You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Helena Mylise Sørensen and Version 2 by Dean Liu.
Lactic acid bacteria (LAB) are capable of synthesising metabolites known as exopolysaccharides (EPS) during fermentation. Traditionally, EPS plays an important role in fermented dairy products through their gelling and thickening properties, but they can also be beneficial to human health.
- exopolysaccharides
- lactic acid bacteria
- functional food
- health benefits
1. Introduction
The foods that resesarchwers consume have ascended from being appetizing and nutritious as their primary requirements to now being an active tool used to improve human health through added functionality [1]. Diet is an important factor in general human health and is considered one of the first lines of defence to prevent many diseases including cancer [2], heart disease [3] and osteoporosis [4]. With an increasing interest in the connection between food and health, products characterized as functional foods are growing in popularity.
Functional foods have no universally agreed definition; however, they are often defined as foods that provide a variety of health benefits when consumed. Japan was the first country to recognise functional food as a unique category and defined it as Food for Specified Health Use (FOSHU) [5]. In Europe, the term has been described by The European Commission’s Concerted Action on Functional Food Science in Europe (FuFoSE), coordinated by International Life Science Institute (ILSI):
“A food can be regarded as ‘functional’ if it is satisfactorily demonstrated to affect beneficially one or more target functions in the body, beyond adequate nutritional effects, in a way that is relevant to either an improved state of health and well-being and/or reduction of risk of disease. Functional foods must remain foods and they must demonstrate their effects in amounts that can normally be expected to be consumed in the diet: they are not pills or capsules, but part of a normal food pattern” [6].
Functional food can be classified into four categories based on this definition: conventional foods, modified foods, foods for special dietary requirements and medicinal foods [7]. Whole foods including grains, fish, fruits, and vegetables are examples of conventional foods that naturally include bioactive food components, which also act as a foundation for the next three functional food groups. Foods that have been enhanced, supplemented, or fortified are known as modified foods. Milk enriched with useful components such as minerals and vitamins [8] and bread enriched with folic acid [9] are examples of this category. Foods for special dietary requirements include commercially available foods such as infant formula [10], gluten-free products and lactose-free dairy products to accommodate food intolerances and allergies [7]. The last group of medical foods differ from foods for special dietary requirements by being strictly administered under the consultation and supervision of a physician. These foods are specially formulated to be supplemented/free of one/several compound/s for those of a particular medical disposition (e.g., phenylalanine-free foods for patients with phenylketonuria) [11].
As such, there is growing interest in the characterisation and subsequent implementation of bioactive ingredients into foods in order to provide populations with sustenance which meets medically defined criteria. One novel approach gaining attention is fermentates; a powdered preparation derived from fermentation reactions that can consist of the fermenting microorganisms themselves or metabolites and bioactive components excreted in the fermentation broth.
2. Market Value of Functional Foods
The functional foods market is experiencing large growth in Europe. The market itself is experiencing growing attention with an increase of publications related to functional food every year as well as continuous development of new products released to the market each year [12]. Several health-oriented factors have contributed to this market growth, including an increase in life expectancy, an increase in the cost of health care, and a general focus on nutrition for quality of life and well-being [13].
The lack of definition of functional foods complicates the estimation of the actual market value for functional foods [14]. Euromonitor has estimated the worth of the global functional food market to be USD 177.4 billion in 2021 and expects the market to reach USD 219.5 billion by 2026 (Figure 1). This equates to an annual increase in the market of 4.3–4.5%. The functional food market saw a steep increase between 2020 and 2021 of 5% compared to just 0.3% from 2019 to 2020 (Figure 1) [15]. This is most likely due to the increased focus on health caused by the COVID-19 pandemic but is a trend that appears to continue through the rest of the decade. According to a report made by Euromonitor in 2021, functional food is now one of the top emerging trends among consumers [16].
