Erythropoietin (EPO) is known as a hormone for erythropoiesis in response to anemia and hypoxia. EPO could interact with its heterodimer receptor (EPOR/βcR) to exert its anti-apoptosis, anti-inflammation and anti-oxidation effects in preventing retinal ganglion cells death through different intracellular signaling pathways.
- erythropoietin
- neuroprotection
- retinal ganglion cell
- optic neuropathy
- optic nerve protection
1. Introduction
2. EPOR: Different Isoforms with Pleiotropic Functions
The structure of EPOR consists of a cytoplasmic domain with 235 amino acids, a single transmembrane domain with 23 amino acids, and an extracellular domain with 225 amino acids [17][10]. There are two subdomains, D1 and D2, in the extracellular domain, both of which are necessary for EPO binding [17][10]. Different isoforms of EPOR have been identified and characterized to have pleiotropic functions:2.1. The Homodimer Isoform: EPOR
2
The homodimer isoform is present in erythroblasts [17][10]. During hematopoiesis, EPO binds to its receptor and results in homodimerization of EPOR (Figure 1) [18][11]. Following binding, Janus kinase-2 (JAK-2) activates several secondary signal molecules [17,19][10][12], such as STAT5 [20][13], MAPK, and PI3-K/Akt [21][14]. The activation of these molecules contributes to the differentiation and maturation of erythroid progenitor cells [22][15].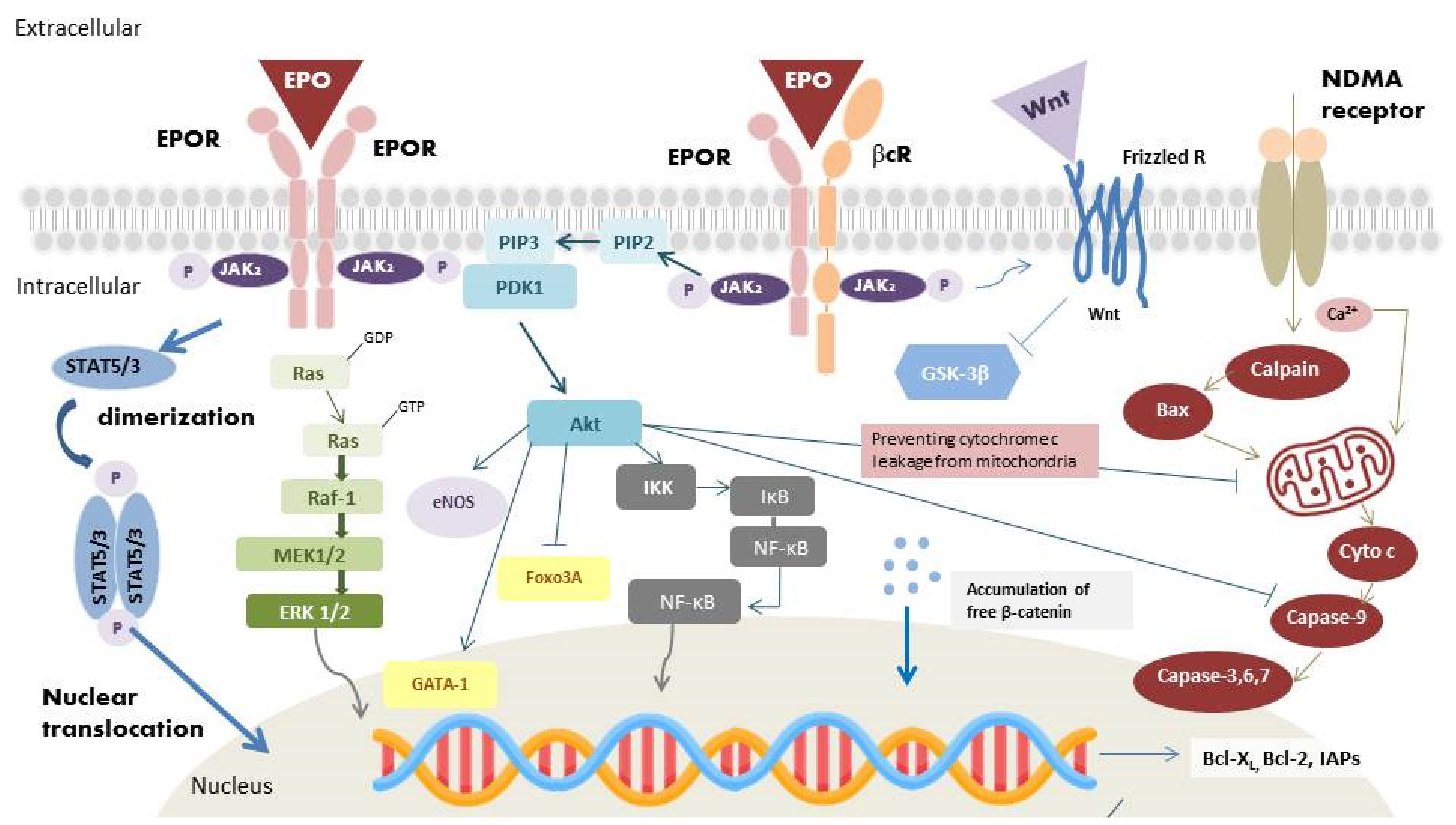
2.2. Heterodimer Isoform: EPOR/βcR
2.3. Extracellular Soluble Isoform: sEPOR
The extracellular soluble isoform of EPOR (sEPOR), which lacks the transmembrane and cytoplasmic domains, is found in human plasma [30][21]. During hypoxia, in contrast to the expression of the full-length form increased through HIF transduction, the expression of the sEPOR is downregulated. In many studies, sEPOR is viewed as an endogenous antagonist of EPO, which blocks the neuroprotective effects of EPO. This form interacts with EPO without further activation of any downstream pathways. Moreover, its binding with EPO restricts the interaction of EPO with other receptor isoforms, resulting in a lower availability and bioactivity of EPO [10,31][22][23].3. Effects of EPO
3.1. Angiogenic Effects
The transcription factor, HIF, has a vital role in hematopoiesis. In normoxic conditions, prolyl hydroxylase domain proteins (PHDs) hydroxylate all HIF-α subunit. After binding to von Hippel–Lindau tumor suppressor protein (VHL), hydroxylated HIF-α is then ubiquitinated. The ubiquitination of hydroxylated HIFs results in its degradation by the proteasome. In hypoxic conditions, the action of PHDs is inhibited. The stabilized HIF-α thus binds to HIF-β and translocates into the nucleus to regulate erythropoiesis via regulation of the expression of the EPO gene, vascular endothelial growth factor (VEGF) [32][24], as well as genes coding for proteins involved in iron metabolism [33][25], which are important for tissue oxygenation. After translation of EPO, the EPO is secreted into the circulation to reach hematopoietic cells. EPO binds with EPOR on erythroid cells, which triggers homodimerization of EPOR and activates the EPOR-associated JAK-2 by autophosphorylation (Figure 1) [17][10]. The active kinase JAK-2 results in the phosphorylation of tyrosine residues on the cytoplasmic portion of the EPOR [17][10]. The phospho-tyrosine residues recruit various proteins, which subsequently activate a series of pathways, including JAK-2/STAT5 (STAT3), PI3-K/Akt, and MAPK pathway [20,21][13][14]. JAK-2 phosphorylates STAT5 or STAT3, once it binds to the cytoplasmic portion of EPOR. STAT5 (STAT3) homodimerizes and translocates into the nucleus as a gene transcription factor. Activating the JAK-2/STAT5 (STAT3) pathway also leads to the upregulation of the antiapoptotic B-cell lymphoma-extra-large (Bcl-XL) protein, therefore protecting proerythroblasts from apoptosis [34][26]. After the activation of Ras by adaptor proteins, initiation of RAF/mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway would occur. RAF-1 protein kinase phosphorylates MEK, which subsequently phosphorylates MAPK/ERK1/2 [35][27]. The last molecules in the cascades translocate into the nucleus and activate various gene transcription factors for erythropoiesis regulation. PI3-K/Akt pathway is one of the main activating signaling pathways. PI3-K could lead to the phosphorylation of Akt, which could activate other proteins involved in erythropoiesis regulation. Akt phosphorylates the transcription factor GATA binding protein-1 (GATA-1), which is an important transcription factor for the anti-apoptotic Bcl-XL expression and erythroid-specific genes. Phosphorylation of GATA-1 could enhance GATA-1 activity in erythroid cell [36][28]. The forkhead box O3A (Foxo3A), another Akt targeted transcription factor, has proapoptotic functions; in contrast, phosphorylation of Foxo3A inhibits its transcriptional activity [37][29]. Figure 1 illustrates the intracellular signaling pathway of EPO. Dysfunction in these signaling pathway leads to abnormal erythropoiesis by disrupting cells proliferation and apoptosis.3.2. Antiapoptotic Effects
