Dong, H.; Zhang, L.; Li, J.; et al. Coupling of Cu(100) and (110) Facets Promotes Carbon Dioxide Conversion to Hydrocarbons and Alcohols. Angew. Chem. Int. Ed. 2021, 60, 4879–4885.
- Zhong, M.; Tran, K.; Min, Y.; Wang, C.; Wang, Z.; Dinh, C.-T.; De Luna, P.; Yu, Z.; Rasouli, A.S.; Brodersen, P.; et al. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 2020, 581, 178.
- Lv, X.; Shang, L.; Zhou, S.; Li, S.; Wang, Y.; Wang, Z.; Sham, T.-K.; Peng, C.; Zheng, G. Electron-Deficient Cu Sites on Cu3Ag1 Catalyst Promoting CO2 Electroreduction to Alcohols. Adv. Energy Mater. 2020, 10, 2001987.
- Wei, X.; Yin, Z.; Lyu, K.; Li, Z.; Gong, J.; Wang, G.; Xiao, L.; Lu, J.; Zhuang, L. Highly Selective Reduction of CO2 to C2+ Hydrocarbons at Copper/Polyaniline Interfaces. ACS Catal. 2020, 10, 4103–4111.
- Li, F.; Thevenon, A.; Rosas-Hernandez, A.; Wang, Z.; Li, Y.; Gabardo, C.M.; Ozden, A.; Cao Thang, D.; Li, J.; Wang, Y.; et al. Molecular tuning of CO2-to-ethylene conversion. Nature 2020, 577, 509.
- Ren, D.; Deng, Y.; Handoko, A.D.; Chen, C.S.; Malkhandi, S.; Yeo, B.S. Selective Electrochemical Reduction of Carbon Dioxide to Ethylene and Ethanol on Copper(I) Oxide Catalysts. ACS Catal. 2015, 5, 2814–2821.
- Popović, S.; Smiljanić, M.; Jovanovič, P.; Vavra, J.; Buonsanti, R.; Hodnik, N. Stability and Degradation Mechanisms of Copper-Based Catalysts for Electrochemical CO2 Reduction. Angew. Chem. Int. Ed. 2020, 59, 14736–14746.
- Wang, X.; Xu, A.N.; Li, F.W.; Hung, S.F.; Nam, D.H.; Gabardo, C.M.; Wang, Z.Y.; Xu, Y.; Ozden, A.; Rasouli, A.S.; et al. Efficient Methane Electrosynthesis Enabled by Tuning Local CO2 Availability. J. Am. Chem. Soc. 2020, 142, 3525–3531.
- Qiu, Y.L.; Zhong, H.X.; Xu, W.B.; Zhang, T.T.; Li, X.F.; Zhang, H.M. Tuning the electrocatalytic properties of a Cu electrode with organic additives containing amine group for CO2 reduction. J. Mater. Chem. A 2019, 7, 5453–5462.
- Chernyshova, I.V.; Somasundaran, P.; Ponnurangam, S. On the origin of the elusive first intermediate of CO2 electroreduction. Proc. Natl. Acad. Sci. USA 2018, 115, E9261.
- Zhang, S.; Fan, Q.; Xia, R.; Meyer, T.J. CO2 Reduction: From Homogeneous to Heterogeneous Electrocatalysis. Acc. Chem. Res. 2020, 53, 255–264.
- Bagger, A.; Ju, W.; Varela, A.S.; Strasser, P.; Rossmeisl, J. Electrochemical CO2 Reduction: A Classification Problem. ChemPhysChem 2017, 18, 3266–3273.
- Cheng, T.; Xiao, H.; Goddard, W.A. Free-Energy Barriers and Reaction Mechanisms for the Electrochemical Reduction of CO on the Cu(100) Surface, Including Multiple Layers of Explicit Solvent at pH 0. J. Phys. Chem. Lett. 2015, 6, 4767–4773.
- Nie, X.; Esopi, M.R.; Janik, M.J.; Asthagiri, A. Selectivity of CO2 Reduction on Copper Electrodes: The Role of the Kinetics of Elementary Steps. Angew. Chem. Int. Ed. 2013, 52, 2459–2462.
- Peterson, A.A.; Abild-Pedersen, F.; Studt, F.; Rossmeisl, J.; Nørskov, J.K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 2010, 3, 1311–1315.
