Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Xin Min.
Single-atom catalysts (SACs) are defined as single or isolated metal atoms with catalytic activity anchored on the support that forms a composite catalyst with catalytic activity, which is a forward direction in the field of heterogeneous catalysis.
- tungsten
- single-atom catalysts
- nanocatalysts
1. Single Tungsten Atom Catalysts
In recent years, there has been an increasing amount of literature on single-atom catalysts (SACs). The first serious discussions and analyses of SACs had been discovered by Qiao [20][1]. SACs are defined as single or isolated metal atoms with catalytic activity anchored on the support that forms a composite catalyst with catalytic activity, which is a forward direction in the field of heterogeneous catalysis. Compared to nanocatalysts, SACs have the advantage of more excellent metal–support interactions [21][2], larger surface-free energy, higher selectivity, lower usage and so on, due to its particle size. As is displayed in Figure 51a,b [22][3], the orange particles are both connected with the support and the reactant. On the contrary, the yellow particles only touch the reactant, which indicates that SAC’s utilization is much better than the nanocatalysts. Herein, Xu et al. [23][4] found that the catalytic activity of SACs are highly correlated with the local environment of the metal center. Meanwhile, Li et al. [24][5] reported that the existence of a single atom could reduce the reaction energy barrier and weaken the competitive reaction adsorption, so as to promote the catalytic reaction. The above research provides a specific direction for the later research on SACs. The results show that [25][6] the microstructure, such as size effect, support effect, coordination effect and electronic effect, play an important role in catalytic performance.
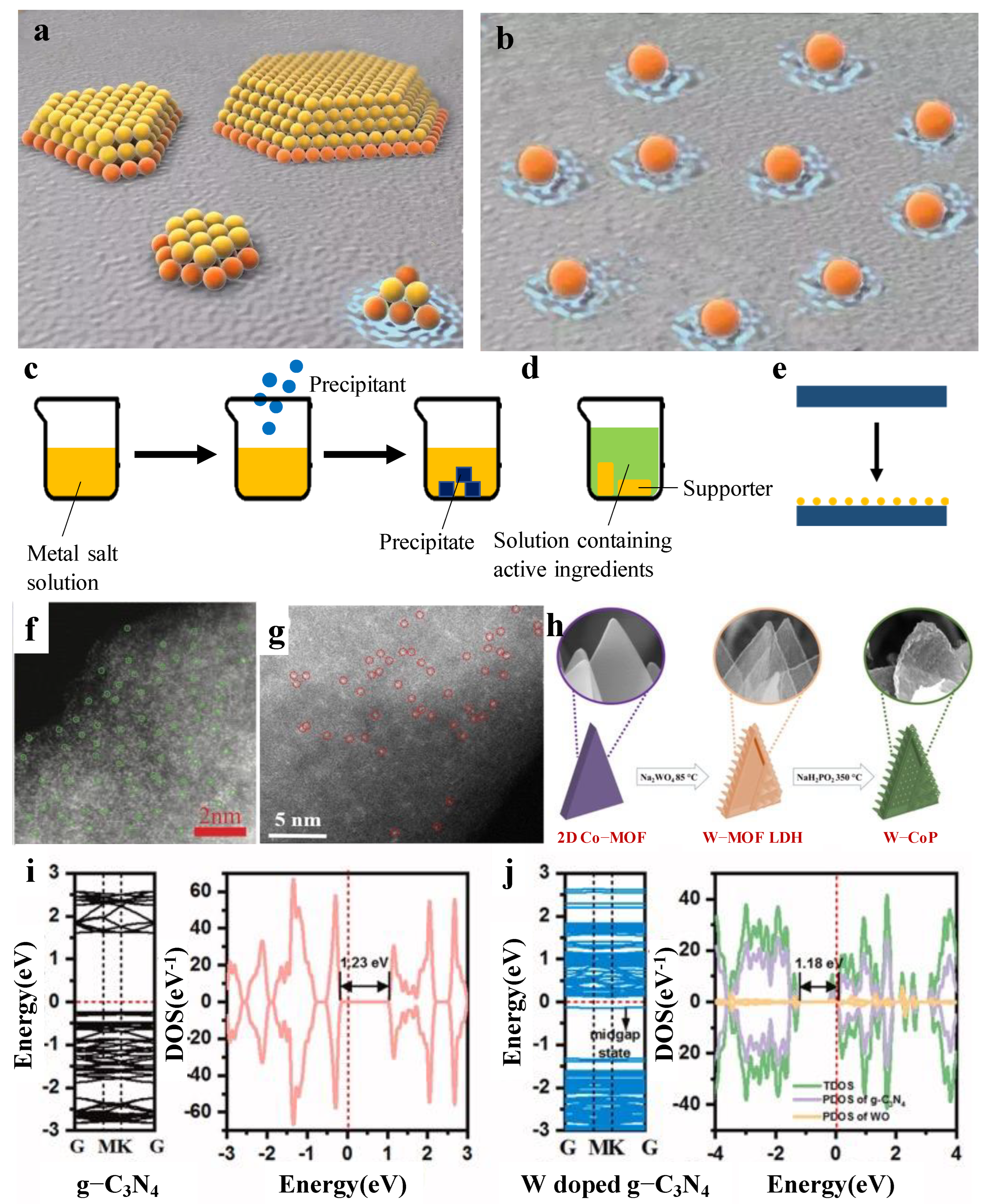
Figure 51. (a) Structural diagram of nanocatalysts. (b) Structural diagram of SACs. Diagram of the preparation method: (c) coprecipitation; (d) immersion; (e) atomic layer deposition. (f) Illustration of the formation of W-SAC. (g) HAADF-STEM image of the W-SAC. (h) HAADF-STEM image of the W-N-C catalysts. Band structures, total and projected density of states for (i) pure g-C3N4 and (j) W-doped g-C3N4.