Binding of EPO to EPOR results in JAK2 phosphorylation and initiates STAT5 (STAT3), MAPK, PI3-K/Akt and nuclear factor kappa-light-chain-enhancer (NF-κB) downstream pathways, which execute the antiapoptotic effect of EPO (Figure 1) [38][30]. The last molecules in the STAT5 (STAT3) and MAPK pathways could translocate into the nucleus and activate the apoptotic regulators of Bcl-2 family, antiapoptotic Bcl-2 and Bcl-XL, to inhibit apoptosis [39][31]. Activation of PI3-K/Akt pathway also prevents cell apoptosis. Cell death signaling can be initiated by caspases or mitochondrial membrane depolarization. When the mitochondria membrane is depolarized, cytochrome c would be released into the cytoplasm and form the apoptosome complex with apoptotic protease activating factor-1 (Apaf-1) [40][32]. Pro-caspase-9 is activated by the apoptosome, which initiates downstream caspase activation. The activated caspases would cause DNA fragmentation and lead to cell apoptosis [40][32]. Activation of PI3-K/Akt pathway could inhibit caspase activity by preventing cytochrome c leakage from the mitochondria [41][33]. IκB kinase (IKK), another Akt target, is also associated with cell survival. In resting cells, NF-κB is held by the IκB. Activation of the IKK complex phosphorylates IκB, resulting in its ubiquitination and degradation, and in the releases of bound NF-κB. NF-κB exerts its protective effects through the increase in inhibitors of apoptotic protein (IAPs) [42][34], blocking of caspase activity [42][34], suppression of TNF-α related apoptosis [42][34], direct enhancing activation of Bcl-XL, and removal of cellular reactive oxygen species (ROS) [43][35].3.3. Anti-Inflammatory Effects
In inflammatory conditions, EPO was detected at the borders of the injury sites. Hence, the potential anti-inflammatory effect of EPO has also been investigated. EPO was found to decrease pro-inflammatory cytokine production, including intercellular adhesion molecule-1 (ICAM-1) [46][36], interleukin-6 (IL-6) [47][37], and TNF-α [48,49][38][39]. EPO also increased the production of the anti-inflammatory cytokine IL-10 [48][38]. Additionally, EPO could increase endothelial nitric oxide synthase (eNOS) protein expression (Figure 1) [50][40], which increase nitric oxide production. Nitric oxide could increase blood flow, and attenuate regional injury [51][41]. As regards the innate immune system, EPO could facilitate phagocytosis in macrophages [52][42], mediate dendritic cell maturation and immunomodulation [53][43], and reduce inflammation caused by mast cells [54][44].3.4. Antioxidant Effects
EPO has the ability to attenuate oxidative stress, allowing it to be categorized as a cytoprotective agent [59,60][45][46]. EPO could induce heme oxygenase-1 expression via PI3K/Akt pathway [61][47], which could provide a cytoprotective effect in astrocytes [62][48]. EPO also increases the level of glutathione peroxidase, a potent antioxidant protein, which can decrease the toxic activity of ROS [63][49].4. Current Strategy of EPO for Optic Nerve Protection and Repair
Encouraging results of EPO from basic research support the possibility of integrating its therapeutic effects in glaucomatous optic neuropathy, optic neuritis, non-arteritic anterior ischemic optic neuropathy (NAION), and traumatic optic neuropathy (TON). Glaucomatous optic neuropathy, a neurodegenerative disease, is characterized by progressive loss of RGCs. Elevated intraocular pressure (IOP) is considered the most important risk factor of glaucomatous optic neuropathy. However, some patients experienced continued RGCs loss despite good intraocular pressure control, suggesting the presence of other complicated mechanisms stimulating RGC death. Multifactorial mechanisms have been postulated for glaucomatous optic neuropathy, including vascular insufficiency, inflammation [66[50][51],67], excitotoxicity [68][52] and neurotrophic factor withdrawal [69][53]. Due to the complex pathogenesis of glaucoma, EPO was developed to prevent the IOP-independent RGCs loss. Several studies have reported that the EPO level in the aqueous humor increased in patients with glaucoma [70][54]. The cause of the elevated aqueous EPO in glaucomatous eyes might be related to the ischemia, hypoxia, or elevated ROS caused by glaucomatous damage [71][55]. The increase in EPO is identified as a compensatory response due to the presence of glutamate, nitric oxide and the free radicals after the glaucomatous damage [72][56]. EPO is found to have neuroprotective effects regardless of the EPO administration methods. However, the discussion of EPO in the treatment of glaucoma is limited to animal studies. In humans, there are only a few observational studies investigating the correlation between EPO and glaucoma, especially neovascular glaucoma [79,80,81][57][58][59]. To date, human studies using EPO for