- Peng, C.; Xu, Z.; Luo, G.; Yan, S.; Zhang, J.; Li, S.; Chen, Y.; Chang, L.Y.; Wang, Z.; Sham, T.-K.; et al. Highly-Exposed Single-Interlayered Cu Edges Enable High-Rate CO2-to-CH4 Electrosynthesis. Adv. Energy Mater. 2022, 12, 2200195.
- Calle-Vallejo, F.; Koper, M.T.M. Theoretical Considerations on the Electroreduction of CO to C2 Species on Cu(100) Electrodes. Angew. Chem. Int. Ed. 2013, 52, 7282–7285.
- Lum, Y.; Cheng, T.; Goddard, W.A.; Ager, J.W. Electrochemical CO Reduction Builds Solvent Water into Oxygenate Products. J. Am. Chem. Soc. 2018, 140, 9337–9340.
- Yang, K.D.; Lee, C.W.; Jin, K.; Im, S.W.; Nam, K.T. Current Status and Bioinspired Perspective of Electrochemical Conversion of CO2 to a Long-Chain Hydrocarbon. J. Phys. Chem. Lett. 2017, 8, 538–545.
- Kortlever, R.; Shen, J.; Schouten, K.J.; Calle-Vallejo, F.; Koper, M.T. Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082.
- Zhang, J.; Cai, W.; Hu, F.X.; Yang, H.; Liu, B. Recent advances in single atom catalysts for the electrochemical carbon dioxide reduction reaction. Chem. Sci. 2021, 12, 6800–6819.
- Hori, Y.; Takahashi, R.; Yoshinami, Y.; Murata, A. Electrochemical Reduction of CO at a Copper Electrode. J. Phys. Chem. B 1997, 101, 7075–7081.
- Ren, D.; Wong, N.T.; Handoko, A.D.; Huang, Y.; Yeo, B.S. Mechanistic Insights into the Enhanced Activity and Stability of Agglomerated Cu Nanocrystals for the Electrochemical Reduction of Carbon Dioxide to n-Propanol. J. Phys. Chem. Lett. 2016, 7, 20–24.
- Ma, W.; He, X.; Wang, W.; Xie, S.; Zhang, Q.; Wang, Y. Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts. Chem. Soc. Rev. 2021, 50, 12897–12914.
- Montoya, J.H.; Peterson, A.A.; Nørskov, J.K. Insights into C-C Coupling in CO2 Electroreduction on Copper Electrodes. ChemCatChem 2013, 5, 737–742.
- Bagger, A.; Arnarson, L.; Hansen, M.H.; Spohr, E.; Rossmeisl, J. Electrochemical CO Reduction: A Property of the Electrochemical Interface. J. Am. Chem. Soc. 2019, 141, 1506–1514.
- Zheng, W.; Yang, J.; Chen, H.; Hou, Y.; Wang, Q.; Gu, M.; He, F.; Xia, Y.; Xia, Z.; Li, Z.; et al. Atomically Defined Undercoordinated Active Sites for Highly Efficient CO2 Electroreduction. Adv. Funct. Mater. 2020, 30, 1907658.
- Zhang, H.; Li, J.; Xi, S.; Du, Y.; Hai, X.; Wang, J.; Xu, H.; Wu, G.; Zhang, J.; Lu, J.; et al. A Graphene-Supported Single-Atom FeN5 Catalytic Site for Efficient Electrochemical CO2 Reduction. Angew. Chem. Int. Ed. 2019, 58, 14871–14876.
- Yang, H.B.; Hung, S.-F.; Liu, S.; Yuan, K.; Miao, S.; Zhang, L.; Huang, X.; Wang, H.-Y.; Cai, W.; Chen, R.; et al. Atomically dispersed Ni(i) as the active site for electrochemical CO2 reduction. Nat. Energy 2018, 3, 140–147.
- Gu, J.; Hsu, C.-S.; Bai, L.; Chen, H.M.; Hu, X. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 2019, 364, 1091.
- Pei, J.; Wang, T.; Sui, R.; Zhang, X.; Zhou, D.; Qin, F.; Zhao, X.; Liu, Q.; Yan, W.; Dong, J.; et al. N-Bridged Co-N-Ni: New bimetallic sites for promoting electrochemical CO2 reduction. Energy Environ. Sci. 2021, 14, 3019–3028.
