Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 3 by Theodoros Varzakas.
Mycotoxins are secondary metabolites produced by fungi that infect a wide range of foods worldwide. Nivalenol (NIV), a type B trichothecene produced by numerous Fusarium species, has the ability to infect a variety of foods both in the field and during post-harvest handling and management. NIV is frequently found in cereal and cereal-based goods, and its strong cytotoxicity poses major concerns for both human and animal health.
- nivalenol
- food contamination
- human and animal health
1. Introduction
Mycotoxins, which are secondary metabolites produced by many fungi, can be detrimental to both human and animal’s health when ingested through contaminated food [1][2][3][4][5][6]. The contamination of crops and their processed products is a major public health and economic concern. More than 80% of the agricultural produce is contaminated by at least one mycotoxin. Several genera of fungi, including Cephalosporium, Cyclindrocarpon, Myrothecium, Phomopsis, Stachybotrys, Trichoderma, Trichotecium, and Verticimonosporium species, are mainly responsible for the mycotoxin production among which the trichothecene group of mycotoxins share a major proportion, and hence pose wider concerns [7]. The trichothecene group of mycotoxins are categorized as type A, B, C, and D, which includes more than 170 different toxins, but their toxicity potential depends upon the type of mycotoxin being produced by the fungi [8].
Nivalenol (NIV) is one of the major mycotoxins, produced by several Fusarium species and belongs to the type B trichothecene. It is a natural contaminant found worldwide in various foodstuffs. Due to the potent cytotoxicity, NIV constitutes a serious health risk to both humans and animals. Its contamination is observed very frequently in agricultural products. Crops, such as wheat, barley, and corn, are usually contaminated with NIV, as these crops are more prone to the growth of NIV-producing fungal species [9][10][11]. Furthermore, NIV is also resistant against food processing conditions, thereby leading to the further spread of NIV-induced mycotoxicosis in consumers [12]. Controlling NIV exposure is especially important because of how frequently it contaminates foods and how easily it can harm mammals through immunotoxicity and hemotoxicity [13]. NIV can produce certain complications, such as inhibition of cell proliferation, induction of CXCL8/interleukin (IL) -8 secretions, and the involvement of stress-activated mitogen-activated protein kinases [14][15][16].
Chemically, the structure of NIV is very similar to DON (deoxynivalenol) mycotoxin of the type B trichothecene group. The only difference between NIV and DON is one oxygen atom at the C-4 position (Figure 1); however, the toxicity of these oxides of carbon completely differs from each other. The co-occurrence of NIV and DON has been reported extensively, but the oxidative stress and toxicity induced by NIV contamination are higher than that described for DON [7][12]. Several studies have reported the contamination of NIV in foods, including wheat [17][18], barley [19][20], groundnuts [21], maize and their by-products [22][23][24], but the level of contamination varies with the type of crop and ecological factors, such as temperature and humidity. Magan et al. [25] reported that environmental factors, including farm practices, handling, storage, processing, and distribution of food grains, largely influence the spread of NIV contamination. Therefore, the knowledge of climatic conditions on the growth of Fusarium species, along with its behavior and mycotoxin production, is essential to control the risk of NIV contamination in crops [26].
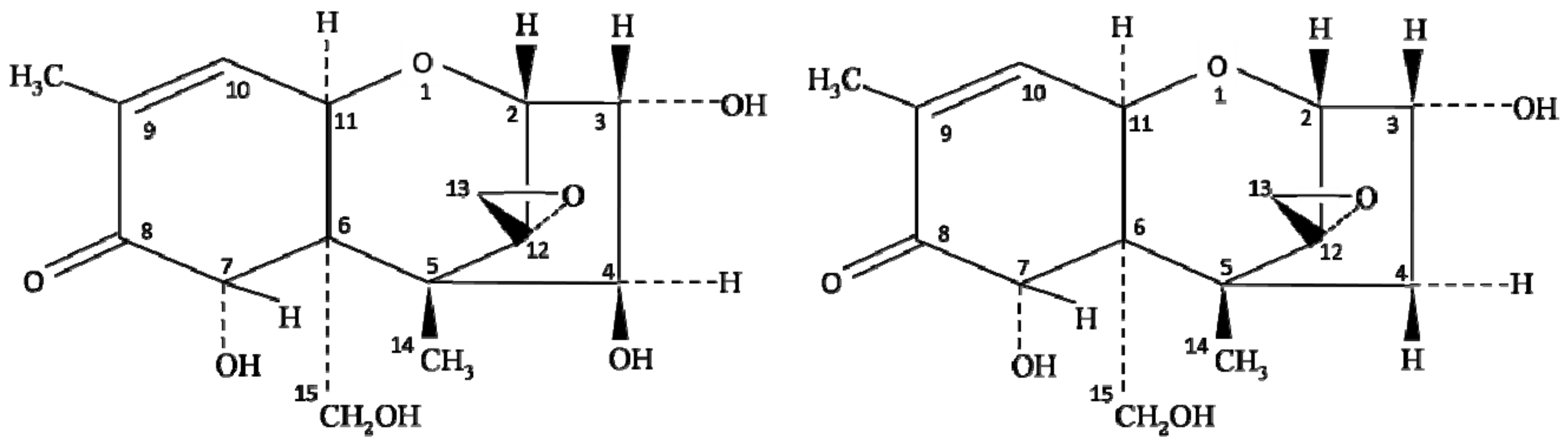
Figure 1. Chemical structure of nivalenol (NIV) and deoxynivalenol (DON).