the treatment of glaucoma are still lacking. Future studies could focus the application of EPO in patients with primary open-angle glaucoma to see if EPO exhibits the same neuroprotective effects in animal experiments. Optic neuritis is another high occurring disease among the world population. For optic neuritis, methylprednisolone is the standard treatment in clinical practices. Although steroid treatment could accelerate visual acuity recovery, recent study demonstrates that steroids could not influence the visual outcome or atrophy of the optic nerve [82][60]. An animal study even demonstrated that methylprednisolone could increase RGCs degeneration by inhibiting the neurotrophin pathway [83][61]. Since EPO has shown multiple neurotrophin-like properties in various neuronal disorders, the efficacy of EPO is evaluated as an add-on therapy to methylprednisolone in autoimmune optic neuritis by investigators. In an experimental autoimmune encephalomyelitis (EAE) rat model, intraperitoneal injection of EPO (5000 U/kg) significantly increased the survivability and functionality of RGCs in rats afflicted with myelin oligodendrocyte glycoprotein (MOG)-induced optic neuritis [84][62]. In the model of MOG-EAE, Sättler et al. concluded that the PI3-K/Akt pathway plays an important role in RGCs survivability under systemic treatment with EPO [84][62]. Establishment of potentially relevant intracellular conduction pathways might make the application of EPO more feasible in MOG-EAE. Human studies have been performed, but the results were not conclusive. Non-arteritic anterior ischemic optic neuropathy is thought to result from vascular insufficiency. Patients with hypertension or obstructive sleep apnea have higher risk of developing NAION since the disease could result in hypoperfusion of the optic nerve. The hypoperfusion causes ischemia and swelling of the axons, thus increasing the pressure on the nervous tissues confined within the tight borders of the posterior scleral outlet. The axon swelling results in further ischemia and neuron swelling. The vicious cycle leads to severe ganglion cells damage. Due to the evidence showing neuroprotection effect of EPO, investigators also determined the efficacy of EPO in NAION. Modarres et al. conducted a prospective interventional case series by intravitreally injecting EPO (2000 IU/0.2 mL) into thirty-one patients within 1 month of the NAION onset [89][63]. For traumatic optic neuropathy, indirect TON is the more common type. The shearing force could lead to small vessel and neuron axon injury around the optic nerve by inducing ischemia, inflammation, and oxidative stress, all of which result in ganglion cell death. Currently, the common treatments are observation, corticosteroids and optic canal decompression. However, none of these managements are proven to be effective. Since EPO has shown to be neuroprotective, EPO might play a role in treating indirect TON. Intravenous EPO was first commenced in patients with indirect TON by Kashkouli et al. in 2011 [92][64]. Indirect TON patients with intravenous EPO (10,000 IU in 3 days) were compared to indirect TON patients without treatment. They found that the EPO-treated group has higher BCVA than that in the observation group. They advocated intravenous EPO may be a new effective and safe treatment in patients with indirect TON [92][64].5. Advances in EPO Derivatives
Epoetin alfa (Epogen), a type of ESA medicine, has been the standard of care for patients with kidney disease and cancer-related anemia. Epoetin alfa-epbx (RetacritTM) shares the same amino acid sequence and similar carbohydrate composition as epoetin alfa (EpogenTM). In 2018, the protein was approved by the FDA, making it the first biosimilar EPO molecules approved in the USA [97][65]. Darbepoetin alfa (DA, Aranesp), an alternative agent of Epoetin alfa and a hyperglycosylated EPO analog, is a novel ESA with two additional N-glycosylation sites accompanied by 22 sialic acid moieties. In the attempt to extend the molecule’s half-life by three-fold longer than EPO in vivo, glycoengineering was conducted to increase the structure’s resistance to degradation. Darbepoetin alfa was approved for treating anemia resulting from renal diseases and cancer chemotherapy. The treatment protocol only requires a once-per-week visit and is accompanied by lower clinical costs [98,99][66][67]. C.E.R.A. (continuous erythropoietin receptor activator), a third-generation ESA, is an EPO (~34 kDa) integrated with methoxypolyethylene glycol (PEG, 30 kDa). Compared with other EPO derivatives, C.E.R.A. has a unique pharmacological profile with the longest half-life and slowest clearance rate. These unique pharmacological properties exist because of methoxypolyethylene glycol (PEG) integration into EPO. Notably, EPO pegylation (the process of connecting a hydrophilic polymer to EPO) significantly prolongs the duration of EPO action, and enhances proteolytic resistance in cell-free plasma [100][68].
