Carotenoids having ≥10 π-π-conjugated C=C double bonds serve as an objective marker of the antioxidant status of human stratum corneum (SC) in vivo according to the principle: the higher the concentration of carotenoids the higher the antioxidant status of the entire SC. The exposure to doses of radiation in the visible (>50 J/cm2) and near-infrared (>120 J/cm2) spectral ranges cause the formation of free radicals in human skin, which can be determined in vivo by a decrease in the concentration of carotenoids in the SC. The topical application of sunscreen containing antioxidants has a protective effect on the skin. A diet containing antioxidants, in particular fruit and vegetables or food supplements, leads to an increase in the carotenoid concentration and the antioxidant status of the SC. The concentration of carotenoids in the SC can reflect the individual lifestyle habits and health status. Resonance Raman spectroscopy and diffuse reflectance spectroscopy are optical methods that provide a rapid and non-invasive screening of the kinetics of carotenoids and changes in the antioxidant status of the human SC, which can be useful in in vivo skin research.
- beta-carotene
- lycopene
- zeaxanthin
- stratum corneum
- antioxidant status
- free radicals
- reactive oxygen species
- lipid oxygen species
- aging
- nutrition
1. Antioxidants in the Human Skin
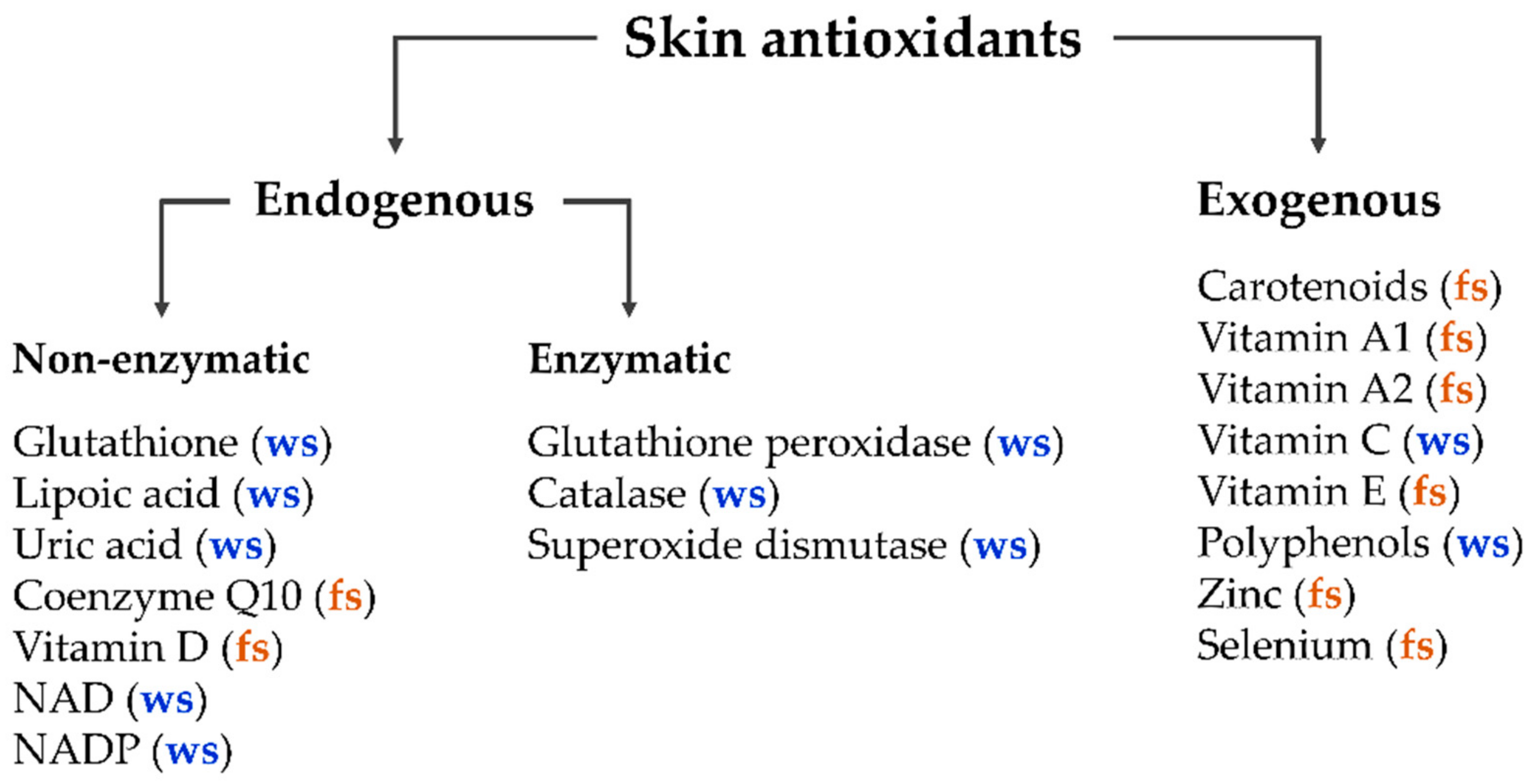
The human body in general and the skin in particular contain a balanced set of antioxidants, which can be divided into two main classes—endogenous and exogenous antioxidants [[1],[2]]. The major enzymatic endogenous antioxidants include glutathione peroxidase, catalase, and superoxide dismutase [[3],[4],[5]]. Non-enzymatic endogenous antioxidants include glutathione, lipoic acid, uric acid, coenzyme Q10, vitamin D, intracellular reducing agents nicotinamide adenine dinucleotide (NAD), and nicotinamide adenine dinucleotide phosphate (NADP). Exogenous antioxidants enter the human organism only by nutrition (dietary antioxidants), such as carotenoids; vitamins A1, A2, C, and E; polyphenols; zinc; and selenium [[1],[6],[7],[8],[9],[10],[11],[12]]. Additionally, antioxidants can be divided into water-soluble and fat-soluble antioxidants (Figure 1).