2. Chemistry and Biosynthesis of Nivalenol
Trichothecenes are sesquiterpenoids with a variable pattern of oxygen and ester groups on a core tricyclic skeleton containing an epoxide function, also known as the 12,13-epoxytrichothec-9-ene (EPT) [27]. A large variety of trichothecene have been found in diverse fungal taxa and are grouped into four classes (types A-D) based on the substitution pattern on the fundamental EPT core [28][29]. Numerous research has been conducted to understand the various impacts of trichothecenes and to study the underlying mechanisms to mitigate the huge losses caused by Fusarium diseases globally [30][31][32][33], which endanger the viability of farms and entire rural communities.
Fusarium’s two primary chemotypes are connected to organisms that either generate deoxynivalenol or nivalenol [34]. The functioning of two genes, TRI13 that expresses a trichothecene 4-hydroxylase and TRI7 that encodes a trichothecene 4-acetyltransferase, has been shown to be the genetic difference between deoxynivalenol and nivalenol chemotypes. Both genes are present but inactive in Fusarium chemotypes that produce deoxynivalenol [35][36]. Compared to its type B companion, DON and NIV, which are more abundant in Asia, contaminate grains at greater levels and cause more severe acute and chronic toxicities. In F. graminearum, twelve trichothecene genes are grouped within a 25-kb area on chromosome II that is inherited as a single core TRI cluster [37][38]. Two more loci are involved, including the single gene Tri101 and the two-gene Tri1-Tri16 locus, both of which are situated in unrelated chromosomal areas [39]. In F. graminearum, the presence of both functioning Tri7 and Tri13 genes is needed for NIV-producing chemotypes [35][40].
Biosynthetic clusters are used in fungi to produce secondary or specialized metabolites [41]. As a result, the proteins that catalyze the biosynthesis of distinct trichothecenes are likewise arranged in biosynthetic clusters (TRI) [37][38], where many of the genes appear to be coordinately expressed and controlled. Intermediates from two separate trichothecene routes, deoxynivalenol biosynthesis and T-2 toxin generation, can feed the nivalenol process. The entry processes from the deoxynivalenol biosynthesis into the nivalenol biosynthesis are mediated by TRI13, which codes for a trichothecene 4-hydroxylase, and TRI7, which codes for a trichothecene 4-acetyltransferase and is non-functional in Fusarium species of the deoxynivalenol chemotype.
The gene TRI13 encodes a trichothecene 4-hydroxylase that has been identified and described from Fusarium graminearum strain 88-1, which represents the nivalenol chemotype. This enzyme catalyzes the conversion of 3,15-acetyldeoxynivalenol to 3,15-diacetylnivalenol, hence functioning as a switch between deoxynivalenol-producing and nivalenol-producing Fusarium graminearum strains [35][42]. The TRI7-encoded trichothecene C-4-acetyltransferase then performs C-4 acetylation, leading to the formation of 3,4,15-triacetylnivalenol [35][42][43]. The same intermediate can also be produced via the trichothecene 7,8-dihydroxylase (TRI1), which acts as 3,4,15-triacetoxyscirpenol 7,8-dihydroxylase and converts 3,4,15-triacetoxyscirpenol, an intermediate of the T-2 toxin biosynthesis, into 7,8-dihydroxy-3,4,15-triacetoxyscirpenol, followed by a currently unknown protein that confers the carbonyl function to the C-8 of 3,4,15-triacetylnivalenol [44][45]. In Fusarium graminearum strain 79A1 [46], the trichothecene C-3 esterase (TRI8) deacylates 3,4,15-triacetylnivalenol, generating 4,15-diacetylnivalenol, which is subsequently deacylated at C-4 by an unknown protein. Furthermore, in the NIV chemotype, Tri13 hydroxylates 3,15- diacetyldeoxynivalenol to yield 3,15-diacetylnivalenol, which is further transformed into NIV [47].
3. Occurrence of Nivalenol in Food and Feed
Several species of Fusarium, including F. poae, F. culmorum, F. grainearum, F. cortaderae, F. asiaticum, F. nivale, F crookwellense etc., are mainly responsible for producing NIV and its acetyle derivatives in foods [18][24][48][49]. The Fusarium species easily grow on cereal grain and produce Fusarium head blight disease under favorable environmental conditions. In general, moderate temperature and high air humidity are ideal for the infection and growth of these Fusarium species. However, sometimes it may vary from species to species, such as F. graminearum are usually found in the warmer and more humid regions (Australia, Eastern Europe, North America, South China), whereas cold climatic regions (Western Europe) are suitable for the growth of F. culmorum species [50]. NIV is reported in various cereals and cereal-based products worldwide (Table 1). It is also interesting to note that NIV mostly co-occurs with DON and is detected simultaneously in different food matrices. Furthermore, most of the methods mentioned in Table 1 were used for the multi-mycotoxin analysis.Table 1. Occurrence of nivalenol in food and feed around the world.