Size effect is one of the most major influences in catalytic performance. The most representative is a series of Pt-based catalysts with different sizes prepared by Cheng et al. [26][7] through atomic layer deposition. By comparing the catalytic activities of nanoparticles, subnanoparticles and single atoms, it is proven that single Pt catalysts show the best catalytic performance, and the activity is 37 times that of commercial Pt/C. In terms of support effect, the carrier plays a decisive role. It not only provides a region for anchoring active species but also optimizes the local geometric and electronic structure of active metals. It is important to adjust the relationship between the metal carrier interaction and the catalytic performance.
The coordination effect describes differences in an adsorbate’s coordination with surface metal atoms due to different crystal facets and defects of support. Relevant theories show that the coordination environment of a single metal atom will change the adsorption affinity of the metal center, thus changing the catalytic performance. The most representative is the research conducted by Jakub et al. [27][8], who explored the difference of adsorption behavior of CO on a single iridium (Ir) atom under different coordination environments on the Fe3O4(001) surface.
As for the influence of the electronic effect on catalytic performance, the most typical catalyst is a catalyst composed of dispersed monatomic Fe reported by Gu et al. [28][9]. The research shows that Fe3+ has higher activity than traditional Fe2+. All the above studies provide theoretical guidance to improve the activity of SACs and to open up new opportunities.
Inspired by this, it was found that there are two main catalytic modes of single tungsten atom catalysts. There are common tungsten single atom catalysts; conversely, tungsten can be used as a single atom to optimize other catalysts; that is, it plays the role of a co-catalyst. The following are expanded from these two aspects.
2. Tungsten Single Atom Catalysts
SACs were studied to achieve application in many fields, such as hydrogen evolution reaction (HER, OER, ORR), oxygen precipitation reaction (OER), oxygen reduction reactions (ORR) and photocatalysis by using coprecipitation, immersion, atomic layer deposition, etc. [29,30,31][10][11][12] (Figure 51c–e). With different types of supports, different properties are shown. The typical supports include metal organic framework (MOF) and carbon material, to name a few. Therefore, the central task is to understand the principle of the structures with atomic-level precision and then design excellent SACs to promote the catalysts’ properties. Electrochemical parameter of single tungsten atom catalysts currently reported are exhibited in Table 1.
Table 1.