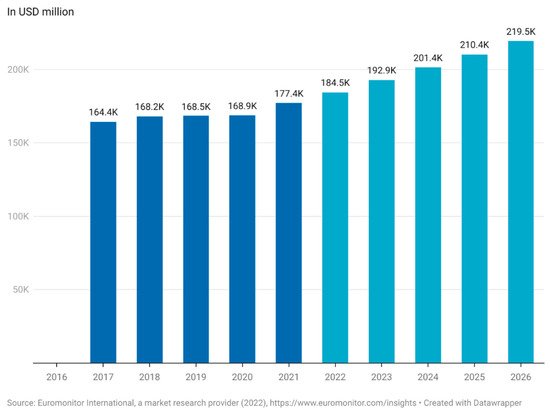
Figure 1. Market size of functional food worldwide (Euromonitor International, a market research provider (2022), (in USD million). This figure was made by the authors with raw data obtained from Euromonitor. Approval for use of data has been obtained from Euromonitor.
The fortification of foodstuffs with vitamin D is an example of functional foods that gained attention during the pandemic, as vitamin D insufficiency has been related to an increased risk of respiratory tract infections [17].
The three most significant regions for functional food sales are the USA, Europe and Japan. As a whole, the European market is less developed than in Japan and the USA due to the historically legislative and regulative frameworks associated with the approval of products in the EU and also a regional distrust of marketed health benefits of processed foods within the region [18]. The UK is the largest market in Europe as of 2021, followed by Germany and France (Figure 2). The market for functional foods is higher in Western Europe than in Eastern Europe, although Eastern Europe has established itself as an emerging market [19]. The estimated market value for the Eastern European market was USD 5213 million while the Western European market had an estimated value of USD 23,033 million. Both markets saw a steep increase between 2020 and 2021 with 6% for Eastern Europe and 7% for Western Europe [15].
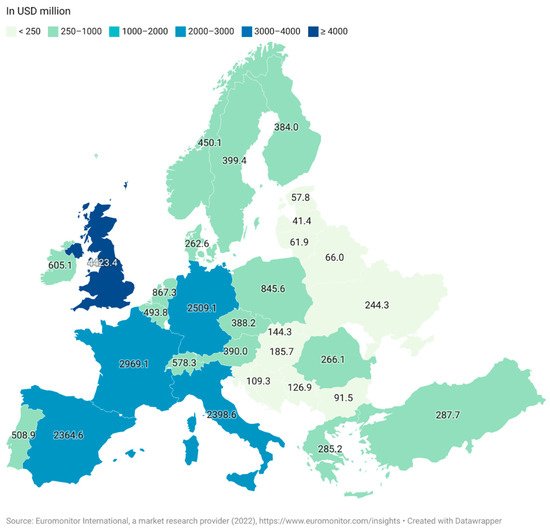
Figure 2. Retail sale value of functional foods in Europe in 2021 (Euromonitor International, a market research provider (2022), (in USD million).
3. Current Commercial and Research Interest
The commercial market of functional foods is complex as it varies with region, but one market of wide interest is the functional dairy market [14]. Dairy products make up a large portion of the market and are estimated to account for 33% of the entire global market [20]. The main reason for this market share is the natural presence of dairy products in a balanced daily diet, as they organically represent a rich source of macromolecular nutrients, vitamins and minerals with known benefits to human health. Adding extra nutritional content to milk-based products simply means modifying or enriching the already naturally healthy base [21]. Milk products (excluding butter) are the fourth largest food group produced worldwide with an annual worldwide production of 857 million tonnes produced, with Asia and Europe being the largest production continents (Figure 3).
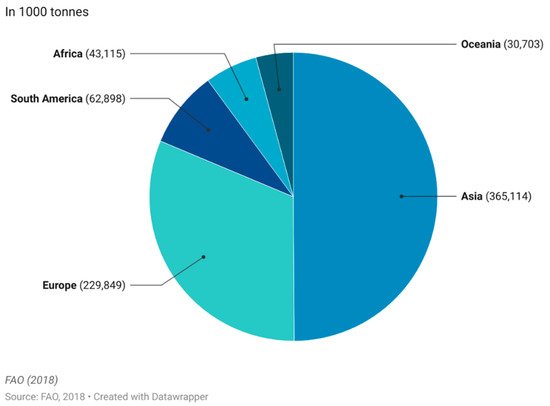
Figure 3. Worldwide production quantity of milk products (excl. butter) in 2018. This figure was made by the authors with raw data from FAO.