Asialerythropoietin (asialoEPO) was evaluated to be a safe drug for clinical treatments. However, asialoEPO’s half-life (t1/2~1.14 min) is much shorter than that of EPO (t1/2~5.6 h). The short half-life gives asialoEPO insufficient persistence time to stimulate hematopoiesis. Based on the above concept, researchers found that chemical modification of the EPO binding sites could abolish erythropoiesis function but retain the tissue-protective effect. Carbamylated EPO (cEpo), a chemically modified derivative of EPO’s lysine residues, was found to act through the heterodimeric EPOR/βcR rather than classical EPOR2 primarily because of the modified structure of cEpo. The sItudy has confirmed that cEpo possesses neuron anti-apoptotic effects similar to EPO but instead does not induce neovascularization [101][69].
6. Advances in EPO Delivery
6.1. Protein-Based Ocular Delivery
Many EPO studies involve frequent injections of ophthalmic proteins via invasive procedures, which might result in a variety of adverse effects and increase the probability of irreversible damage to the patient’s eye. Topical sustain released formulations are non-invasive drugs, that effectively reaches the posterior segment of the eye. According to Silva et al., mucoadhesive polymers such as chitosan and hyaluronic acid can improve the ocular bioavailability of drugs with the support of nanoparticulate delivery systems [106][70]. The formulation was found to be non-cytotoxic toward ARPE-19 and HaCa T cell lines. CS/HA6-rhEPO may be a promising topical formulation after enhancing its bioavailability through different ocular barriers. For the intraocular route of administration, De Julius et al. developed two polymer microparticles, poly (propylene sulfide) (PPS) and poly (lactic-co-glycolic acid) (PLGA), to prolong His-tagged rhEPO-R76E (42kDa) release [107][71]. The rhEPO-R76E was loaded into the polymeric microparticles to prolong in vivo release for at least 28 days to resolve the issue involving short half-life of the rhEPO-R76E (t1/2~13 min). PPS-based microparticles platform is especially promising because it is degradable by ROS. The delivery system provides extended neuroprotection and inherent antioxidant benefits, which reinforces its ability in ocular delivery of EPO.6.2. Gene-Based Ocular Delivery
Under the gene delivery approaches, EPO has significant therapeutic potential in neurodegenerative diseases due to its neuroprotective effects. However, recombinant EPO is limited in clinical treatment of glaucoma patients due to its short half-life. As regards this issue, Bond et al. constructed a viral gene delivery system for EPO-R76E [108][72]. Treatment with recombinant adeno-associated virus (rAAV) provides sustainable, long-term delivery of EPO-R76E without a critical rise in hematocrit [108,109][72][73]. AAV-mediated long-term EPO expression is achievable in animal models with the primary functions of promoting red blood cells proliferation and neuroprotection. Another challenge in applying gene therapy in humans is the improvement of drug selectivity. For systemically secreted hormone, such as EPO, it is vital to use an inducible genetic delivery system to avoid excess expression and side effects. However, precise expression control is highly desirable when maintaining steady-state red blood cell counts within a narrow therapeutic window. Hines-Beard et al. packaged EPOR76E into a recombinant adeno-associated viral vector under the control of the tetracycline inducible promoter [110][74]. In the retina, tetracycline-controlled expression of green fluoresce protein (GFP) in retinal pigmented epithelium and photoreceptor cells becomes apparent in rats following subretinal injections of rAAV-2/2 vector. The outer nuclear layer in the eyes was approximately 8 μm thicker in mice that received doxycycline water as compared to the control groups.6.3. Surface Receptor-Targeted Ocular Delivery
Through surface receptor-targeted delivery results, most suggest that the tissue-protective effect of EPO and injury response are mediated by the EPOR/βcR heterodimer and not by the EPOR homodimer [112][75]. In addition to some well-known derivatives of EPO with better affinity for the EPOR/βcR heterodimer, such as asialoEPO, cEpo and HBSP (see above), traptamers of transmembrane domain (TMD) proteins of EPOR/βcR are also an option. He et al. constructed ELI-3 traptamer that specifically targets the TMD of human EPOR and triggers cooperative JAK/STAT signaling for proliferation and tissue protection [113][76].6.4. Cell-Based Ocular Delivery
Mesenchymal stem cell (MSC) therapy is potential in treating optic neuropathies. MSCs have demonstrated to possess neuroprotective effects in numerous neurodegenerative diseases while maintaining retinal morphology [114,115][77][78]. They could also regulate inflammatory responses [116][79] and increase the secretion of neurotrophic factors [117][80]. Furthermore, they could transdifferentiate into retinal progenitor cells [118][81]. MSCs could be the vector of EPO because these cells could cross the brain-retinal barrier and localize into the inflamed sites [121][82]. The phenomenon emphasizes a mutualistic relationship between MSCs and EPO. Hence, some investigators attempt to evaluate the efficacy of EPO-expressing MSC in treating retinal degenerative diseases. Ding et al. transduced MSCs with lentiviral particles encoding EPO. They found that co-treatment with EPO and MSCs instead of only with MSC could attenuate human retinal neuron apoptosis by restoring the mitochondrial membrane potential and protect human retinal neurons from glutamate neurotoxicity [122][83]. The ability for EPO to synchronize with MSCs may become beneficial in producing medical protocols involving the treatment of patients with glaucomatous optic neuropathy. Given their endogenous long-term therapeutic effects, MSCs-based therapy could be the future direction in optic nerve repair.References
- Annese, T.; Tamma, R.; Ruggieri, S.; Ribatti, D. Erythropoietin in tumor angiogenesis. Exp. Cell Res. 2019, 374, 266–273.
- Tirpe, A.A.; Gulei, D.; Ciortea, S.M.; Crivii, C.; Berindan-Neagoe, I. Hypoxia: Overview on hypoxia-mediated mechanisms with a focus on the role of HIF genes. Int. J. Mol. Sci. 2019, 20, 6140.