| Food Matrix | Country | Range (μg/kg) | Detection Technique | References |
|---|---|---|---|---|
| Food | ||||
| Adlay millet | South Korea | 12.6–337.6 | HPLC-UV | [51] |
| Baby foods | Spain | 75–100 | HPLC-MS/MS | [52] |
| Baby formula | South Korea | 4.4–1000 | HPLC-UV | [53] |
| Barley | England | 10–1088 | GC/MS | [54] |
| South Korea | 10.4–110.3 | HPLC-UV | [51] | |
| Italy | 21.7–106 | LC-MS/MS | [55] | |
| Spain | 12.47 | GC-MS | [56] | |
| Baked snacks | Spain | 55.7 | GC-MS | [57] |
| Barley grain | Poland | 5 | TLC and HPLC | [58] |
| Barley and barley products | Germany | 0.87–19 | LC-MS/MS | [59] |
| Beer | Czech Republic | 4–6 | UHPLC-APCI-Orbitrap MS | [60] |
| Beer | Spain | 10–15 | UHPLC-APCI-Orbitrap MS | [61] |
| Black bean paste (Chunjang) | South Korea | 83.8 | HPLC-UV | [51] |
| Breakfast cereals | Spain | 51.1–106.5 | GC-MS | [57] |
| Breakfast cereals | South Korea | 1096.8 | GC-MS | [62] |
| Brown rice | South Korea | 47.4 | HPLC-UV | [51] |
| Cereals | Finland | 185–300 | LC-MS/MS | [63] |
| Cereals | Czech Republic | 50 | UHPLC-ESI-ToF-MS | [64] |
| Cereal based products | Czech Republic | 25 | UHPLC-APCI-MS/MS | [65] |
| Cereal based products | Switzerland | 100 | HPLC-ESI-MS/MS | [66] |
| Cereals and cereal-based products | Spain | 121–176 | LC-MS/MS | [67] |
| Corn | South Korea | 0–181.41 * | HPLC-PAD | [68] |
| Corn | France | 7–340 | HPLC | [69] |
| De-hulled and naked barley | Spain | 1.1–7.6 | LC-MS/MS | [59] |
| Durum wheat | France | 60 | HPLC | [69] |
| Durum wheat flour | Denmark | 83–440 | GC-ECD | [70] |
| Flour bread | Italy | 5–8 | LC-MS/MS | [55] |
| Foxtail millet | South Korea | 27.4–370.8 | HPLC-UV | [51] |
| Ground wheat | Italy | 3.5–63.5 | LC-APCI-MS/MS | [71] |
| Groundnut-maize based snacks | Nigeria | 1.8–2.5 | LC-MS/MS | [72] |
| Grain-based product | Italy | 30 | GC-MS | [73] |
| Groundnut | Nigeria | 1.0 | LC-MS/MS | [72] |
| Malting barley | Spain | 35 | LC-MS/MS | [59] |
| Maize | South Korea | 51.3 | HPLC-UV | [51] |
| Nigeria | 0.8 | LC-MS/MS | [72] | |
| China | 2.1–15.3 | UHPLC-MS/MS | [74] | |
| Austria | 22.3–250 | LC-MS/MS | [75] | |
| Spain | 6.4 | GC-MS/MS | [76] | |
| Germany | 4.41–20 | GC | [77] | |
| UK | 5–10 | HPLC | [78] | |
| Poland | 2 | TLC and HPLC | [58] | |
| Maize flour | Germany | 39 | GC-MS | [79] |
| Maize-based breakfast cereal | Spain | 16–60.2 | GC-MS | [57] |
| Multicereal Flour | Spain | 75 | LC-MS/MS | [80] |
| Mixed paste | South Korea | 15.9–100.6 | HPLC-UV | [51] |
| Mixed grains | South Korea | 88.9 | GC-MS | [62] |
| Oats | South Korea | 23.5 | HPLC-UV | [51] |
| Italy | 45.5–50.4 | LC-MS/MS | [55] | |
| Germany | 17 | LC-MS/MS | [81] | |
| Italy | 8–20 | LC-MS/MS | [82] | |
| Austria | 100 | HPLC | [69] | |
| England | 10–112 | GC/MS | [83] | |
| Oats grain | Poland | 6 | TLC and HPLC | [58] |
| Pearl barley | Spain | 0.18 | LC-MS/MS | [59] |
| Popcorn | South Korea | 68.7 | HPLC-UV | [51] |
| Red chili paste (Gochujang) | South Korea | 8.5–120.2 | HPLC-UV | [51] |
| Rice | South Korea | 10 | HPLC | [84] |
| Rice | Thailand | 0.50–15.00 | UHPLC-MS/MS | [85] |
| Rice wine | South Korea | 2.5 | HPLC-UV | [53] |
| Rye | Italy | 33.9–34.4 | LC-MS/MS | [55] |
| Germany | 1.8 | LC-MS/MS | [81] | |
| France | 2–48 | HPLC | [69] | |
| Rye flour | Denmark | 38–48 | GC-ECD | [70] |
| Rye grain | Poland | 5 | TLC and HPLC | [58] |
| Sesame butter | China | 0.05–7.25 | UHPLC-MS/MS | [86] |
| Semolina | Germany | 36 | GC-MS | [79] |
| Wheat | Italy | 12–106 | LC-MS/MS | [55] |
| Japan | 0.2 | HPLC-AAPI-MS/MS | [87] | |
| Spain | 53.6 | GC-MS/MS | [76] | |
| Germany | 33 | GC-MS | [88] | |
| Poland | 10 | GC-GC-ToF-MS | [89] | |
| Argentina | 0.11–0.40 | HPLC | [90] | |
| England | 10–330 | GC/MS | [91] | |
| Wheat flour | South Korea | 31.8 | GC-MS | [62] |
| Denmark | 10–189 | GC-ECD | [70] | |
| Spain | 30 | HPLC-ESI-MS/MS | [92] | |
| Wheat semolina | Spain | 8.8–13.6 | GC-MS/MS | [93] |
| Winter barley | Spain | 5.6–6.5 | LC-MS/MS | [59] |
| Winter wheat | Italy | 70 | HPLC-MS/MS | [94] |
| Spelt | Italy | 23 | LC-MS/MS | [55] |
| Feed | ||||
| Bran | South Korea | 11.1–36.9 | HPLC-UV | [84] |
| Cattle feed | South Korea | 0–111.52 * | HPLC-PAD | [68] |
| Chicken feed | South Korea | 0–101.23 * | HPLC-PAD | [68] |
| Maize silages | Denmark | 122 | LC-MS/MS | [95] |
| Pig feed | South Korea | 0–84.21 * | HPLC-PAD | [68] |