Electrochemical parameter of single tungsten atom catalysts currently reported.
| Support | Reaction | Overpotential (mV) at Current Density (mA/cm2) |
Tafel Slope (mV/dec) | Ref. |
|---|---|---|---|---|
| N-doped carbon |
HER | 85 at 10 (0.1 M KOH) 105 at 10 (0.5 M H2SO4) |
53 (1 M KOH) 58 (0.5 M H2SO4) |
[15][13] |
| ZIFs | ORR | - | 71 (KOH) | [32][14] |
| GO | nitrogen reduction (NRR) | - | 8.35% at −0.70 V | [33][15] |
| CoP | HER | 40 at 10 (1.0 M KOH) 48 at 10 (0.5 M H2SO4) |
47 (1 M KOH) 56 (0.5 M H2SO4) |
[34][16] |
| NiS0.5Se0.5 | HER | 39 at 10 (1.0 M KOH) | 51 (1 M KOH) | [35][17] |
| NiS0.5Se0.5 | OER | 171 at 10 (1.0 M KOH) | 41 (1 M KOH) | [35][17] |
2.1. Tungsten Single Atom on Metal Organic Framework
Metal organic frameworks (MOFs), also known as porous coordination polymers (PCPs), are a kind of crystalline composite formed by the connection of metal centers or metal clusters with organic ligands in the form of coordination bonds. Compared with most microporous materials, MOFs hold better performance, and the specific surface area of MOFs is larger. At the same time, its structure and porosity can also be adjusted and controlled with different metals or ligands to realize functional modification.
Among single tungsten atom catalysts, the most representative research using MOF as a carrier was performed by Chen et al. [15][13]. They calculated the ΔGH* of their W-SAC (a single W atom is supported on a derived N-doped carbon by pyrolysis strategy). An excellent electrocatalyst for HER usually gives a ΔGH* value close to 0 eV. The results revealed that the W-SAC has a low ΔGH* value of only 0.033 eV. In comparison, WC and WN possess a much higher ΔGH* of −0.290 and −0.246 eV, respectively. These results suggested that the W-SAC was more of an advantage to HER than other W species. The study by Chen et al. [15][13] is the first example of single W atom catalysts. The obtained W-SAC exhibits high electrochemical HER performance under both alkaline and acidic media. Under a 0.1 m KOH solution, a low overpotential of 85 mV at a current density of 10 mA/cm2 was shown, which was close to that of commercial Pt/C. In this kind of solution, the Tafel slope was calculated to be 53 mV/dec, only 5 mV higher than that of commercial Pt/C. However, a low overpotential of 105 mV at a current density of 10 mA/cm2 and a microscopic Tafel slope of 58 mV/dec (in 0.5 m H2SO4 solution) was calculated. It is easy to find that single atoms were observed in HRTEM, mainly because amine groups in derived N-doped carbons prevent the aggregation of W species, making single tungsten atoms much easier to form. On this basis, further research was conducted by Jiang et al. [32][14]. They prepared W-SACs by loading tungsten onto zeolite imidazoline frameworks (ZIFs). The results showed that without amine groups, clusters can be observed (Figure 51f,g).
2.2. Tungsten Single Atom on Carbon Materials
Hu et al. [36][18] found that the combination of the active center of the transition metal atom and the carbon support will give the transition metal catalysts excellent characteristics. Common carbon materials include graphene and fullerene. Graphene is a two-dimensional carbon nanomaterial with a hexagonal honeycomb lattice composed of carbon atoms with sp2 hybrid orbitals. It has the characteristics of simple preparation, good biocompatibility, excellent electronic transmission performance, and so on. Fullerene is similar to graphite in structure, which is a hollow molecule completely composed of carbon. Fullerenes have been widely used in catalysts in recent years [37][19] because of their excellent chemical properties, such as thermal stability and high hydrogen storage capacity. In recent years, carbon material has attracted more and more attention and is one of the more excellent supports. When the dispersed metal atoms are firmly anchored on the surface of carbon material, such electrocatalysts will show ultra-high specific surface area and excellent electronic performance [38][20], which provide great potential for innovation in the field of electrocatalysis.
The first to report the application of single-atom tungsten supported on graphene in the NRR was Gu et al. [33][15], based on the theory that the tungsten atoms have high NRR activity and selectivity for N2 fixation [39][21]. Gu et al. fabricate tungsten on graphene oxide (GO) through resin-chelating and self-template method. The reason for choosing GO as the support is that the introduction of O coordination weakens the W-N bond strength, which is a benefit for NRR. After DFT, the results showed that the single tungsten atoms are the origin of activity of the high NRR activity, which provides a theoretical basis for the application of single tungsten atom catalysts in NRR.