Most antioxidants are able to neutralize several free radical attacks before being destroyed [[14],[15],[16]]. Some combinations of antioxidants, such as carotenoids and vitamin E [[17]], carotenoids and polyphenols [[18]], superoxide dismutase and catalase [[1],[3]], lycopene, β-carotene, and vitamins C and E [[19],[20]], act in synergy, significantly increasing the number of neutralized free radicals and thus improving the efficiency of antioxidant protection.
Human skin contains a balanced set of antioxidants. Their concentration may vary with the seasons [[21],[22]] and depends on lifestyle habits [[5],[22]]. All of the known methods for determining the concentration of enzymes in the skin are invasive or minimally invasive [[7],[21]]. The mechanisms of SC saturation with enzymatic antioxidants are not well understood; however, superoxide dismutase and catalase are known to be non-homogeneously distributed throughout the human SC, having a minimum near the surface [[4],[21]]. Non-enzymatic endogenous intracellular reducing agents are mainly present in the viable epidermis and in the dermis in various concentrations. The SC contains significantly lower concentrations of enzymatic and non-enzymatic endogenous antioxidants. Thus, non-enzymatic exogenous antioxidants predominate in the SC, continuously entering the cells of the viable epidermis from the bloodstream and saturating the entire SC in the course of keratinization. However, this pathway is not unique, as vitamin E [[23],[24]], the vitamins A1 and A2 [[9]], and carotenoids [[25],[26]] reach the skin surface as part of the sweat and/or sebum secretion and thus increase antioxidant concentrations in the superficial SC [[25],[27],[28]].
2. Carotenoids in Human Skin
Carotenoids are fat-soluble compounds, which are categorized into two classes—xanthophylls containing oxygen and carotenes, which do not contain oxygen [[29]]. Most carotenoids contain 40 carbon atoms and are capable of neutralizing reactive oxygen species (ROS) due to their antioxidant properties as shown in vitro [[30],[31],[32],[33],[34],[35],[36],[37]]. Among carotenoids, lycopene has the highest antioxidant efficiency [[38],[39]], followed by α-carotene, β-cryptoxanthin, β-carotene, zeaxanthin, and lutein [[40]]. Furthermore, carotenoids are natural filters of high-energy visible light, because due to the presence of more than 10 π-π-conjugated C=C double bonds [[41]] they effectively absorb light in the violet-green spectral range [[32],[42]].
In the human body, apart from the skin, carotenoids are found in the blood plasma and are constantly circulating in the bloodstream [[43],[44]]; they are also stored in the subcutaneous fatty tissue [[9],[45]] and the liver [[46]]. The skin carotenoids α-carotene, β-carotene, and γ-carotene are pro-vitamin-active [[47],[48]].
In the human skin, the highest concentration of carotenoids is found within adipocytes in the fat-rich subcutaneous tissue [[49],[50]] and in the SC within the lipid lamellae [[25],[51]]. The carotenoid concentration is non-homogeneously distributed in the SC and has two maxima—near the surface and near the bottom of the SC [[25],[52]], which is explained by the two independent delivery pathways: from the inside due to keratinization and from the outside with sweat and/or sebum secretion [[26],[27],[28],[53]].
3. Methods of Non-Invasive In Vivo Determination of Carotenoids in the SC
Carotenoids can be determined by analyzing skin biopsies using high-pressure liquid chromatography [[45],[54]], but this method is invasive and does not provide accurate information due to preparation-induced oxidation of carotenoids. Non-invasive methods to assess the carotenoid content in the SC in vivo are advantageous and currently popular in skin research due to their practicability and reliability. The most effective non-invasive methods to measure carotenoids in the human SC are limited to optical methods, which include resonance Raman spectroscopy (RRS) [[54],[55],[56],[57]], confocal Raman micro-spectroscopy [[25],[27],[52],[58],[59]], skin color measurements [[60],[61],[62],[63],[64],[65]], and diffuse reflectance spectroscopy [[66],[67],[68]], reviewed in detail in [[69]].
As the concentration of carotenoids in the SC is the highest compared to viable epidermis and dermis [[9]], it is advantageous to measure on the palms of hands, which provide a thick SC and are easily accessible. Skin on other body areas is also suitable for in vivo measurements using exemplary methods.
4. Carotenoids Are Marker Substances of the Antioxidant Status of the Human SC In Vivo
The human SC contains various antioxidants forming the antioxidant status of the SC (Figure 1). Determining the antioxidant status of the SC in vivo requires determining the efficiency of neutralizing free radicals. Electron paramagnetic resonance (EPR) spectroscopy is a non-invasive method for the detection of radical formation in tissues, organs, and cell cultures. The topical application of the spin probe nitroxide TEMPO (2,2,6,6-tetramethylpiperidine-1-yl-oxyl) on the skin allows statements about the redox state in the SC as shown for in vivo measurements on the skin of the human inner forearm [[70]]. The intensity of the EPR signal is proportional to the concentration of the spin probe TEMPO in the SC and decreases exponentially with time. A decrease in the spin probe concentration, according to Hee et al. [[71],[72]], can be described by the radical marker quenching rate constant k, which directly depends on the antioxidant status of the SC.
Thus, the higher the antioxidant status of the SC, the faster the neutralization of TEMPO and the faster the EPR signal intensity decreases and, consequently, the higher is the rate constant k. A positive correlation (R = 0.65) between the concentration of carotenoids and the decrease of the skin probe TEMPO is given implying that the more carotenoids are in the SC, the higher the rate constant k, i.e., the higher is the antioxidant status of the SC. These dependencies confirm that carotenoids can be considered as marker substances of the entire antioxidant status of the human SC of non-smokers in vivo [[73]], which was indirectly confirmed in other studies [[74],[75],[76]]. The obtained results are important for an easier investigation of the changes in the entire antioxidant status of the human SC in vivo based on the determination of the carotenoid concentration, which substantially extends the number of practical applications.
5. Factors Resulting in the Reduction of Carotenoids in the SC
External and internal stress-factors on human skin can result in the formation of free radicals which cause the decrease of antioxidant concentrations in all skin layers [[74]].