| Wheat germ | Germany | 26 | GC-MS | [79] |
| Wheat bran | Germany | 37 | GC-MS | [79] |
* ng/kg of samples. GC: gas chromatography; GC-MS: gas chromatography–mass spectrometry; GC-ECD: gas chromatography with electron capture detection; GC-GC-ToF-MS: two-dimensional gas chromatography/time-of-flight-mass spectrometry; GC-MS/MS: gas chromatography coupled with tandem mass spectrometry; HPLC-AAPI-MS/MS: high performance liquid chromatography/atmospheric pressure ionization/tandem mass spectrometry; HPLC-APCI-MS/MS: high-performance liquid chromatography–atmospheric pressure chemical ionization–tandem mass spectrometry; HPLC-ESI-MS/MS: high-performance liquid chromatography/electrospray ionization tandem mass spectrometry; HPLC-MS/MS: high performance liquid chromatography and tandem mass spectrometry; HPLC-PAD: high-performance liquid chromatography-photodiode array detector; HPLC-UV: high performance liquid chromatographic method coupled with ultraviolet detector; LC-APCI-MS/MS: liquid chromatography coupled with atmospheric pressure chemical ionization triple quadrupole mass spectrometry; LC-MS/MS: liquid chromatography–tandem mass spectrometry; TLC: thin layer chromatography; HPLC: high performance liquid chromatography; UFLC-MS/MS: ultra-fast liquid chromatography tandem mass spectrometry; UHPLC-APCI-MS/MS: ultra-performance liquid chromatography–atmospheric pressure chemical ionization tandem mass spectrometry; UHPLC-APCI-Orbitrap MS: ultrahigh-performance liquid chromatography electrospray ionization quadrupole Orbitrap high-resolution mass spectrometry; UHPLC-ESI-ToF-MS: ultra-high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry; UHPLC-MS/MS: ultra-high performance liquid chromatography tandem mass spectrometry; UHPLC-QqLIT-MS: ultra-high-performance liquid chromatography quadrupole linear ion trap mass spectrometry.
References
- Mahato, D.K.; Devi, S.; Pandhi, S.; Sharma, B.; Maurya, K.K.; Mishra, S.; Dhawan, K.; Selvakumar, R.; Kamle, M.; Mishra, A.K. Occurrence, impact on agriculture, human health, and management strategies of zearalenone in food and feed: A review. Toxins 2021, 13, 92.
- Mahato, D.K.; Kamle, M.; Sharma, B.; Pandhi, S.; Devi, S.; Dhawan, K.; Selvakumar, R.; Mishra, D.; Kumar, A.; Arora, S. Patulin in food: A mycotoxin concern for human health and its management strategies. Toxicon 2021, 198, 12–23.
- Kumar, P.; Mahato, D.K.; Sharma, B.; Borah, R.; Haque, S.; Mahmud, M.M.C.; Shah, A.K.; Rawal, D.; Bora, H.; Bui, S. Ochratoxins in food and feed: Occurrence and its impact on human health and management strategies. Toxicon 2020, 187, 151–162.
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in food and feed: An overview on prevalence, detection and control strategies. Front. Microbiol. 2019, 10, 2266.
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 2170.
- Agriopoulou, S.; Koliadima, A.; Karaiskakis, G.; Kapolos, J. Kinetic study of aflatoxins’ degradation in the presence of ozone. Food Control 2016, 61, 221–226.
- Aupanun, S.; Poapolathep, S.; Phuektes, P.; Giorgi, M.; Zhang, Z.; Oswald, I.P.; Poapolathep, A. Individual and combined mycotoxins deoxynivalenol, nivalenol, and fusarenon-X induced apoptosis in lymphoid tissues of mice after oral exposure. Toxicon 2019, 165, 83–94.
- Mahato, D.K.; Pandhi, S.; Kamle, M.; Gupta, A.; Sharma, B.; Panda, B.K.; Srivastava, S.; Kumar, M.; Selvakumar, R.; Pandey, A.K.; et al. Trichothecenes in food and feed: Occurrence, impact on human health and their detection and management strategies. Toxicon 2022, 208, 62–77.
- Eckard, S.; Wettstein, F.E.; Forrer, H.-R.; Vogelgsang, S. Incidence of Fusarium species and mycotoxins in silage maize. Toxins 2011, 3, 949–967.
- Schollenberger, M.; Müller, H.-M.; Ernst, K.; Sondermann, S.; Liebscher, M.; Schlecker, C.; Wischer, G.; Drochner, W.; Hartung, K.; Piepho, H.-P. Occurrence and distribution of 13 trichothecene toxins in naturally contaminated maize plants in Germany. Toxins 2012, 4, 778–787.
- De Boevre, M.; Jacxsens, L.; Lachat, C.; Eeckhout, M.; Di Mavungu, J.D.; Audenaert, K.; Maene, P.; Haesaert, G.; Kolsteren, P.; De Meulenaer, B. Human exposure to mycotoxins and their masked forms through cereal-based foods in Belgium. Toxicol. Lett. 2013, 218, 281–292.
- Nagashima, H. Deoxynivalenol and nivalenol toxicities in cultured cells: A review of comparative studies. Food Saf. 2018, 6, 51–57.