In comparison with the prevalent 2D material-supported SACs, the design of SACs is still challenging. Li et al. [40][22] introduced a new type of SAC, which a recently identified all-boron fullerene (B40) employed as the support. Among a series of candidates, the single tungsten atom supported B40 is screened out as the most feasible catalyst for the NRR with a low overpotential and high selectivity to passivate the competitive hydrogen evolution process. These results offer a promising NRR electrocatalyst and a hopeful pathway for a performance enhancement strategy.
3. Tungsten Single Atom Co-Catalysts
Tungsten is also widely used as a single atom to optimize other catalysts. The most representative work in HER is performed by Wu et al. [34][16]. Their work focused on preparing another kind of W-SAC, in which the support is cobalt phosphide (CoP). W-CoP was prepared by etching and phosphating (Figure 51h). First, in 1.0 M KOH, it showed the overpotentials of 40 mV to realize 10 mA/cm2 and reached a smaller Tafel slope of 47 mV/dec. Conversely, a low overpotential of 48 mV at a current density of 10 mA/cm2 and a microscopic Tafel slope of 56 mV/dec (in 0.5 m H2SO4 solution) was calculated. Compared with other W-SACs, the surface of W-CoP is coarser because of the 3D shape of the CoP. It is speculated that there may be more surface-active sites. The above study provides a research direction about single tungsten used as a co-catalyst.
Developing a kind of electrocatalyst toward HER/OER is crucial for the spread of hydrogen energy industrialization. Herein, Wang et al. [35][17] prepared a W single-atom-doped heterostructure grown on nickel foam (NF), denoted as W-NiS0.5Se0.5. The HER performances were tested in 1.0 M KOH, showing that W-NiS0.5Se0.5 exhibits a lower overpotential of 39 mV to reach 10 mA/cm2, which is comparable to commercial Pt/C (36 mV). Furthermore, W-NiS0.5Se0.5 shows a Tafel slope of 51 mV/dec and a turnover frequency (TOF) of 1.105/s at −100 mV (RHE), which is approximate to commercial Pt/C (36 mV/dec) and higher than commercial Pt/C (0.222/s), respectively. In OER, the LSV curves showed that W-NiS0.5Se0.5 exhibited a lower overpotential (171 M V) at 10 mA/cm2 than that of IrO2 on NF (337 mV). Furthermore, W-NiS0.5Se0.5 shows a Tafel slope of 41 mV/dec and a turnover frequency (TOF) of 1.85/s at −250 mV (RHE), which is smaller than those of IrO2 on NF (92 mV/dec) and better than IrO2 on NF 0.0017/s. Finally, W-NiS0.5Se0.5 is expected to assemble a two-electrode water splitting system by using it as a cathode and anode, opening the application direction of the tungsten single atom as a co-catalyst of bifunctional catalysts.
Tungsten single atom as a co-catalyst cannot only be used in electrocatalysis, but can also be used in photocatalysis. Photocatalysis is considered as a promising strategy to produce hydrogen. The most representative research is performed by Zhang et al. [41][23]. A single W atom-dispersed graphitic carbon nitride (g-C3N4) was prepared in combination with a thermal oxidation etching process. Importantly, no significant clusters could be observed in the HAADF-STEM images, which suggests that a single W atom appeared. Meanwhile, the Brunauer–Emmett–Teller (BET) results show that the mesoporous structure was generated, which usually renders more active sites, had a higher mass transfer efficiency, and was favorable for improving the photocatalytic activity. Based on the results of the DFT calculations, it can be concluded that the bandgap of the W-doped g-C3N4 decreases compared to the pure g-C3N4 through density of states (DOS) results (Figure 51i,j). This work paves an efficient path toward a high-loading W-SAC and opens new insight into its application.
On this basis, Gu et al. [42][24] further innovated a novel kind of W-SAC with a single W atom supported on g-C3N4, compared with the study by Zhang et al., which was focused on for a one-step calcination of mixed precursors, and this method is more convenient. The results obtained are roughly consistent with those of their predecessors. They also reported that single W atoms broaden the visible-light absorption of pure CN. The bandgap energy from 2.66 eV (pure CN) reduces to 2.47 eV (W-CN).