One of the main external stress factors is solar radiation. The effect of sunlight on the skin depends on the energy of the photons, the radiation dose and the penetration depth, which is minimal for UV and maximal for the IR-A spectral range. Air pollution by various gases and particulate matter has a negative effect on human skin [[77]] and can also act as an external stressor, leading to the formation of free radicals in human skin [[78]]. To protect the skin from the negative effects of external pollutants, topical application of antioxidants is effective [[79]]. Internal stressors include lifestyle habits such as smoking, alcohol abuse, high-intensity exercise, stress, and dietary habits, as well as diseases.
5.1. Effect of UV Radiation
Under the irradiation with UV-B (dose 0.03 J/cm2: light intensity 0.3 mW/cm2, irradiation time 100 s), a decrease of the carotenoid concentration in the human SC over time was demonstrated in vivo using RRS [[80]]. A decrease of the lycopene / ß-carotene concentration is observed 0–30 / 30–90 min after termination of irradiation, which is probably caused by different quenching rates in the neutralization of ROS. The maximal decrease was observed 2–3 h post-irradiation, which seems to be related to the induction of inflammatory reactions and the subsequent oxidative stress. The recovery of carotenoids in the SC to the initial level lasted up to three days [[80]].
Sunscreens are often used to protect the skin from solar UV radiation [[81]]. They contain absorbers, reflectors, and scatterers of UV light. Plant extracts [[82]] and other antioxidants [[83]] are also used to enhance skin protection [[84],[85]] and to increase the photo-stability of sunscreens [[86]].
5.2. Effect of Visible Light
Using in vivo RRS, Vandersee et al. [[87]] showed that the concentration of carotenoids in the human SC decreased by 13–21% after irradiation with light of the violet-blue spectral range (400–495 nm; dose 50 or 100 J/cm2: light intensity 100 mW/cm2, irradiation time 500 or 1000 s). A decrease in the carotenoid concentration was observed immediately after irradiation, and recovery to the initial value lasted between 2 up to 24 h, depending on the irradiation dose (50 or 100 J/cm2). It was suggested that violet-blue light causes ROS formation in the skin, which induces a decrease of the carotenoid concentration in the SC, was confirmed in an in vivo study of human skin using EPR spectroscopy [[88]] and in numerous studies evaluating oxidative stress-induced changes in the skin [[89],[90],[91]].
To protect the skin from the effects of radiation in the visible spectral range, the use of sunscreens that protect in the UV spectral range is ineffective. The incorporation of filters that absorb, reflect, or scatter light in the visible spectral range is rarely used in practice because visible light filters frequently have a dense texture and are not transparent, thus adding an undesirable color to the cosmetic product and, after application, to the skin [[92]]. Recent human skin studies in vivo demonstrated that topical application of sunscreens containing 0.005% Glycyrrhiza inflata root extract, namely the phenolic compound licochalcone A, has a protective antioxidant effect on human skin by neutralizing the violet-blue radiation-induced ROS [[93]]. Thus, the addition of antioxidants to cream formulations is effective in neutralizing ROS, which was also confirmed previously [[84],[90]].
5.3. Effect of Near-Infrared Radiation
The decrease of the carotenoid concentration in the human SC after irradiation with near-IR light (600–3000 nm; dose 306–342 J/cm2: light intensity 170–190 mW/cm2, irradiation time 30 min) was shown in vivo using RRS [[94]]. It was hypothesized that the decrease in the carotenoid concentration might be due to the neutralization of the formed ROS, and this assumption was later confirmed by a temperature-controlled ex vivo study on porcine skin using EPR spectroscopy [[95]]. These results were preceded by the work of Zastrow et al. [[96]], in which the free radical action spectrum of excised human skin was demonstrated—about 50% of free radicals (predominantly ROS) are formed in the skin by irradiation in the visible and near-IR spectral ranges. ROS are formed in the skin as a result of irradiation in the IR-A and IR-B spectral ranges, which is confirmed by the immediate decrease of carotenoid concentration in the SC after the termination of irradiation [[97]]. Recovery to the initial value lasted up to 24 h and starts in the superficial SC depth [[28]].
To protect the skin from IR-A and IR-B radiation, the use of absorbers, reflectors, and scatterers as part of sunscreens is less effective compared to protection in the UV spectral range. An effective protection includes the topical application of antioxidants (carotenoids) that neutralize IR-generated ROS [[98],[99],[100]].
5.4. Impact of Internal Stress Factors and Diseases
Using non-invasive RRS and diffuse reflectance spectroscopy it was shown in human skin in vivo that the consumption of high doses of alcohol [[101]], excessive physical activity [[102]], domestic and work stress [[22],[103],[104]], and other stress factors [[104],[105]] result in a reduction of the carotenoid concentration in the human SC. It has been noticed that the concentration of carotenoids in the SC of smokers is significantly lower than in non-smokers [[106],[107],[108]], which is in agreement with the literature data [[109],[110],[111]]. The concentration of carotenoids in the SC of women is significantly higher than in men [[108]] and tends to decrease with increasing body mass index to values >30, which is also consistent with published data [[110],[111],[112]].
It is known that various diseases can cause a disturbance of the redox balance, which leads to the development of oxidative stress that is accompanied by a decrease in the antioxidant concentration [[113],[114],[115]].
A number of studies have shown an association between a diet rich in carotenoids, e.g., fruit and vegetables, and the risk of developing certain types of cancer according to the following principle: the more carotenoids in a diet, the lower the probability of cancer development [[116],[117],[118]]. The same relationship has been observed between a carotenoid-rich diet and the risk of cardiovascular [[119],[120]], eye [[121]], and other diseases [[30],[122]]. The inverse relationship for the development of lung cancer has been reported for heavy smoking individuals after supplementation of a high single dose of ß-carotene, which strongly exceeds the physiological concentration [[123]].
It was shown that the concentration of carotenoids in the SC of various cancer patients measured before chemotherapy was significantly lower than that of healthy individuals [[124]], probably due to the psychological burden and anxiety that cancer patients suffered from or the accumulating long-lasting effect of previous chemotherapy cycles [[125]]. Although the concentration of carotenoids in the SC does not correlate with the age of a healthy person [[108]], such a correlation has been found for the skin of cancer patients, which was decreasing with increasing patient age [[126]].