The three biggest food categories are sugar cane (1.93 billion tonnes), maize and products (1.13 billion tonnes) and vegetable (other) (960 million tonnes).
One approach to enriching foods is through fermentates which are defined as a powdered preparation derived from fermentation that can consist of either the whole lysed microorganisms themselves, metabolites, or bioactive components also known as postbiotics [22]. Promising postbiotics known as EPS will be explored in this review, including methods for production, isolation purification, and quantification. Several health benefits have been associated with EPS including increased prebiotic activity, improved digestion, immunostimulatory, antiviral, antioxidant, anti-tumoral and cholesterol-lowering properties [23][24][25][26][27][28][23,24,25,26,27,28]. This alludes to the promising potential of LAB-produced EPS as a functional food ingredient. The EPS produced by LAB varies greatly in structure, and understanding these differences is key to assessing the functionality of EPS.
4. Exopolysaccharides as a Functional Food Ingredient
The microbial cell envelope is composed of glycan molecules that are either capsular and linked tightly to the cell surface, or in the form of EPS that can either be loosely attached or excreted into the environment of the cells. The ability to produce and excrete EPS is exhibited by many species of bacteria including LAB, propionibacteria and bifidobacteria during growth [29]. EPS harbours a diverse role in the bacterial environment mostly related to the protection of the cell against environmental factors such as desiccation, pH, osmotic pressure, light, metal ions, bacteriocins, phagocytosis, protozoa and toxic compounds [30][31][32][33][30,31,32,33]. EPS can also function as a carbon source for some organisms, but most bacteria that produce EPS do not have genes encoding EPS degradation and are therefore not capable of catabolising the polysaccharides [34].
When utilized as a functional food ingredient, EPS acts as a postbiotic. Postbiotics are defined as metabolic products released into the fermentation matrix or at cell lysis that can confer health benefits and also includes vitamins, peptides, acids and proteins in addition to EPS [35]. Postbiotics are advantageous over probiotics as a food ingredient, as it not required for the cells to be viable and therefore circumvent some of the processing, storage and shelf-life challenges of probiotics [35][36][35,36]. Prebiotics is another type of food ingredient and is defined by Gibson and Roberfroid (1995) as “a nondigestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, and thus improves host health” [37]. These non-digestible substrates include oligosaccharides, dietary fibres, polyphenols and short-chain fatty acids [38]. In conclusion, prebiotics act as the substrate for the commensal gut microbiome, probiotics constitute the live cell fraction and postbiotics refer to the metabolites excreted by bacteria during fermentation.
When EPS is added to food products, the matrix of the product is affected, resulting in changes to the texture, rheology, and viscosity. The degree and in what way EPS will have an influence on the physiochemical properties of food products will depend on the molecular weight, charge, and structure of the polymer [39][40][39,40]. Therefore, when applied as an additive, EPS has the potential to increase creaminess, viscosity, and thickness, decrease fat content, and replace emulsion, thickening, and stabilizer agents [40]. A study found that with the increasing addition of LAB-derived EPS an increase in the zeta potential of sodium caseinate could be observed. This would indicate the potential for increased stability of dairy products containing sodium caseinate in a dose-dependent manner of EPS [41].
5. Exopolysaccharide Structures and Biosyntheses
The structure of EPS can be divided into two different groups: homopolysaccharides and heteropolysaccharides. The homopolysaccharide type is comprised entirely of a single type of monosaccharide, whereas heteropolysaccharides consist of multiple types of monosaccharides [34]. Owing to these differences, the size profile of each type can be very different with the molecular weight of homopolysaccharides lying within a range of 10 kDa and 6000 kDa, while heteropolysaccharides vary more in molecular weight between 10 kDa and 10,000 kDa [42][43][42,43].