- Yasuoka, Y.; Fukuyama, T.; Izumi, Y.; Nakayama, Y.; Inoue, H.; Yanagita, K.; Oshima, T.; Yamazaki, T.; Uematsu, T.; Kobayashi, N.; et al. Erythropoietin production by the kidney and the liver in response to severe hypoxia evaluated by Western blotting with deglycosylation. Physiol. Rep. 2020, 8, e14485.
- Kimáková, P.; Solár, P.; Solárová, Z.; Komel, R.; Debeljak, N. Erythropoietin and its angiogenic activity. Int. J. Mol. Sci. 2017, 18, 1519.
- Ostrowski, D.; Heinrich, R. Alternative erythropoietin receptors in the nervous system. J. Clin. Med. 2018, 7, 24.
- Klopsch, C.; Skorska, A.; Ludwig, M.; Lemcke, H.; Maass, G.; Gaebel, R.; Beyer, M.; Lux, C.; Toelk, A.; Müller, K.; et al. Intramyocardial angiogenetic stem cells and epicardial erythropoietin save the acute ischemic heart. Dis. Models Mech. 2018, 11, dmm033282.
- Bretz, C.A.; Ramshekar, A.; Kunz, E.; Wang, H.; Hartnett, M.E. Signaling through the erythropoietin receptor affects angiogenesis in retinovascular disease. Investig. Ophthalmol. Vis. Sci. 2020, 61, 23.
- Samson, F.P.; He, W.; Sripathi, S.R.; Patrick, A.T.; Madu, J.; Chung, H.; Frost, M.C.; Jee, D.; Gutsaeva, D.R.; Jahng, W.J. Dual switch mechanism of erythropoietin as an antiapoptotic and pro-angiogenic determinant in the retina. ACS Omega 2020, 5, 21113–21126.
- García-Ramírez, M.; Hernández, C.; Simó, R. Expression of erythropoietin and its receptor in the human retina: A comparative study of diabetic and nondiabetic subjects. Diabetes Care 2008, 31, 1189–1194.
- Constantinescu, S.N.; Ghaffari, S.; Lodish, H.F. The erythropoietin receptor: Structure, activation and intracellular signal transduction. Trends Endocrinol. Metab. TEM 1999, 10, 18–23.
- Watowich, S.S.; Hilton, D.J.; Lodish, H.F. Activation and inhibition of erythropoietin receptor function: Role of receptor dimerization. Mol. Cell. Biol. 1994, 14, 3535–3549.
- Kim, A.R.; Ulirsch, J.C.; Wilmes, S.; Unal, E.; Moraga, I.; Karakukcu, M.; Yuan, D.; Kazerounian, S.; Abdulhay, N.J.; King, D.S.; et al. Functional selectivity in cytokine signaling revealed through a pathogenic EPO mutation. Cell 2017, 168, 1053–1064.
- Tóthová, Z.; Tomc, J.; Debeljak, N.; Solár, P. STAT5 as a key protein of erythropoietin signalization. Int. J. Mol. Sci. 2021, 22, 7109.
- Tóthová, Z.; Šemeláková, M.; Solárová, Z.; Tomc, J.; Debeljak, N.; Solár, P. The role of PI3K/AKT and MAPK signaling pathways in erythropoietin signalization. Int. J. Mol. Sci. 2021, 22, 7682.
- Sanghera, K.P.; Mathalone, N.; Baigi, R.; Panov, E.; Wang, D.; Zhao, X.; Hsu, H.; Wang, H.; Tropepe, V.; Ward, M.; et al. The PI3K/Akt/mTOR pathway mediates retinal progenitor cell survival under hypoxic and superoxide stress. Mol. Cell. Neurosci. 2011, 47, 145–153.
- Masuda, S.; Nagao, M.; Takahata, K.; Konishi, Y.; Gallyas, F., Jr.; Tabira, T.; Sasaki, R. Functional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cells. J. Biol. Chem. 1993, 268, 11208–11216.
- Kebschull, L.; Theilmann, L.F.C.; Mohr, A.; Uennigmann, W.; Stoeppeler, S.; Heitplatz, B.; Spiegel, H.U.; Bahde, R.; Palmes, D.M.; Becker, F. EPOR2/βcR2-independendent effects of low-dose epoetin-α in porcine liver transplantation. Biosci. Rep. 2017, 37, BSR20171007.
- Jubinsky, P.T.; Krijanovski, O.I.; Nathan, D.G.; Tavernier, J.; Sieff, C.A. The beta chain of the interleukin-3 receptor functionally associates with the erythropoietin receptor. Blood 1997, 90, 1867–1873.
- Peng, B.; Kong, G.; Yang, C.; Ming, Y. Erythropoietin and its derivatives: From tissue protection to immune regulation. Cell Death Dis. 2020, 11, 79.
- Colella, P.; Iodice, C.; Di Vicino, U.; Annunziata, I.; Surace, E.M.; Auricchio, A. Non-erythropoietic erythropoietin derivatives protect from light-induced and genetic photoreceptor degeneration. Hum. Mol. Genet. 2011, 20, 2251–2262.
- Vizcardo-Galindo, G.; León-Velarde, F.; Villafuerte, F.C. High-altitude hypoxia decreases plasma erythropoietin soluble receptor concentration in lowlanders. High Alt. Med. Biol. 2020, 21, 92–98.
- Dreixler, J.C.; Hagevik, S.; Hemmert, J.W.; Shaikh, A.R.; Rosenbaum, D.M.; Roth, S. Involvement of erythropoietin in retinal ischemic preconditioning. Anesthesiology 2009, 110, 774–780.
- Khankin, E.V.; Mutter, W.P.; Tamez, H.; Yuan, H.T.; Karumanchi, S.A.; Thadhani, R. Soluble erythropoietin receptor contributes to erythropoietin resistance in end-stage renal disease. PLoS ONE 2010, 5, e9246.