Thus, it could be concluded that the action of internal stressors, including various diseases, leads to a decrease in the concentration of carotenoids in the SC, which entails a decrease of the entire antioxidant status.
5.5. Effect of Chemotherapy
Doxorubicine and its metabolites appeared within the sweat glands spreading continuously over the skin surface of palms and soles, which exhibit a high density of sweat glands. The fluorescent signal of doxorubicine could be detected 1–2 h after starting the intravenous infusion and remained there for at least 4.5 h [[127]]. Before the invention of targeted therapies and immune therapy, many common chemotherapeutic agents were based on or caused radical formation, e.g., cytostatics inducing in particular ROS, increasing the amount of oxidation products in the body [[128],[129]]. Cutaneous antioxidants neutralize part of the chemotherapy-induced free radicals and are further destroyed. Thus, the higher the concentration of antioxidants in the patient’s skin, the more effective the neutralization of free radicals and protective potential regarding skin toxicities.
A chemotherapy including intravenous infusion of paclitaxel (175 mg/m2 every 3-weeks), docetaxel (30–35 mg/m2 every 3-weeks), or 5-fluorouracil (2400 mg/m2 every 2-weeks), leads to a significant decrease of the carotenoid concentration in the palm immediately after a single infusion by ≈5, ≈12, and ≈7% on average, respectively, which is an indirect indicator of increased oxidative processes [[126]].
Thus, it has been shown, directly and indirectly, that systemically applied chemotherapeutic agents can reach the skin surface as part of the sweat, where they re-penetrate into the SC, interacting with its components, and reducing the carotenoid concentration [[124],[126]].
6. Factors Leading to An Increase of Carotenoids in the SC
As demonstrated earlier, the carotenoid concentration in the SC decreases as a result of different stress factors. On the other hand, there is always a compensatory effect—the restoration of the carotenoid concentration in the SC, which is considerably slower compared to the degradation process [[80],[87],[94],[101]] and strongly depends on nutrition and on the carotenoids that are stored in the body.
6.1. Effect of a Carotenoid-Rich Nutrition on the Carotenoid Concentration in the SC
In vivo studies on humans show that the controlled consumption of dietary carotenoid-containing supplements leads to a significant dose-dependent increase in the carotenoid concentration in the blood plasma (on average by 200%) and in the SC (on average by 25%) [[130],[131],[132],[133]]. The same effect is observed with the consumption of carotenoid-containing food, in particular fruit and vegetables [[134],[135],[136],[137],[138],[139]]. At the same time, the carotenoid concentrations in the blood plasma and in the SC are strictly correlated [[64],[67],[140],[141],[142],[143]]. Using RRS, it was shown in vivo that an 8-week course of daily oral intake of plant antioxidants including carotenoids, has a long-term effect of increasing the carotenoid concentration in the SC [[131]]. After finishing the intake, the carotenoid concentration in the SC is reduced to its initial value within 5-weeks, which exceeds the 2–4-weeks of the SC renewal. Therefore, it has to be assumed that the carotenoids saturate the SC by continuously diffusing from the storage in the body.
In a placebo-controlled randomized study using diffuse reflectance spectroscopy [[144]] in 29 healthy women of Fitzpatrick skin Type II [[145]], the daily oral supplementation of a green cabbage carotenoid-rich extract (1.65 mg carotenoids/day) shows a significant increase of the carotenoid concentration in the SC of the palm by ≈10–22%. In addition, a significant increase in the concentration of collagen I after 5- and 10-months of green cabbage extract supplementation was shown on the cheek (increase by ≈18 and ≈17%, respectively) and the inner forearm (increase by ≈20 and ≈12%, respectively) [[144]]. Thus, increased concentrations of carotenoids in the SC lead to slower degradation of collagen I and, probably, promote the production of new collagen I by fibroblasts.
6.2. Effect of a Carotenoid-Rich Nutrition on the Antioxidant Status of the SC
The intake of the verum carotenoid-rich supplement (4.45 mg carotenoids/day) within 4- and 8-weeks resulted in a significant increase in the carotenoid concentration in the SC [[107]], which is in agreement with previous results with a 4-weeks supplementation (9 mg carotenoids/day) [[132]]. A total of 12-weeks after the termination of the dietary supplementation, the carotenoid concentration in the SC had decreased to the initial level. In the placebo group the carotenoid concentrations in the SC did not significantly change over the total duration of the studyresearch. The rate constant k correlates with the carotenoid concentration: unchanged in the placebo group, increasing after 4- and 8-weeks in the verum group, and decreasing to the initial value 12-weeks after the intake termination. After an 8-week intake of the verum supplement, when the difference in the carotenoid concentration and antioxidant status in the SC was the highest between the two groups, all the volunteers were exposed to a sunlight simulator (420–2000 nm; dose 72 J/cm2: light intensity 120 mW/cm2, exposure time 10 min) to induce free radicals. The free radicals were recorded in vivo by EPR spectroscopy using the stable nitroxide spin probe PCA (3-carboxy-2,2,5,5-tetramethyl-1-pyrrolidine-1-oxyl), which does not react with antioxidants of the human epidermis [[5]]. The results show that the amount of free radicals generated in the SC is significantly lower (by ≈34%) in the verum than in the placebo group. This effect is related to the antioxidant property of carotenoids to neutralize free radicals, further confirming the increased antioxidant status of the SC in the verum compared to the placebo group. These results explain the reduction of the UV-B light-induced erythema intensity, which had been reported in other studies for the group of volunteers taking carotenoid supplements [[65],[101],[146]] and further support the conclusion that carotenoids can be considered as marker substances for the entire antioxidant status of the human SC in vivo [[73]]. Consequently, a diet that is enriched with carotenoids leads to an increase in their concentration in the skin, and also to an increase in the antioxidant status of the SC [[107]].