Homopolysaccharides are largely constructed from sucrose, by the polymerization of glucose or fructose, and have been further divided into four subgroups: α-glucans, β-glucan, β-fructans and α-galactan [44]. Both glucan types consist solely of glucose, but their different linkages make it possible to further distinguish their structure. The α-glucan type homopolysaccharide is linked with either α-1,2, α-1,3 α-1,4 or α-1,6 or glycosidic bonds and is classified as dextrans (α-1,6), alternans (1,3 α and α-1,6), mutans (1,3 α and α-1,6) and reuterans (1,4 and α-1,6). While four subtypes of α-glucans are known, only one β-glucan is described which is linked with β-1,2, β-1,3 and β-1,4 glycosidic bonds (Figure 4) [44][45][44,45].
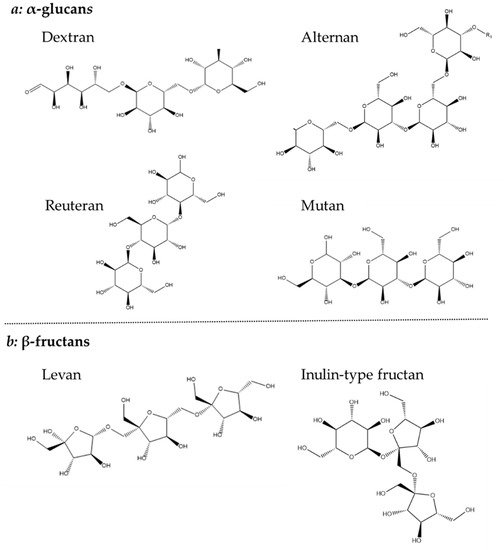
Figure 4. Chemical structure of (a) α-glucans and (b) β-fructans.
The β-fructans contain units of fructose linked with either β-2,1 or β-2,6 and osidic bonds, which are divided into inulin-type (β-2,1) and levan (β-2,6). Finally, the less common β-galactan is comprised of galactose units linked with either β-1,3 or β-1,6 [44]. Both glucans, fructans and galactans are produced by LAB belonging to the genera of Lactobacillus, Streptococcus and Leuconostoc (Figure 4) [34].
Most of the EPS produced by LAB belongs to the group of heteropolysaccharides which are typically composed of 3–8 units of glucose, galactose or rhamnose typically. However, they can also contain other monosaccharides including fructose, mannose, fucose, glucuronic acid and N-acetylglucosides, and additionally isoform-specific modifications to the monosaccharides that comprise them in the form of acetyl and phosphate groups (Figure 5) [45][46][45,46]. In this work, 201 LAB strains were evaluated in terms of heteropolysaccharides production with the following LAB strains evaluated for EPS production: Lactobacillus. delbrueckii subsp. bulgaricus, L. delbrueckii subsp lactis, L. helveticus, L. casei, L. paracasei, L. rhamnosus, Enterococcus. faecalis, E. faecium and Streptococcus. thermophilus [47]. IThis study emphasized the large structural diversity and molecular weight differences (8 kD to 5000 kD) of heteropolysaccharides [47].
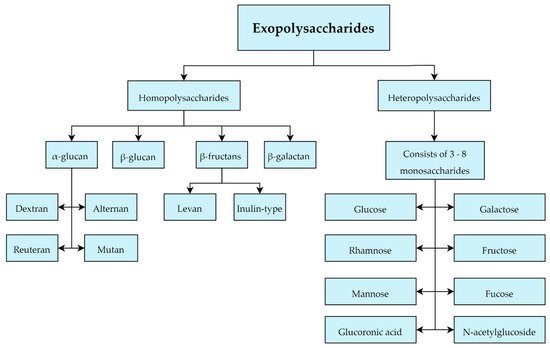
Figure 5. Classification of exopolysaccharides produced by Lactobacillus sp.
The biosynthesis of homopolysaccharides is carried out by extracellular enzymes called glycansucrases that are anchored to the cell wall. These are further divided into glycosyltransferase (GTF) and fructosyltransferase (FTF). GTF catalyzes the transfer of glucose while FTF catalyzes the transfer of fructose to a growing chain of homopolysaccharides. In addition, these enzymes are specific to forming different types of linkages [48] (Figure 6).