3. Summary
Atomic tungsten is the origin of catalyst activity instead of other W-based species; thus, W-SACs have broad prospects. However, there are still many deficiencies. When the metal particles are reduced to the single atomic level, the specific surface area will increase sharply, resulting in an increase in metal surface free energy. It is easy to agglomerate and form large clusters during preparation and reaction, resulting in the deactivation of the catalysts. Therefore, the stability and load of SACs are great challenges. To overcome these problems, many strategies have been proposed, including defect engineering and carrier selection. At present, choosing an appropriate support is the most effective way. Zhang et al. [43][25] explored a new type of monatomic layer cluster for advanced catalytic fields. Similarly to SACs, this kind of catalytic material is still in the early stages of research, but it may still bring greater opportunities and challenges to the catalytic field in the future.
References
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom Catalysis of CO Oxidation Using Pt1/FeOx. Nat. Chem. 2011, 8, 634–641.
- Wang, L.; Li, H.; Zhang, W.; Zhao, X.; Qiu, J.; Li, A.; Zheng, X.; Hu, Z.; Si, R.; Zeng, J. Supported Rhodium Catalysts for Ammonia–borane Hydrolysis: Dependence of the Catalytic Activity on the Highest Occupied State of the Single Rhodium Atoms. Angew. Chem. Int. Edit. 2017, 56, 4712–4718.
- Today, Let's Talk About a Familiar Chemical Term. Available online: https://mp.weixin.qq.com/s/-av2t-VK5NlE_QkRvz42QA (accessed on 5 August 2018).
- Xu, H.; Cheng, D.; Cao, D.; Zeng, X.C. A Universal Principle for a Rational Design of Single-atom Electrocatalysts. Nat. Catal. 2018, 7, 339–348.
- Li, F.; Li, Y.; Zeng, X.C.; Chen, Z. Exploration of High-performance Single-atom Catalysts on Support M1/FeOX for CO Oxidation via Computational Study. ACS Catal. 2015, 5, 544–552.
- Pan, Y.; Zhang, C.; Liu, Z.; Chen, C.; Li, Y. Structural Regulation with Atomic-level Precision: From Single-atomic Site to Diatomic and Atomic Interface Catalysis. Matter 2020, 2, 78–110.
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.-K.; Liu, L.-M.; et al. Platinum Single-atom and Cluster Catalysis of the Hydrogen Evolution Reaction. Nat. Commun. 2016, 7, 13638.
- Jakub, Z.; Jan, H.; Matthias, M.; Roland, B.; Florian, K.; Martin, S.; Michael, S.; Ulrike, D.; Cesare, F.; Gareth, S.P. Local Structure and Coordination Define Adsorption in a Model Ir1/Fe3O4 Single-atom Catalys. Angew. Chem. 2019, 131, 14099–14106.
- Gu, J.; Hsu, C.S.; Bai, L.; Chen, H.M.; Hu, X. Atomically Dispersed Fe3+ Sites Catalyze Efficient CO2 Electroreduction to CO. Science 2019, 364, 1091–1094.
- Lin, J.; Wang, A.; Qiao, B.; Liu, X.; Yang, X.; Wang, X.; Liang, J.; Li, J.; Liu, J.; Zhang, T. Remarkable Performance of Ir1/FeOx Single-atom Catalyst in Water Gas Shift Reaction. J. Am. Chem. Soc. 2013, 125, 15314–15317.
- Moses-DeBusk, M.; Yoon, M.; Allard, L.F.; Mullins, D.R.; Wu, Z.; Yang, X.; Veith, G.; Stocks, G.M.; Narula, C.K. CO Oxidation on Supported Single Pt Atoms: Experimental and ab Initio Density Functional Studies of CO Interaction with Pt Atom on θ-Al2O3(010) Surface. J. Am. Chem. Soc. 2013, 135, 12634–12645.
- Xing, J.; Chen, J.F.; Li, Y.H.; Yuan, W.T.; Zhou, Y.; Zheng, L.R.; Wang, H.F.; Hu, P.; Wang, Y.; Zhao, H.J.; et al. Stable Isolated Metal Atoms as Active Sites for Photocatalytic Hydrogen Evolution. Chem. A Eur. J. 2014, 20, 2138–2144.