6.3. Effect of a Carotenoid-Rich Cosmetics on the Carotenoid Concentration in the SC
The fastest way to increase the concentration of antioxidants in the SC is the topical application of a cream containing antioxidants, which increases the skin protection against the effects of IR [[81],[84],[98],[99],[100]] and visible [[81],[84],[93],[98]] radiation. It was shown that the daily application of a cream containing antioxidants, including 0.2% carotenoids, for 8-weeks has a short-term effect. After termination of the cream application the carotenoid concentration in the SC is reduced to the initial value within 10-days [[131]]. It has been demonstrated that combined oral and topical application of carotenoids leads to the most efficient and prolonged (up to 5-weeks) increase in the concentration of carotenoids in the SC [[131]]. These results are in agreement with the current data in the literature [[111],[147]].
6.4. Biofeedback as Motivation for a Healthier Lifestyle
Diffuse reflectance spectroscopy was used twice a week for 7-months to investigate changes in the concentration of carotenoids in the SC of the palm in 50 high school students. Before starting the restudyearch, basement values of the cutaneous carotenoids were assessed. Afterwards, an intervention was conducted; the students received lectures on the importance of a healthy nutrition and lifestyle. Furthermore, the function of antioxidants and the factors influencing its change were explained, which subsequently served as a motivating factor for a healthier lifestyle. After each measurement a questionnaire was completed including an assessment of the amount of fruit and vegetables in the daily diet, the current subjective stress level (including social and personal stress, exams, lack of sleep), health status (illness, fatigue), smoking, and alcohol consumption. The results showed that the concentration of carotenoids in the SC significantly increased after the intervention was conducted [[148]].
Another important result of this restudyearch was the observation of an even competitive motivation for higher antioxidant values among the participants. Comparing their skin carotenoid values with each other, the students started to compete for higher concentrations, thus striving for a healthier lifestyle. The rapid and non-invasive feedback by their antioxidant values gave the participants additional motivation to take part in the studyresearch and to improve their dietary and lifestyle habits [[148]].
References
- Flora SJ.; Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. . Oxid Med Cell Longev 2009, 2, 191.
- Allemann IB, Baumann L.; Botanicals in skin care products. . Int J Dermatol 2009, 48, 923.
- Masaki H.; Role of antioxidants in the skin: Anti-aging effects. . J Dermatol Sci 2010, 58, 85.
- Thiele JJ, Schroeter C, Hsieh SN, Podda M, Packer L.; The antioxidant network of the stratum corneum. . Curr Probl Dermatol 2001, 29, 26.
- Haag, S.F.; Bechtel, A.; Darvin, M.E.; Klein, F.; Groth, N.; Schafer-Korting, M.; Bittl, R.; Lademann, J.; Sterry, W.; Meinke, M.C.; et al. Comparative study of carotenoids, catalase and radical formation in human and animal skin.. Skin. Skin Pharmacol. Physiol. 2010, 23, 306, 10.1159/000313539.
- Clarke, K.A.; Dew, T.P.; Watson, R.E.B.; Farrar, M.D.; Osman, J.E.; Nicolaou, A.; Rhodes, L.E.; Williamson, G.; Green Tea Catechins and Their Metabolites in Human Skin before and after Exposure to Ultraviolet Radiation.. J Nutr Biochem 2016, 27, 203, 10.1016/j.jnutbio.2015.09.001.
- Markina, M.; Lebedeva, E.; Neudachina, L.; Stozhko, N.; Brainina, K.; Determination of Antioxidants in Human Skin by Capillary Zone Electrophoresis and Potentiometry.. Anal Lett 2016, 49, 1804, 10.1080/00032719.2015.1124111.
- Roth, E.; Manhart, N.; Wessner, B.; Assessing the Antioxidative Status in Critically Ill Patients.. Curr Opin Clin Nutr 2004, 7, 161, 10.1097/00075197-200403000-00010.
- Vahlquist, A.; Lee, J.B.; Michaelsson, G.; Rollman, O.; Vitamin A in Human Skin: II Concentrations of Carotene, Retinol and Dehydroretinol in Various Components of Normal Skin. . J. Invest. Dermatol. 1982, 79, 94, 10.1111/1523-1747.ep12500033.
- Woodby, B.; Penta, K.; Pecorelli, A.; Lila, M.A.; Valacchi, G.; Skin Health from the inside Out. . Annu. Rev. Food Sci. Technol. 2020, 11, 235, 10.1146/annurev-food-032519-051722.
- Rao, A.V.; Rao, L.G.; Carotenoids and Human Health. . Pharmacol Res 2007, 55, 207, 10.1016/j.phrs.2007.01.012.
- Yonekura, L.; Nagao, A.; Intestinal Absorption of Dietary Carotenoids.. Mol. Nutr. Food Res. 2007, 51 , 107, 10.1002/mnfr.200600145.
- Darvin, M.E.; Lademann, J.; von Hagen, J.; Lohan, S.B.; Kolmar, H.; Meinke, M.C.; Jung, S.; Carotenoids in Human Skin In Vivo: Antioxidant and Photo-Protectant Role against External and Internal Stressors. . Antioxidants 2022, 11, 1451, 10.3390/antiox11081451.
- Biesalski, H.K.; Hemmes, C.; Hopfenmuller, W.; Schmid, C.; Gollnick, H.P.; Effects of Controlled Exposure of Sunlight on Plasma and Skin Levels of Beta-Carotene.. Free Radic. Res. 1996, 24, 215, 10.3109/10715769609088019.
- Pisoschi, A.M.; Pop, A.; The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review.. Eur J Med Chem 2015, 97, 55, 10.1016/j.ejmech.2015.04.040.
- Ribaya-Mercado, J.D.; Garmyn, M.; Gilchrest, B.A.; Russell, R.M.; Skin Lycopene Is Destroyed Preferentially over Beta-Carotene during Ultraviolet Irradiation in Humans.. J. Nutr. 1995, 125, 1854, 10.1093/jn/125.7.1854.
- Stahl, W.; Heinrich, U.; Jungmann, H.; Sies, H.; Tronnier, H.; Carotenoids and Carotenoids plus Vitamin E Protect against Ultraviolet Light-Induced Erythema in Humans.. Am J Clin Nutr 2000, 71, 795.