- Zingales, V.; Fernández-Franzón, M.; Ruiz, M.-J. Occurrence, mitigation and in vitro cytotoxicity of nivalenol, a type B trichothecene mycotoxin–Updates from the last decade (2010–2020). Food Chem. Toxicol. 2021, 152, 112182.
- Thuvander, A.; Wikman, C.; Gadhasson, I. In vitro exposure of human lymphocytes to trichothecenes: Individual variation in sensitivity and effects of combined exposure on lymphocyte function. Food Chem. Toxicol. 1999, 37, 639–648.
- Nagashima, H.; Nakagawa, H.; Iwashita, K. Cytotoxic effects of nivalenol on HL60 cells. Mycotoxins 2006, 56, 65–70.
- Sugita-Konishi, Y.; Pestka, J.J. Differential upregulation of TNF-α, IL-6, and IL-8 production by deoxynivalenol (vomitoxin) and other 8-ketotrichothecenes in a human macrophage model. J. Toxicol. Environ. Health Part A. 2001, 64, 619–636.
- Lindblad, M.; Gidlund, A.; Sulyok, M.; Börjesson, T.; Krska, R.; Olsen, M.; Fredlund, E. Deoxynivalenol and other selected Fusarium toxins in Swedish wheat—Occurrence and correlation to specific Fusarium species. Int. J. Food Microbiol. 2013, 167, 284–291.
- Umpiérrez-Failache, M.; Garmendia, G.; Pereyra, S.; Rodríguez-Haralambides, A.; Ward, T.J.; Vero, S. Regional differences in species composition and toxigenic potential among Fusarium head blight isolates from Uruguay indicate a risk of nivalenol contamination in new wheat production areas. Int. J. Food Microbiol. 2013, 166, 135–140.
- Beccari, G.; Senatore, M.T.; Tini, F.; Sulyok, M.; Covarelli, L. Fungal community, Fusarium head blight complex and secondary metabolites associated with malting barley grains harvested in Umbria, central Italy. Int. J. Food Microbiol. 2018, 273, 33–42.
- Garmendia, G.; Pattarino, L.; Negrín, C.; Martínez-Silveira, A.; Pereyra, S.; Ward, T.J.; Vero, S. Species composition, toxigenic potential and aggressiveness of Fusarium isolates causing Head Blight of barley in Uruguay. Food Microbiol. 2018, 76, 426–433.
- Oyedele, O.A.; Ezekiel, C.N.; Sulyok, M.; Adetunji, M.C.; Warth, B.; Atanda, O.O.; Krska, R. Mycotoxin risk assessment for consumers of groundnut in domestic markets in Nigeria. Int. J. Food Microbiol. 2017, 251, 24–32.
- Duan, C.; Qin, Z.; Yang, Z.; Li, W.; Sun, S.; Zhu, Z.; Wang, X. Identification of pathogenic Fusarium spp. causing maize ear rot and potential mycotoxin production in China. Toxins 2016, 8, 186.
- Reisinger, N.; Schürer-Waldheim, S.; Mayer, E.; Debevere, S.; Antonissen, G.; Sulyok, M.; Nagl, V. Mycotoxin occurrence in maize silage—A neglected risk for bovine gut health? Toxins 2019, 11, 577.
- Vandicke, J.; De Visschere, K.; Croubels, S.; De Saeger, S.; Audenaert, K.; Haesaert, G. Mycotoxins in Flanders’ fields: Occurrence and correlations with Fusarium species in whole-plant harvested maize. Microorganisms 2019, 7, 571.
- Magan, N.; Aldred, D.; Mylona, K.; Lambert, R.J.W. Limiting mycotoxins in stored wheat. Food Addit. Contam. 2010, 27, 644–650.
- Shen, C.-M.; Hu, Y.-C.; Sun, H.-Y.; Li, W.; Guo, J.-H.; Chen, H.-G. Geographic distribution of trichothecene chemotypes of the Fusarium graminearum species complex in major winter wheat production areas of China. Plant Dis. 2012, 96, 1172–1178.
- Wu, Q.; Dohnal, V.; Kuca, K.; Yuan, Z. Trichothecenes: Structure-toxic activity relationships. Curr. Drug Metab. 2013, 14, 641–660.
- Alexander, N.J.; Proctor, R.H.; McCormick, S.P. Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. Toxin Rev. 2009, 28, 198–215.
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From simple to complex mycotoxins. Toxins 2011, 3, 802–814.
- Burlakoti, R.R.; Ali, S.; Secor, G.A.; Neate, S.M.; McMullen, M.P.; Adhikari, T.B. Comparative mycotoxin profiles of Gibberella zeae populations from barley, wheat, potatoes, and sugar beets. Appl. Environ. Microbiol. 2008, 74, 6513–6520.
- Xu, X.; Nicholson, P. Community ecology of fungal pathogens causing wheat head blight. Annu. Rev. Phytopathol. 2009, 47, 83–103.
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health B 2005, 8, 39–69.
- Escrivá, L.; Font, G.; Manyes, L. In vivo toxicity studies of Fusarium mycotoxins in the last decade: A review. Food Chem. Toxicol. 2015, 78, 185–206.
- Miller, J.D.; Greenhalgh, R.; Wang, Y.; Lu, M. Trichothecene chemotypes of three Fusarium species. Mycologia 1991, 83, 121–130.
- Lee, T.; Han, Y.-K.; Kim, K.-H.; Yun, S.-H.; Lee, Y.-W. Tri13 and Tri7 determine deoxynivalenol-and nivalenol-producing chemotypes of Gibberella zeae. Appl. Environ. Microbiol. 2002, 68, 2148–2154.