- Chen, W.; Pei, J.; He, C.T.; Wan, J.; Ren, H.; Wang, Y.; Dong, J.; Wu, K.; Cheong, W.-C.; Mao, J.; et al. Single Tungsten Atoms Supported on MOF-drived N-doped Carbon for Robust Electrochemical Hydrogen Evolution. Adv. Mater. 2018, 30, 1800396.
- Jiang, B.; Sun, H.; Yuan, T.; He, W.; Zheng, C.; Zhang, H.J.; Yang, J.; Zheng, S. A Framework-derived Tungsten Single-atom Catalyst for Oxygen Reduction Reaction. Am. Chem. Soc. 2021, 35, 8173–8180.
- Gu, Y.; Xi, B.; Tian, W.; Zhang, H.; Fu, Q.; Xiong, S. Boosting Selective Nitrogen Reduction via Geometric Coordination Engineering on Single-tungsten-atom. Catal. Adv. Mater. 2021, 33, 2100429.
- Wu, J.; Han, N.; Ning, S.; Chen, T.; Zhu, C.; Pan, C.; Wu, H.; Pennycook, S.J.; Guan, C. Single-atom Tungsten-doped CoP Nanoarrays as a High-efficiency pH-Universal Catalyst for Hydrogen Evolution Reaction. Am. Chem. Soc. 2020, 8, 12832–14825.
- Wang, Y.; Li, X.; Zhang, M.; Zhang, J.; Chen, Z.; Zheng, X.; Zhao, N.; Tian, Z.; Han, X.; Zaghib, K.; et al. Single-atom Tungsten Doped Nis0.5Se0.5 Heterostructures Catalyze Water Splitting Highly Active and Durable. Res. Sq. 2021, 7, 369139.
- Hu, C.; Lin, Y.; Connell, J.W.; Cheng, H.; Gogotsi, Y.; Titirici, M.; Dai, L. Carbon-based Metal-free Catalysts for Energy Storage and Environmental Remediation. Adv. Mater. 2019, 31, e1806128.
- Fernandez-Delgado, O.; Santiago, A.R.P.; Betancourth, J.G.; Sanad, M.F.; Sreenivasan, S.T.; Echegoyen, L. Diazonium Functionalized Fullerenes: A New Class of Efficient Molecular Catalysts for the Hydrogen Evolution Reaction. Nanoscale 2022, 14, 3858–3864.
- Zhang, Q.; Zhang, X.; Wang, J.; Wang, C. Graphene Supported Single-atom Catalysts and Applications on Electrocatalysis. Nanotechnology 2020, 32, 1361–6528.
- Liu, X.; Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Building Up a Picture of the Electrocatalytic Nitrogen Reduction Activity of Transition Metal Single-atom Catalysts. J. Am. Soc. 2019, 141, 9664–9672.
- Li, W.Y.; Sun, Y.B.; Li, M.Y.; Zhang, X.Y.; Zhao, X.; Dang, J.S. Anchored Atomic Tungsten on a B40 Cage: A Highly Active and Selective Single-atom Catalyst for Nitrogen Reduction. Phys. Chem. Chem. Phys. PCCP 2021, 23, 2469–2474.
- Zhang, F.; Zhang, J.; Wang, H.; Li, J.; Liu, H.; Jin, X.; Wang, X.; Zhang, G. Single Tungsten Atom Steered Band-gap Engineering for Graphitic Carbon Nitride Ultrathin Nanosheets Boots Visible-light Photocatalytic H2 Evolution. Chem. Eng. J. 2021, 424, 130004.
- Gu, Y.; Xu, T.; Chen, X.; Chen, W.; Lu, W. High-loading Single-atom Tungsten Anchored on Graghitic Carbon Nitride (melon) for Efficient Oxidation of Emerging Contaminants. Chem. Eng. J. 2022, 427, 131973.
- Zhang, B.-W.; Ren, L.; Wang, Y.-X.; Du, Y.; Jiang, L.; Dou, S.-X. New Monatomic Layer Clusters for Advanced Catalysis Materials. Sci. China Mater. 2018, 62, 149–153.
More