- Calniquer, G.; Khanin, M.; Ovadia, H.; Linnewiel-Hermoni, K.; Stepensky, D.; Trachtenberg, A.; Sedlov, T.; Braverman, O.; Levy, J.; Sharoni, Y.; et al. Combined Effects of Carotenoids and Polyphenols in Balancing the Response of Skin Cells to UV Irradiation.. Molecules 2021, 26, 1931, 10.3390/molecules26071931.
- Darvin, M.E.; Sterry, W.; Lademann, J.; Resonance Raman Spectroscopy as an Effective Tool for the Determination of Antioxidative Stability of Cosmetic Formulations.. J. Biophotonics 2010, 3 , 82, 10.1002/jbio.200910060.
- Liu, D.H.; Shi, J.; Ibarra, A.C.; Kakuda, Y.; Xue, S.J.; The Scavenging Capacity and Synergistic Effects of Lycopene, Vitamin E, Vitamin C, and Beta-Carotene Mixtures on the DPPH Free Radical.. Lwt-Food Sci Technol 2008, 41, 1344, 10.1016/j.lwt.2007.08.001.
- Hellemans, L.; Corstjens, H.; Neven, A.; Declercq, L.; Maes, D.; Antioxidant Enzyme Activity in Human Stratum Corneum Shows Seasonal Variation with an Age-Dependent Recovery.. J. Invest. Dermatol. 2003, 120, 434, 10.1046/j.1523-1747.2003.12056.x.
- Darvin, M.E.; Patzelt, A.; Knorr, F.; Blume-Peytavi, U.; Sterry, W.; Lademann, J.; One-Year Study on the Variation of Carotenoid Antioxidant Substances in Living Human Skin: Influence of Dietary Supplementation and Stress Factors. . J. Biomed. Opt. 2008, 13, 044028, 10.1117/1.2952076.
- Thiele, J.J.; Weber, S.U.; Packer, L.; Sebaceous Gland Secretion Is a Major Physiologic Route of Vitamin E Delivery to Skin.. J Invest Dermatol 1999, 113, 1006, 10.1046/j.1523-1747.1999.00794.x.
- Ekanayake-Mudiyanselage, S.; Thiele, J.; Sebaceous glands as transporters of vitamin E.. Hautarzt 2006, 57, 291, 10.1007/s00105-005-1090-7.
- Choe, C.; Ri, J.; Schleusener, J.; Lademann, J.; Darvin, M.E.; The Non-Homogenous Distribution and Aggregation of Carotenoids in the Stratum Corneum Correlates with the Organization of Intercellular Lipids in Vivo.. Exp. Dermatol. 2019, 28, 1237, 10.1111/exd.14018.
- Darvin, M.E.; Fluhr, J.W.; Caspers, P.; van der Pool, A.; Richter, H.; Patzelt, A.; Sterry, W.; Lademann, J.; In Vivo Distribution of Carotenoids in Different Anatomical Locations of Human Skin: Comparative Assessment with Two Different Raman Spectroscopy Methods.. Exp. Dermatol. 2009, 18, 1060, 10.1111/j.1600-0625.2009.00946.x.
- Lademann, J.; Caspers, P.J.; van der Pol, A.; Richter, H.; Patzelt, A.; Zastrow, L.; Darvin, M.; Sterry, W.; Fluhr, J.W.; In Vivo Raman Spectroscopy Detects Increased Epidermal Antioxidative Potential with Topically Applied Carotenoids.. Laser Phys Lett 2009, 6, 76, 10.1002/lapl.200810092.
- Fluhr, J.W.; Caspers, P.; van der Pol, J.A.; Richter, H.; Sterry, W.; Lademann, J.; Darvin, M.E.; Kinetics of Carotenoid Distribution in Human Skin in Vivo after Exogenous Stress: Disinfectant and WIRA-Induced Carotenoid Depletion Recovers from Outside to Inside. . J. Biomed. Opt. 2011, 16, 035002, 10.1117/1.3555183.
- Maghsoudi, S.; Taghavi Shahraki, B.; Rabiee, N.; Fatahi, Y.; Bagherzadeh, M.; Dinarvand, R.; Ahmadi, S.; Rabiee, M.; Tahriri, M.; Hamblin, M.R.; et al.et al. The Colorful World of Carotenoids: A Profound Insight on Therapeutics and Recent Trends in Nano Delivery Systems.. Critical Reviews in Food Science and Nutrition 2022, 62, 3658, 10.1080/10408398.2020.1867958.
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G.; The Benefits and Risks of Certain Dietary Carotenoids That Exhibit Both Anti- and Pro-Oxidative Mechanisms-A Comprehensive Review.. Antioxidants-Basel 2020, 9, 264, 10.3390/antiox9030264.
- Jomova, K.; Valko, M.; Health Protective Effects of Carotenoids and Their Interactions with Other Biological Antioxidants.. Eur J Med Chem 2013, 70, 102, 10.1016/j.ejmech.2013.09.054.
- Melendez-Martinez, A.J.; Stinco, C.M.; Mapelli-Brahm, P.; Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene.. Nutrients 2019, 11, 1093, 10.3390/nu11051093.
- Krinsky, N.I.; Carotenoids as Antioxidants. . Nutrition 2001, 17, 815, 10.1016/S0899-9007(01)00651-7.
- Paiva, S.A.R.; Russell, R.M.; Beta-Carotene and Other Carotenoids as Antioxidants. . J Am Coll Nutr 1999, 18, 426, 10.1080/07315724.1999.10718880.
- Stahl, W.; Sies, H.; Antioxidant Activity of Carotenoids. . Mol Aspects Med 2003, 24, 345, 10.1016/S0098-2997(03)00030-X.
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al.et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. . Antioxidants-Basel 2020, 9, 706, 10.3390/antiox9080706.
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A.; Vital Roles of Carotenoids in Plants and Humans to Deteriorate Stress with Its Structure, Biosynthesis, Metabolic Engineering and Functional Aspects.. Curr Plant Biol 2021, 26, 100203, ARTN 100203 10.1016/j.cpb.2021.100203.