- Zhang, D.; Lv, F.L.; Wang, G.H. Effects of HIF-1α on diabetic retinopathy angiogenesis and VEGF expression. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5071–5076.
- Ogawa, C.; Tsuchiya, K.; Tomosugi, N.; Maeda, K. A hypoxia-inducible factor stabilizer improves hematopoiesis and iron metabolism early after administration to treat anemia in hemodialysis patients. Int. J. Mol. Sci. 2020, 21, 7153.
- Socolovsky, M.; Nam, H.; Fleming, M.D.; Haase, V.H.; Brugnara, C.; Lodish, H.F. Ineffective erythropoiesis in Stat5a(-/-)5b(-/-) mice due to decreased survival of early erythroblasts. Blood 2001, 98, 3261–3273.
- Tu, P.S.; Lin, E.C.; Chen, H.W.; Chen, S.W.; Lin, T.A.; Gau, J.P.; Chang, Y.I. The extracellular signal-regulated kinase 1/2 modulates the intracellular localization of DNA methyltransferase 3A to regulate erythrocytic differentiation. Am. J. Transl. Res. 2020, 12, 1016–1030.
- Dai, T.Y.; Lan, J.J.; Gao, R.L.; Zhao, Y.N.; Yu, X.L.; Liang, S.X.; Liu, W.B.; Sun, X. Panaxdiol saponins component promotes hematopoiesis by regulating GATA transcription factors of intracellular signaling pathway in mouse bone marrow. Ann. Transl. Med. 2022, 10, 38.
- Das, T.P.; Suman, S.; Alatassi, H.; Ankem, M.K.; Damodaran, C. Inhibition of AKT promotes FOXO3a-dependent apoptosis in prostate cancer. Cell Death Dis. 2016, 7, e2111.
- Digicaylioglu, M.; Lipton, S.A. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature 2001, 412, 641–647.
- Shen, J.; Wu, Y.; Xu, J.Y.; Zhang, J.; Sinclair, S.H.; Yanoff, M.; Xu, G.; Li, W.; Xu, G.T. ERK- and Akt-dependent neuroprotection by erythropoietin (EPO) against glyoxal-AGEs via modulation of Bcl-xL, Bax, and BAD. Investig. Ophthalmol. Vis. Sci. 2010, 51, 35–46.
- Chong, Z.Z.; Kang, J.Q.; Maiese, K. Apaf-1, Bcl-xL, cytochrome c, and caspase-9 form the critical elements for cerebral vascular protection by erythropoietin. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2003, 23, 320–330.
- Wang, Z.Y.; Shen, L.J.; Tu, L.; Hu, D.N.; Liu, G.Y.; Zhou, Z.L.; Lin, Y.; Chen, L.H.; Qu, J. Erythropoietin protects retinal pigment epithelial cells from oxidative damage. Free Radic. Biol. Med. 2009, 46, 1032–1041.
- Wang, C.Y.; Mayo, M.W.; Korneluk, R.G.; Goeddel, D.V.; Baldwin, A.S., Jr. NF-kappaB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 1998, 281, 1680–1683.
- Chen, C.; Edelstein, L.C.; Gélinas, C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L). Mol. Cell. Biol. 2000, 20, 2687–2695.
- Kwak, J.; Kim, J.H.; Jang, H.N.; Jung, M.H.; Cho, H.S.; Chang, S.H.; Kim, H.J. Erythropoietin ameliorates ischemia/reperfusion-induced acute kidney injury via inflammasome suppression in mice. Int. J. Mol. Sci. 2020, 21, 3453.
- Gong, Q.; Zeng, J.; Zhang, X.; Huang, Y.; Chen, C.; Quan, J.; Ling, J. Effect of erythropoietin on angiogenic potential of dental pulp cells. Exp. Ther. Med. 2021, 22, 1079.
- Lin, X.; Ma, X.; Cui, X.; Zhang, R.; Pan, H.; Gao, W. Effects of erythropoietin on lung injury induced by cardiopulmonary bypass after cardiac surgery. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e920039.
- Cui, J.; Zhang, F.; Cao, W.; Wang, Y.; Liu, J.; Liu, X.; Chen, T.; Li, L.; Tian, J.; Yu, B. Erythropoietin alleviates hyperglycaemia-associated inflammation by regulating macrophage polarization via the JAK2/STAT3 signalling pathway. Mol. Immunol. 2018, 101, 221–228.
- Elshiekh, M.; Kadkhodaee, M.; Seifi, B.; Ranjbaran, M.; Askari, H. Up-regulation of nitric oxide synthases by erythropoietin alone or in conjunction with ischemic preconditioning in ischemia reperfusion injury of rat kidneys. Gen. Physiol. Biophys. 2017, 36, 281–288.
- Cruz Navarro, J.; Pillai, S.; Ponce, L.L.; Van, M.; Goodman, J.C.; Robertson, C.S. Endothelial nitric oxide synthase mediates the cerebrovascular effects of erythropoietin in traumatic brain injury. Front. Immunol. 2014, 5, 494.
- Govindappa, P.K.; Elfar, J.C. Erythropoietin promotes M2 macrophage phagocytosis of Schwann cells in peripheral nerve injury. Cell Death Dis. 2022, 13, 245.
- Einwächter, H.; Heiseke, A.; Schlitzer, A.; Gasteiger, G.; Adler, H.; Voehringer, D.; Manz, M.G.; Ruzsics, Z.; Dölken, L.; Koszinowski, U.H.; et al. The innate immune response to infection induces erythropoietin-dependent replenishment of the dendritic cell compartment. Front. Immunol. 2020, 11, 1627.
- Korkmaz, T.; Kahramansoy, N.; Kilicgun, A.; Firat, T. The effect of erythropoietin to pulmonary injury and mast cells secondary to acute pancreatitis. BMC Res. Notes 2014, 7, 267.
- Shokrzadeh, M.; Etebari, M.; Ghassemi-Barghi, N. An engineered non-erythropoietic erythropoietin-derived peptide, ARA290, attenuates doxorubicin induced genotoxicity and oxidative stress. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2020, 66, 104864.