- Brown, D.W.; McCormick, S.P.; Alexander, N.J.; Proctor, R.H.; Desjardins, A.E. A genetic and biochemical approach to study trichothecene diversity in Fusarium sporotrichioides and Fusarium graminearum. Fungal Genet. Biol. 2001, 32, 121–133.
- Kimura, M.; Tokai, T.; O’Donnell, K.; Ward, T.J.; Fujimura, M.; Hamamoto, H.; Shibata, T.; Yamaguchi, I. The trichothecene biosynthesis gene cluster of Fusarium graminearum F15 contains a limited number of essential pathway genes and expressed non-essential genes. FEBS Lett. 2003, 539, 105–110.
- Brown, D.W.; Dyer, R.B.; McCormick, S.P.; Kendra, D.F.; Plattner, R.D. Functional demarcation of the Fusarium core trichothecene gene cluster. Fungal Genet. Biol. 2004, 41, 454–462.
- Torres, A.M.; Palacios, S.A.; Yerkovich, N.; Palazzini, J.M.; Battilani, P.; Leslie, J.F.; Logrieco, A.; Chulze, S.N. Fusarium head blight and mycotoxins in wheat: Prevention and control strategies across the food chain. World Mycotoxin J. 2019, 12, 333–355.
- Jennings, P.; Coates, M.E.; Turner, J.A.; Chandler, E.A.; Nicholson, P. Determination of deoxynivalenol and nivalenol chemotypes of Fusarium culmorum isolates from England and Wales by PCR assay. Plant Pathol. 2004, 53, 182–190.
- Wisecaver, J.H.; Rokas, A. Fungal metabolic gene clusters—Caravans traveling across genomes and environments. Front. Microbiol. 2015, 6, 161.
- Brown, D.W.; McCormick, S.P.; Alexander, N.J.; Proctor, R.H.; Desjardins, A.E. Inactivation of a cytochrome P-450 is a determinant of trichothecene diversity in Fusarium species. Fungal Genet. Biol. 2002, 36, 224–233.
- Lee, T.; Oh, D.-W.; Kim, H.-S.; Lee, J.; Kim, Y.-H.; Yun, S.-H.; Lee, Y.-W. Identification of deoxynivalenol-and nivalenol-producing chemotypes of Gibberella zeae by using PCR. Appl. Environ. Microbiol. 2001, 67, 2966–2972.
- McCormick, S.P.; Harris, L.J.; Alexander, N.J.; Ouellet, T.; Saparno, A.; Allard, S.; Desjardins, A.E. Tri1 in Fusarium graminearum encodes a P450 oxygenase. Appl. Environ. Microbiol. 2004, 70, 2044–2051.
- McCormick, S.P.; Alexander, N.J.; Proctor, R.H. Heterologous expression of two trichothecene P450 genes in Fusarium verticillioides. Can. J. Microbiol. 2006, 52, 220–226.
- Alexander, N.J.; McCormick, S.P.; Waalwijk, C.; van der Lee, T.; Proctor, R.H. The genetic basis for 3-ADON and 15-ADON trichothecene chemotypes in Fusarium. Fungal Genet. Biol. 2011, 48, 485–495.
- Maeda, K.; Tanaka, Y.; Matsuyama, M.; Sato, M.; Sadamatsu, K.; Suzuki, T.; Matsui, K.; Nakajima, Y.; Tokai, T.; Kanamaru, K. Substrate specificities of Fusarium biosynthetic enzymes explain the genetic basis of a mixed chemotype producing both deoxynivalenol and nivalenol-type trichothecenes. Int. J. Food Microbiol. 2020, 320, 108532.
- Pasquali, M.; Giraud, F.; Brochot, C.; Cocco, E.; Hoffmann, L.; Bohn, T. Genetic Fusarium chemotyping as a useful tool for predicting nivalenol contamination in winter wheat. Int. J. Food Microbiol. 2010, 137, 246–253.
- Gomes, L.B.; Ward, T.J.; Badiale-Furlong, E.; Del Ponte, E.M. Species composition, toxigenic potential and pathogenicity of Fusarium graminearum species complex isolates from southern B razilian rice. Plant Pathol. 2015, 64, 980–987.
- Bryła, M.; Ksieniewicz-Woźniak, E.; Waśkiewicz, A.; Szymczyk, K.; Jędrzejczak, R. Natural occurrence of nivalenol, deoxynivalenol, and deoxynivalenol-3-glucoside in Polish winter wheat. Toxins 2018, 10, 81.
- Lee, S.Y.; Woo, S.Y.; Tian, F.; Song, J.; Michlmayr, H.; Kim, J.-B.; Chun, H.S. Occurrence of deoxynivalenol, nivalenol, and their glucosides in Korean market foods and estimation of their population exposure through food consumption. Toxins 2020, 12, 89.
- Rubert, J.; Soler, C.; Mañes, J. Application of an HPLC–MS/MS method for mycotoxin analysis in commercial baby foods. Food Chem. 2012, 133, 176–183.
- Lee, S.Y.; Woo, S.Y.; Malachová, A.; Michlmayr, H.; Kim, S.-H.; Kang, G.J.; Chun, H.S. Simple validated method for simultaneous determination of deoxynivalenol, nivalenol, and their 3-β-D-glucosides in baby formula and Korean rice wine via HPLC-UV with immunoaffinity cleanup. Food Addit. Contam. Part A 2019, 36, 964–975.