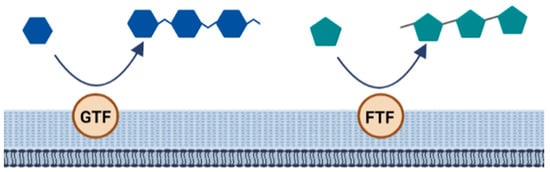
Figure 6. Biosynthesis of homopolysaccharides.
For heteropolysaccharide biosynthesis in LAB, the Wzx/Wzy pathway is utilized which consists of five steps (Figure 7): (1) Saccharides will be transported into the cell and are phosphorylated to either glucose-1-phosphate or glucose-6-phosphate. (2) The sugar nucleotides uridine-diphosphate-glucose (UDP-glucose), UDP-galactose and deoxythymidine-diphospho-rhamnose (dTDP-rhamnose) are formed intracellularly. (3) Individual repeating units are attached to an undecaprenol diphosphate anchor (UDA) that is embedded in the cell membrane and the units are synthesised through several GTFs to form. (4) The Wxz flippase protein translocates the repeating sugar units to the outer membrane. (5) The outer membrane protein Wxy polymerises the sugar units into heteropolysaccharides and they are released into the extracellular environment [43][49][43,49].
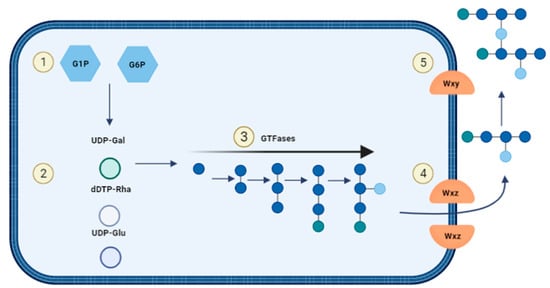
Figure 7. Biosynthesis of heterosaccharides.
6. Industrial Applications
The majority of industrially applied polysaccharides are currently derived from plant or algae sources, with Xanthan being the only significant bacterially derived EPS commercially available polysaccharide, constituting 6% of the global EPS market [50]. Bacterial-derived EPS however have some unique strengths over plant or algae-derived ones. Bacterial EPS can be produced from renewable sources and by producing EPS through controlled fermentation a reproducible, high quality and high titer product can be obtained [51]. These qualities have seen the application and integration of EPS in the food industry with preparations used to improve the stability, rheology and texture of many foodstuffs and beverages. Currently, EPS is used in yoghurt, kefir, cheeses, gluten-free products and cereal-based products either in situ or as an ingredient [32][42][52][53][32,42,52,53]. However, the production of EPS in situ is not always desired in food products, and EPS is well-known for the spoilage of cider and wine [42].
For applications of EPS in situ and as a food ingredient, continuous effort to improve the production process is needed.
7. Legal Status of EPS as a Novel Food Ingredient
There are, to date, 39 LAB species and 5 Bifidobacteria species on the qualified presumption of safety list (QPS list) issued by the European Food Safety Authority (EFSA) that also have generally regarded as safe (GRAS) status. These are of specific importance because they may facilitate easier application in food matrices [54]. However, there is no EFSA or FDA health claim for the use of EPS from lactic acid bacteria in food products. A few microbial EPS are approved by EFSA and include xanthan gum from Xanthomonas campestris, β-glucans from Saccharomyces cerevisiae and chitin from Aspergillus niger while pullulan from Aureobasidium pullulans is currently under investigation to get EFSA approval [55][56][57][58][55,56,57,58]. As several LAB are approved for food use EPS can be used as an ingredient when produced in situ, but to be used as a purified ingredient in the EU several steps would need to be taken to comply with the EFSA regulations on novel food ingredients [59]. Purified EPS falls within this category of novel food ingredients as it has not traditionally been consumed as a food product and will as such need to be approved as safe for human consumption.