- Lin, L.; Li, H.; Yan, C.; Li, H.; Si, R.; Li, M.; Xiao, J.; Wang, G.; Bao, X. Synergistic Catalysis over Iron-Nitrogen Sites Anchored with Cobalt Phthalocyanine for Efficient CO2 Electroreduction. Adv. Mater. 2019, 31, 1903470.
- Zu, X.; Li, X.; Liu, W.; Sun, Y.; Xu, J.; Yao, T.; Yan, W.; Gao, S.; Wang, C.; Wei, S.; et al. Efficient and Robust Carbon Dioxide Electroreduction Enabled by Atomically Dispersed Sn delta+ Sites. Adv. Mater. 2019, 31, 1808135.
- Zhang, E.; Wang, T.; Yu, K.; Liu, J.; Chen, W.; Li, A.; Rong, H.; Lin, R.; Ji, S.; Zhene, X.; et al. Bismuth Single Atoms Resulting from Transformation of Metal-Organic Frameworks and Their Use as Electrocatalysts for CO2 Reduction. J. Am. Chem. Soc. 2019, 141, 16569–16573.
- Nellaiappan, S.; Katiyar, N.K.; Kumar, R.; Parui, A.; Malviya, K.D.; Pradeep, K.G.; Singh, A.K.; Sharma, S.; Tiwary, C.S.; Biswas, K. High-Entropy Alloys as Catalysts for the CO2 and CO Reduction Reactions: Experimental Realization. ACS Catal. 2020, 10, 3658–3663.
- Fan, L.; Liu, C.-Y.; Zhu, P.; Xia, C.; Zhang, X.; Wu, Z.-Y.; Lu, Y.; Senftle, T.P.; Wang, H. Proton sponge promotion of electrochemical CO2 reduction to multi-carbon products. Joule 2022, 6, 205–220.
- Ma, W.; Xie, S.; Liu, T.; Fan, Q.; Ye, J.; Sun, F.; Jiang, Z.; Zhang, Q.; Cheng, J.; Wang, Y. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C–C coupling over fluorine-modified copper. Nat. Catal. 2020, 3, 478–487.
- Xu, H.; Rebollar, D.; He, H.; Chong, L.; Liu, Y.; Liu, C.; Sun, C.-J.; Li, T.; Muntean, J.V.; Winans, R.E.; et al. Highly selective electrocatalytic CO2 reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper. Nat. Energy 2020, 5, 623–632.
- Morales-Guio, C.G.; Cave, E.R.; Nitopi, S.A.; Feaster, J.T.; Wang, L.; Kuhl, K.P.; Jackson, A.; Johnson, N.C.; Abram, D.N.; Hatsukade, T.; et al. Improved CO2 reduction activity towards C2+ alcohols on a tandem gold on copper electrocatalyst. Nat. Catal. 2018, 1, 764–771.
- Li, F.; Li, Y.C.; Wang, Z.; Li, J.; Nam, D.-H.; Lum, Y.; Luo, M.; Wang, X.; Ozden, A.; Hung, S.-F.; et al. Cooperative CO2-to-ethanol conversion via enriched intermediates at molecule–metal catalyst interfaces. Nat. Catal. 2020, 3, 75–82.
- Zhu, Q.; Sun, X.; Yang, D.; Ma, J.; Kang, X.; Zheng, L.; Zhang, J.; Wu, Z.; Han, B. Carbon dioxide electroreduction to C2 products over copper-cuprous oxide derived from electrosynthesized copper complex. Nat. Commun. 2019, 10, 3851.
- Zheng, W.; Wang, Y.; Shuai, L.; Wang, X.; He, F.; Lei, C.; Li, Z.; Yang, B.; Lei, L.; Yuan, C.; et al. Highly Boosted Reaction Kinetics in Carbon Dioxide Electroreduction by Surface-Introduced Electronegative Dopants. Adv. Funct. Mater. 2021, 31, 2008146.
- Zhang, B.; Zhang, J.; Shi, J.; Tan, D.; Liu, L.; Zhang, F.; Lu, C.; Su, Z.; Tan, X.; Cheng, X.; et al. Manganese acting as a high-performance heterogeneous electrocatalyst in carbon dioxide reduction. Nat. Commun. 2019, 10, 2980.