- Edwards, S.G. Fusarium mycotoxin content of UK organic and conventional barley. Food Addit. Contam. 2009, 26, 1185–1190.
- Juan, C.; Ritieni, A.; Mañes, J. Occurrence of Fusarium mycotoxins in Italian cereal and cereal products from organic farming. Food Chem. 2013, 141, 1747–1755.
- Ibáñez-Vea, M.; Lizarraga, E.; González-Peñas, E. Simultaneous determination of type-A and type-B trichothecenes in barley samples by GC–MS. Food Control 2011, 22, 1428–1434.
- Castillo, M.-Á.; Montes, R.; Navarro, A.; Segarra, R.; Cuesta, G.; Hernández, E. Occurrence of deoxynivalenol and nivalenol in Spanish corn-based food products. J. Food Compos. Anal. 2008, 21, 423–427.
- Krysinska-Traczyk, E.; Perkowski, J.; Dutkiewicz, J. Levels of fungi and mycotoxins in the samples of grain and grain dust collected from five various cereal crops in eastern Poland. Ann. Agric. Environ. Med. 2007, 14, 159–167.
- Barthel, J.; Gottschalk, C.; Rapp, M.; Berger, M.; Bauer, J.; Meyer, K. Occurrence of type A, B and D trichothecenes in barley and barley products from the Bavarian market. Mycotoxin Res. 2012, 28, 97–106.
- Zachariasova, M.; Cajka, T.; Godula, M.; Malachova, A.; Veprikova, Z.; Hajslova, J. Analysis of multiple mycotoxins in beer employing (ultra)-high-resolution mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 3357–3367.
- Rubert, J.; Soler, C.; Marín, R.; James, K.J.; Mañes, J. Mass spectrometry strategies for mycotoxins analysis in European beers. Food Control 2013, 30, 122–128.
- Ok, H.E.; Choi, S.-W.; Chung, S.H.; Kang, Y.-W.; Kim, D.-S.; Chun, H.S. Natural occurrence of type-B trichothecene mycotoxins in Korean cereal-based products. Food Addit. Contam. Part B 2011, 4, 132–140.
- Kokkonen, M.K.; Jestoi, M.N. A multi-compound LC-MS/MS method for the screening of mycotoxins in grains. Food Anal. Methods. 2009, 2, 128–140.
- Zachariasova, M.; Lacina, O.; Malachova, A.; Kostelanska, M.; Poustka, J.; Godula, M.; Hajslova, J. Novel approaches in analysis of Fusarium mycotoxins in cereals employing ultra performance liquid chromatography coupled with high resolution mass spectrometry. Anal. Chim. Acta. 2010, 662, 51–61.
- Malachova, A.; Dzuman, Z.; Veprikova, Z.; Vaclavikova, M.; Zachariasova, M.; Hajslova, J. Deoxynivalenol, deoxynivalenol-3-glucoside, and enniatins: The major mycotoxins found in cereal-based products on the Czech market. J. Agric. Food Chem. 2011, 59, 12990–12997.
- Desmarchelier, A.; Oberson, J.-M.; Tella, P.; Gremaud, E.; Seefelder, W.; Mottier, P. Development and comparison of two multiresidue methods for the analysis of 17 mycotoxins in cereals by liquid chromatography electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2010, 58, 7510–7519.
- Serrano, A.B.; Font, G.; Ruiz, M.J.; Ferrer, E. Co-occurrence and risk assessment of mycotoxins in food and diet from Mediterranean area. Food Chem. 2012, 135, 423–429.
- Kim, D.-H.; Hong, S.-Y.; Jeon, M.-H.; An, J.-M.; Kim, S.-Y.; Kim, H.-Y.; Yoon, B.R.; Chung, S.H. Simultaneous determination of the levels of deoxynivalenol, 3-acetyldeoxynivalenol, and nivalenol in grain and feed samples from South Korea using a high-performance liquid chromatography–photodiode array detector. Appl. Biol. Chem. 2016, 59, 881–887.
- Schothorst, R.C.; van Egmond, H.P. Report from SCOOP task 3.2. 10 “collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states”: Subtask: Trichothecenes. Toxicol. lett. 2004, 153, 133–143.
- Rasmussen, P.H.; Ghorbani, F.; Berg, T. Deoxynivalenol and other Fusarium toxins in wheat and rye flours on the Danish market. Food Addit. Contam. 2003, 20, 396–404.
- Lattanzio, V.M.T.; Solfrizzo, M.; Visconti, A. Determination of trichothecenes in cereals and cereal-based products by liquid chromatography–tandem mass spectrometry. Food Addit. Contam. 2008, 25, 320–330.
- Kayode, O.F.; Sulyok, M.; Fapohunda, S.O.; Ezekiel, C.N.; Krska, R.; Oguntona, C.R.B. Mycotoxins and fungal metabolites in groundnut-and maize-based snacks from Nigeria. Food Addit. Contam. Part B. 2013, 6, 294–300.
- Jestoi, M.; Somma, M.C.; Kouva, M.; Veijalainen, P.; Rizzo, A.; Ritieni, A.; Peltonen, K. Levels of mycotoxins and sample cytotoxicity of selected organic and conventional grain-based products purchased from Finnish and Italian markets. Mol. Nutr. Food Res. 2004, 48, 299–307.
- Jin, P.G.; Han, Z.; Cai, Z.X.; Wu, Y.J.; Ren, Y.P. Simultaneous determination of 10 mycotoxins in grain by ultra-high-performance liquid chromatography–tandem mass spectrometry using 13C15-deoxynivalenol as internal standard. Food Addit. Contam. Part A 2010, 27, 1701–1713.