- Dang, J.Z.; Tu, Y.F.; Wang, J.; Yang, Y.J. Carbamylated erythropoietin alleviates kidney damage in diabetic rats by suppressing oxidative stress. Curr. Med. Sci. 2021, 41, 513–521.
- Salinas, M.; Wang, J.; Rosa de Sagarra, M.; Martín, D.; Rojo, A.I.; Martin-Perez, J.; Ortiz de Montellano, P.R.; Cuadrado, A. Protein kinase Akt/PKB phosphorylates heme oxygenase-1 in vitro and in vivo. FEBS Lett. 2004, 578, 90–94.
- Diaz, Z.; Assaraf, M.I.; Miller, W.H., Jr.; Schipper, H.M. Astroglial cytoprotection by erythropoietin pre-conditioning: Implications for ischemic and degenerative CNS disorders. J. Neurochem. 2005, 93, 392–402.
- Thompson, A.M.; Farmer, K.; Rowe, E.M.; Hayley, S. Erythropoietin modulates striatal antioxidant signalling to reduce neurodegeneration in a toxicant model of Parkinson’s disease. Mol. Cell. Neurosci. 2020, 109, 103554.
- Pulukool, S.K.; Bhagavatham, S.K.S.; Kannan, V.; Sukumar, P.; Dandamudi, R.B.; Ghaisas, S.; Kunchala, H.; Saieesh, D.; Naik, A.A.; Pargaonkar, A.; et al. Elevated dimethylarginine, ATP, cytokines, metabolic remodeling involving tryptophan metabolism and potential microglial inflammation characterize primary open angle glaucoma. Sci. Rep. 2021, 11, 9766.
- Krishnan, A.; Kocab, A.J.; Zacks, D.N.; Marshak-Rothstein, A.; Gregory-Ksander, M. A small peptide antagonist of the Fas receptor inhibits neuroinflammation and prevents axon degeneration and retinal ganglion cell death in an inducible mouse model of glaucoma. J. Neuroinflamm. 2019, 16, 184.
- Li, Q.; Jin, R.; Zhang, S.; Sun, X.; Wu, J. Group II metabotropic glutamate receptor agonist promotes retinal ganglion cell survival by reducing neuronal excitotoxicity in a rat chronic ocular hypertension model. Neuropharmacology 2020, 170, 108016.
- Cha, Y.W.; Kim, S.T. Serum and aqueous humor levels of brain-derived neurotrophic factor in patients with primary open-angle glaucoma and normal-tension glaucoma. Int. Ophthalmol. 2021, 41, 3869–3875.
- Mokbel, T.H.; Ghanem, A.A.; Kishk, H.; Arafa, L.F.; El-Baiomy, A.A. Erythropoietin and soluble CD44 levels in patients with primary open-angle glaucoma. Clin. Exp. Ophthalmol. 2010, 38, 560–565.
- Arjamaa, O.; Nikinmaa, M. Oxygen-dependent diseases in the retina: Role of hypoxia-inducible factors. Exp. Eye Res. 2006, 83, 473–483.
- Kawakami, M.; Sekiguchi, M.; Sato, K.; Kozaki, S.; Takahashi, M. Erythropoietin receptor-mediated inhibition of exocytotic glutamate release confers neuroprotection during chemical ischemia. J. Biol. Chem. 2001, 276, 39469–39475.
- Zhou, M.; Chen, S.; Wang, W.; Huang, W.; Cheng, B.; Ding, X.; Zhang, X. Levels of erythropoietin and vascular endothelial growth factor in surgery-required advanced neovascular glaucoma eyes before and after intravitreal injection of bevacizumab. Investig. Opthalmol. Vis. Sci. 2013, 54, 3874–3879.
- Sun, Y.; Zhao, H.; Shen, Y.; Guan, W. Comparison of erythropoietin, semaphorins 3A and pigment epithelium derived factor levels in serum and aqueous humor of patients with neovascular glaucoma and cataract. J. Coll. Phys. Surg. Pak. JCPSP 2019, 29, 900–901.
- Watanabe, D.; Suzuma, K.; Matsui, S.; Kurimoto, M.; Kiryu, J.; Kita, M.; Suzuma, I.; Ohashi, H.; Ojima, T.; Murakami, T.; et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N. Engl. J. Med. 2005, 353, 782–792.
- Mackay, D.D. Should patients with optic neuritis be treated with steroids? Curr. Opin. Ophthalmol. 2015, 26, 439–444.
- Diem, R.; Hobom, M.; Maier, K.; Weissert, R.; Storch, M.K.; Meyer, R.; Bähr, M. Methylprednisolone increases neuronal apoptosis during autoimmune CNS inflammation by inhibition of an endogenous neuroprotective pathway. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 6993–7000.
- Sättler, M.B.; Merkler, D.; Maier, K.; Stadelmann, C.; Ehrenreich, H.; Bähr, M.; Diem, R. Neuroprotective effects and intracellular signaling pathways of erythropoietin in a rat model of multiple sclerosis. Cell Death Differ. 2004, 11 (Suppl. 2), S181–S192.
- Modarres, M.; Falavarjani, K.G.; Nazari, H.; Sanjari, M.S.; Aghamohammadi, F.; Homaii, M.; Samiy, N. Intravitreal erythropoietin injection for the treatment of non-arteritic anterior ischaemic optic neuropathy. Br. J. Ophthalmol. 2011, 95, 992–995.
- Kashkouli, M.B.; Pakdel, F.; Sanjari, M.S.; Haghighi, A.; Nojomi, M.; Homaee, M.H.; Heirati, A. Erythropoietin: A novel treatment for traumatic optic neuropathy-a pilot study. Graefe’s Archive Clin. Exp. Ophthalmol. 2011, 249, 731–736.