- Di Mascio, P.; Kaiser, S.; Sies, H.; Lycopene as the Most Efficient Biological Carotenoid Singlet Oxygen Quencher. . Arch Biochem Biophys 1989, 274, 532, 10.1016/0003-9861(89)90467-0.
- Cantrell, A.; McGarvey, D.J.; Truscott, T.G.; Rancan, F.; Bohm, F.; Singlet Oxygen Quenching by Dietary Carotenoids in a Model Membrane Environment.. Arch Biochem Biophys 2003, 412, 47, 10.1016/S0003-9861(03)00014-6.
- Mortensen, A.; Skibsted, L.H.; Reactivity of Beta-Carotene towards Peroxyl Radicals Studied by Laser Flash and Steady-State Photolysis.. Febs Lett 1998, 426, 392, 10.1016/S0014-5793(98)00382-2.
- Novikov, V.S.; Kuzmin, V.V.; Darvin, M.E.; Lademann, J.; Sagitova, E.A.; Prokhorov, K.A.; Ustynyuk, L.Y.; Nikolaeva, G.Y.; Relations between the Raman Spectra and Molecular Structure of Selected Carotenoids: DFT Study of Alpha-Carotene, Beta-Carotene, Gamma-Carotene and Lycopene. . Spectrochim Acta A 2022, 270, 120755, 10.1016/j.saa.2021.120755.
- Darvin, M.E.; Gersonde, I.; Ey, S.; Brandt, N.N.; Albrecht, H.; Gonchukov, S.A.; Sterry, W.; Lademann, J.; Noninvasive Detection of Beta-Carotene and Lycopene in Human Skin Using Raman Spectroscopy.. Laser Phys 2004, , 14, 231.
- Parker, R.S.; Carotenoids in Human Blood and Tissues.. The Journal of Nutrition 1989, 119, 101, 10.1093/jn/119.1.101.
- Kiely, M.; Cogan, P.F.; Kearney, P.J.; Morrissey, P.A.; Concentrations of Tocopherols and Carotenoids in Maternal and Cord Blood Plasma.. Eur J Clin Nutr 1999, 53, 711, 10.1038/sj.ejcn.1600838.
- Talwar, D.; Ha, T.K.; Cooney, J.; Brownlee, C.; O’Reilly, D.S.; A Routine Method for the Simultaneous Measurement of Retinol, Alpha-Tocopherol and Five Carotenoids in Human Plasma by Reverse Phase HPLC. . Clin. Chim. Acta 1998, 270, 85, 10.1016/s0009-8981(97)00224-6.
- Elvira-Torales, L.I.; Garcia-Alonso, J.; Periago-Caston, M.J.; Nutritional Importance of Carotenoids and Their Effect on Liver Health: A Review.. Antioxidants-Basel 2019, 8, 229, 10.3390/antiox8070229.
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.W.; Thurnham, D.; Yin, S.A.; Biesalski, H.K.; Beta-Carotene Is an Important Vitamin A Source for Human. . J Nutr 2010, 140, 2268s, 10.3945/jn.109.119024.
- Mordi, R.C.; Ademosun, O.T.; Ajanaku, C.O.; Olanrewaju, I.O.; Walton, J.C.; Free Radical Mediated Oxidative Degradation of Carotenes and Xanthophylls.. Molecules 2020, 25, 1038, 10.3390/molecules25051038.
- Kaplan, L.A.; Lau, J.M.; Stein, E.A.; Carotenoid Composition, Concentrations, and Relationships in Various Human Organs.. Clin Physiol Biochem 1990, , 8, 1.
- Östh, M.; Öst, A.; Kjolhede, P.; Strålfors, P.; The Concentration of β-Carotene in Human Adipocytes, but Not the Whole-Body Adipocyte Stores, Is Reduced in Obesity.. PLOS ONE 2014, 9, e85610, 10.1371/journal.pone.0085610.
- van de Ven, M.; Kattenberg, M.; van Ginkel, G.; Levine, Y.K.; Study of the Orientational Ordering of Carotenoids in Lipid Bilayers by Resonance-Raman Spectroscopy.. Biophysical Journal 1984, 45, 1203, 10.1016/S0006-3495(84)84269-1.
- Yakimov, B.P.; Venets, A.V.; Schleusener, J.; Fadeev, V.V.; Lademann, J.; Shirshin, E.A.; Darvin, M.E.; Blind Source Separation of Molecular Components of the Human Skin in Vivo: Non-Negative Matrix Factorization of Raman Microspectroscopy Data.. Analyst 2021, 146, 3185, 10.1039/D0AN02480E.
- Fluhr, J.W.; Sassning, S.; Lademann, O.; Darvin, M.E.; Schanzer, S.; Kramer, A.; Richter, H.; Sterry, W.; Lademann, J.; In Vivo Skin Treatment with Tissue-Tolerable Plasma Influences Skin Physiology and Antioxidant Profile in Human Stratum Corneum.. Exp. Dermatol. 2012, 21, 130, 10.1111/j.1600-0625.2011.01411.x.
- Hata, T.R.; Scholz, T.A.; Ermakov, I.V.; McClane, R.W.; Khachik, F.; Gellermann, W.; Pershing, L.K.; Non-Invasive Raman Spectroscopic Detection of Carotenoids in Human Skin.. J. Invest. Dermatol. 2000, 115, 441, 10.1046/j.1523-1747.2000.00060.x.
- Ermakov, I.V.; Ermakova, M.R.; Gellermann, W.; Lademann, J.; Noninvasive Selective Detection of Lycopene and Beta-Carotene in Human Skin Using Raman Spectroscopy.. J Biomed Opt 2004, 9, 332, 10.1117/1.1646172.
- Darvin, M.E.; Gersonde, I.; Albrecht, H.; Gonchukov, S.A.; Sterry, W.; Lademann, J.; Determination of Beta Carotene and Lycopene Concentrations in Human Skin Using Resonance Raman Spectroscopy.. Laser Phys 2005, 15, 295.