- Warth, B.; Parich, A.; Atehnkeng, J.; Bandyopadhyay, R.; Schuhmacher, R.; Sulyok, M.; Krska, R. Quantitation of mycotoxins in food and feed from Burkina Faso and Mozambique using a modern LC-MS/MS multitoxin method. J. Agric. Food Chem. 2012, 60, 9352–9363.
- Rodríguez-Carrasco, Y.; Ruiz, M.J.; Font, G.; Berrada, H. Exposure estimates to Fusarium mycotoxins through cereals intake. Chemosphere 2013, 93, 2297–2303.
- Goertz, A.; Zuehlke, S.; Spiteller, M.; Steiner, U.; Dehne, H.W.; Waalwijk, C.; de Vries, I.; Oerke, E.C. Fusarium species and mycotoxin profiles on commercial maize hybrids in Germany. Eur. J. Plant Pathol. 2010, 128, 101–111.
- Scudamore, K.A.; Patel, S. Occurrence of Fusarium mycotoxins in maize imported into the UK, 2004–2007. Food Addit. Contam. Part A. 2009, 26, 363–371.
- Schollenberger, M.; Müller, H.M.; Drochner, W. Fusarium toxin contents of maize and maize products purchased in the years 2000 and 2001 in Germany. Mycotoxin Res. 2005, 21, 26–28.
- Rubert, J.; Soler, C.; Mañes, J. Evaluation of matrix solid-phase dispersion (MSPD) extraction for multi-mycotoxin determination in different flours using LC–MS/MS. Talanta 2011, 85, 206–215.
- Gottschalk, C.; Barthel, J.; Engelhardt, G.; Bauer, J.; Meyer, K. Simultaneous determination of type A, B and D trichothecenes and their occurrence in cereals and cereal products Food Addit. Contam. Part A 2009, 26, 1273–1289.
- Juan, C.; Ritieni, A.; Mañes, J. Determination of trichothecenes and zearalenones in grain cereal, flour and bread by liquid chromatography tandem mass spectrometry. Food Chem. 2012, 134, 2389–2397.
- Edwards, S.G. Fusarium mycotoxin content of UK organic and conventional oats. Food Addit. Contam. 2009, 26, 1063–1069.
- Ok, H.E.; Lee, S.Y.; Chun, H.S. Occurrence and simultaneous determination of nivalenol and deoxynivalenol in rice and bran by HPLC-UV detection and immunoaffinity cleanup. Food Control 2018, 87, 53–59.
- Koesukwiwat, U.; Sanguankaew, K.; Leepipatpiboon, N. Evaluation of a modified QuEChERS method for analysis of mycotoxins in rice. Food Chem. 2014, 153, 44–51.
- Liu, Y.; Han, S.; Lu, M.; Wang, P.; Han, J.; Wang, J. Modified QuEChERS method combined with ultra-high performance liquid chromatography tandem mass spectrometry for the simultaneous determination of 26 mycotoxins in sesame butter. J. Chromatogr. B 2014, 970, 68–76.
- Tanaka, H.; Takino, M.; Sugita-Konishi, Y.; Tanaka, T.; Toriba, A.; Hayakawa, K. Determination of nivalenol and deoxynivalenol by liquid chromatography/atmospheric pressure photoionization mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 3119–3124.
- Schollenberger, M.; Müller, H.-M.; Rüfle, M.; Suchy, S.; Plank, S.; Drochner, W. Natural occurrence of 16 Fusarium toxins in grains and feedstuffs of plant origin from Germany. Mycopathologia 2006, 161, 43–52.
- Jeleń, H.H.; Wąsowicz, E. Determination of trichothecenes in wheat grain without sample cleanup using comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry. J. Chromatogr. A. 2008, 1215, 203–207.
- Basílico, M.L.Z.; Pose, G.; Ludemann, V.; Fernández Pinto, V.E.; Aríngoli, E.E.; Ritieni, A.; Basílico, J.C. Fungal diversity and natural occurrence of fusaproliferin, beauvericin, deoxynivalenol and nivalenol in wheat cultivated in Santa Fe Province, Argentina. Mycotoxin Res. 2010, 26, 85–91.
- Edwards, S.G. Fusarium mycotoxin content of UK organic and conventional wheat. Food Addit. Contam. 2009, 26, 496–506.
- Sospedra, I.; Blesa, J.; Soriano, J.M.; Mañes, J. Use of the modified quick easy cheap effective rugged and safe sample preparation approach for the simultaneous analysis of type A-and B-trichothecenes in wheat flour. J. Chromatogr. A. 2010, 1217, 1437–1440.
- Rodríguez-Carrasco, Y.; Berrada, H.; Font, G.; Mañes, J. Multi-mycotoxin analysis in wheat semolina using an acetonitrile-based extraction procedure and gas chromatography–tandem mass spectrometry. J. Chromatogr. A. 2012, 1270, 28–40.
- Giraud, F.; Pasquali, M.; Jarroudi, M.E.; Vrancken, C.; Brochot, C.; Cocco, E.; Hoffmann, L.; Delfosse, P.; Bohn, T. Fusarium head blight and associated mycotoxin occurrence on winter wheat in Luxembourg in 2007/2008. Food Addit. Contam. 2010, 27, 825–835.
- Rasmussen, R.R.; Storm, I.M.L.D.; Rasmussen, P.H.; Smedsgaard, J.; Nielsen, K.F. Multi-mycotoxin analysis of maize silage by LC-MS/MS. Anal. Bioanal. Chem. 2010, 397, 765–776.
More