- Anand, S.; Al-Mondhiry, J.; Fischer, K.; Glaspy, J. Epoetin alfa-epbx: A new entrant into a crowded market. a historical review of the role of erythropoietin stimulating agents and the development of the first epoetin biosimilar in the United States. Expert Rev. Clin. Pharmacol. 2021, 14, 1–8.
- Lee, D.E.; Son, W.; Ha, B.J.; Oh, M.S.; Yoo, O.J. The prolonged half-lives of new erythropoietin derivatives via peptide addition. Biochem. Biophys. Res. Commun. 2006, 339, 380–385.
- Powell, J.; Gurk-Turner, C. Darbepoetin alfa (Aranesp). Bayl. Univ. Med. Cent. Proc. 2002, 15, 332–335.
- Aizawa, K.; Kawasaki, R.; Tashiro, Y.; Hirata, M.; Endo, K.; Shimonaka, Y. Epoetin beta pegol, but not recombinant erythropoietin, retains its hematopoietic effect in vivo in the presence of the sialic acid-metabolizing enzyme sialidase. Int. J. Hematol. 2016, 104, 182–189.
- Liu, X.; Zhu, B.; Zou, H.; Hu, D.; Gu, Q.; Liu, K.; Xu, X. Carbamylated erythropoietin mediates retinal neuroprotection in streptozotocin-induced early-stage diabetic rats. Graefe’s Archive Clin. Exp. Ophthalmol. 2015, 253, 1263–1272.
- Silva, B.; Marto, J.; Braz, B.S.; Delgado, E.; Almeida, A.J.; Gonçalves, L. New nanoparticles for topical ocular delivery of erythropoietin. Int. J. Pharm. 2020, 576, 119020.
- DeJulius, C.R.; Bernardo-Colón, A.; Naguib, S.; Backstrom, J.R.; Kavanaugh, T.; Gupta, M.K.; Duvall, C.L.; Rex, T.S. Microsphere antioxidant and sustained erythropoietin-R76E release functions cooperate to reduce traumatic optic neuropathy. J. Control. Release Off. J. Control. Release Soc. 2021, 329, 762–773.
- Bond, W.S.; Hines-Beard, J.; GoldenMerry, Y.L.; Davis, M.; Farooque, A.; Sappington, R.M.; Calkins, D.J.; Rex, T.S. Virus-mediated EpoR76E therapy slows optic nerve axonopathy in experimental glaucoma. Mol. Ther. J. Am. Soc. Gene Ther. 2016, 24, 230–239.
- Tao, Y.; Zhu, Q.; Wang, L.; Zha, X.; Teng, D.; Xu, L. Adeno-associated virus (AAV)-mediated neuroprotective effects on the degenerative retina: The therapeutic potential of erythropoietin. Fundam. Clin. Pharmacol. 2020, 34, 131–147.
- Hines-Beard, J.; Desai, S.; Haag, R.; Esumi, N.; D’Surney, L.; Parker, S.; Richardson, C.; Rex, T.S. Identification of a therapeutic dose of continuously delivered erythropoietin in the eye using an inducible promoter system. Curr. Gene Ther. 2013, 13, 275–281.
- Bohr, S.; Patel, S.J.; Vasko, R.; Shen, K.; Iracheta-Vellve, A.; Lee, J.; Bale, S.S.; Chakraborty, N.; Brines, M.; Cerami, A.; et al. Modulation of cellular stress response via the erythropoietin/CD131 heteroreceptor complex in mouse mesenchymal-derived cells. J. Mol. Med. 2015, 93, 199–210.
- He, L.; Cohen, E.B.; Edwards, A.P.B.; Xavier-Ferrucio, J.; Bugge, K.; Federman, R.S.; Absher, D.; Myers, R.M.; Kragelund, B.B.; Krause, D.S.; et al. Transmembrane protein aptamer induces cooperative signaling by the EPO receptor and the cytokine receptor β-common subunit. iScience 2019, 17, 167–181.
- Liu, W.; Rong, Y.; Wang, J.; Zhou, Z.; Ge, X.; Ji, C.; Jiang, D.; Gong, F.; Li, L.; Chen, J.; et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J. Neuroinflamm. 2020, 17, 47.
- Kim, J.; Lee, Y.; Lee, S.; Kim, K.; Song, M.; Lee, J. Mesenchymal stem cell therapy and alzheimer’s disease: Current status and future perspectives. J. Alzheimer’s Dis. JAD 2020, 77, 1–14.
- Luque-Campos, N.; Contreras-López, R.A.; Jose Paredes-Martínez, M.; Torres, M.J.; Bahraoui, S.; Wei, M.; Espinoza, F.; Djouad, F.; Elizondo-Vega, R.J.; Luz-Crawford, P. Mesenchymal stem cells improve rheumatoid arthritis progression by controlling memory T cell response. Front. Immunol. 2019, 10, 798.
- Jiang, Y.; Gao, H.; Yuan, H.; Xu, H.; Tian, M.; Du, G.; Xie, W. Amelioration of postoperative cognitive dysfunction in mice by mesenchymal stem cell-conditioned medium treatments is associated with reduced inflammation, oxidative stress and increased BDNF expression in brain tissues. Neurosci. Lett. 2019, 709, 134372.
- Hu, Y.; Liang, J.; Cui, H.; Wang, X.; Rong, H.; Shao, B.; Cui, H. Wharton’s jelly mesenchymal stem cells differentiate into retinal progenitor cells. Neural Regen. Res. 2013, 8, 1783–1792.
- Omoto, M.; Katikireddy, K.R.; Rezazadeh, A.; Dohlman, T.H.; Chauhan, S.K. Mesenchymal stem cells home to inflamed ocular surface and suppress allosensitization in corneal transplantation. Investig. Opthalmol. Vis. Sci. 2014, 55, 6631–6638.
- Shirley Ding, S.L.; Kumar, S.; Ali Khan, M.S.; Ling Mok, P. Human mesenchymal stem cells expressing erythropoietin enhance survivability of retinal neurons against oxidative stress: An in vitro study. Front. Cell. Neurosci. 2018, 12, 190.