- Darvin, M.E.; Gersonde, I.; Albrecht, H.; Meinke, M.; Sterry, W.; Lademann, J.; Non-Invasive in Vivo Detection of the Carotenoid Antioxidant Substance Lycopene in the Human Skin Using the Resonance Raman Spectroscopy.. Laser Phys Lett 2006, 3, 460, 10.1002/lapl.200610032.
- Darvin, M.E.; Choe, C.S.; Schleusener, J.; Lademann, J.; Non-Invasive Depth Profiling of the Stratum Corneum in Vivo Using Confocal Raman Microscopy Considering the Non-Homogeneous Distribution of Keratin.. Biomed. Opt. Express 2019, , 10, 3092, 10.1364/BOE.10.003092.
- Darvin, M.E.; Schleusener, J.; Lademann, J.; Choe, C.-S.; Current Views on Noninvasive in Vivo Determination of Physiological Parameters of the Stratum Corneum Using Confocal Raman Microspectroscopy.. Skin Pharmacol Physiol 2022, 35, 125, 10.1159/000521416.
- Ashton, L.M.; Pezdirc, K.B.; Hutchesson, M.J.; Rollo, M.E.; Collins, C.E.; Is Skin Coloration Measured by Reflectance Spectroscopy Related to Intake of Nutrient-Dense Foods? A Cross-Sectional Evaluation in Australian Young Adults.. Nutrients 2017, 10, 11, 10.3390/nu10010011.
- Whitehead, R.D.; Coetzee, V.; Ozakinci, G.; Perrett, D.I.; Cross-Cultural Effects of Fruit and Vegetable Consumption on Skin Color.. Am. J. Public Health 2012, 102, 212, 10.2105/AJPH.2011.300495.
- Whitehead, R.D.; Re, D.; Xiao, D.; Ozakinci, G.; Perrett, D.I.; You Are What You Eat: Within-Subject Increases in Fruit and Vegetable Consumption Confer Beneficial Skin-Color Changes.. PLoS One 2012, 7, e32988, 10.1371/journal.pone.0032988.
- Tan, K.W.; Graf, B.A.; Mitra, S.R.; Stephen, I.D.; Daily Consumption of a Fruit and Vegetable Smoothie Alters Facial Skin Color.. PLoS One 2015, 10, e0133445, 10.1371/journal.pone.0133445.
- Pezdirc, K.; Hutchesson, M.J.; Williams, R.L.; Rollo, M.E.; Burrows, T.L.; Wood, L.G.; Oldmeadow, C.; Collins, C.E.; Consuming High-Carotenoid Fruit and Vegetables Influences Skin Yellowness and Plasma Carotenoids in Young Women: A Single-Blind Randomized Crossover Trial.. J Acad Nutr Diet 2016, 116, 1257, 10.1016/j.jand.2016.03.012.
- Alaluf, S.; Heinrich, U.; Stahl, W.; Tronnier, H.; Wiseman, S.; Dietary Carotenoids Contribute to Normal Human Skin Color and UV Photosensitivity.. J. Nutrition 2002, 132, 399, 10.1093/jn/132.3.399.
- Darvin, M.E.; Sandhagen, C.; Koecher, W.; Sterry, W.; Lademann, J.; Meinke, M.C.; Comparison of Two Methods for Noninvasive Determination of Carotenoids in Human and Animal Skin: Raman Spectroscopy versus Reflection Spectroscopy.. J. Biophotonics 2012, 5, 550, 10.1002/jbio.201100080.
- Darvin, M.E.; Magnussen, B.; Lademann, J.; Kocher, W.; Multiple Spatially Resolved Reflection Spectroscopy for in Vivo Determination of Carotenoids in Human Skin and Blood.. Laser Phys Lett 2016, 13, 095601, Artn 095601 10.1088/1612-2011/13/9/095601.
- Ermakov, I.V.; Gellermann, W.; Dermal Carotenoid Measurements via Pressure Mediated Reflection Spectroscopy.. J Biophotonics 2012, 5, 559, 10.1002/jbio.201100122.
- Darvin, M.E.; Meinke, M.C.; Sterry, W.; Lademann, J.; Optical Methods for Noninvasive Determination of Carotenoids in Human and Animal Skin.. J. Biomed. Opt. 2013, 18, 61230, 10.1117/1.JBO.18.6.061230.
- Herrling, T.; Jung, K.; Fuchs, J.; Measurements of UV-Generated Free Radicals/Reactive Oxygen Species (ROS) in Skin.. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 63, 840, 10.1016/j.saa.2005.10.013.
- He, G.; Samouilov, A.; Kuppusamy, P.; Zweier, J.L.; In Vivo EPR Imaging of the Distribution and Metabolism of Nitroxide Radicals in Human Skin.. J. Magn. Reson. 2001, 148, 155, 10.1006/jmre.2000.2226.
- He, G.; Samouilov, A.; Kuppusamy, P.; Zweier, J.L.; In Vivo Imaging of Free Radicals: Applications from Mouse to Man.. Mol. Cell Biochem. 2002, 234–235, 359.
- Haag, S.F.; Taskoparan, B.; Darvin, M.E.; Groth, N.; Lademann, J.; Sterry, W.; Meinke, M.C.; Determination of the Antioxidative Capacity of the Skin in Vivo Using Resonance Raman and Electron Paramagnetic Resonance Spectroscopy.. Exp. Dermatol. 2011, 20, 483, 10.1111/j.1600-0625.2010.01246.x.
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T.; The Role of Carotenoids in Human Skin.. Molecules 2011, 16, 10491, 10.3390/molecules161210491.
- Lademann, J.; Patzelt, A.; Schanzer, S.; Richter, H.; Meinke, M.C.; Sterry, W.; Zastrow, L.; Doucet, O.; Vergou, T.; Darvin, M.E.; et al. Uptake of Antioxidants by Natural Nutrition and Supplementation: Pros and Cons from the Dermatological Point of View.. Skin Pharmacol Physiol 2011, 24, 269, 10.1159/000328725.
- Lademann, J.; Kocher, W.; Yu, R.; Meinke, M.C.; Na Lee, B.; Jung, S.; Sterry, W.; Darvin, M.E.; Cutaneous Carotenoids: The Mirror of Lifestyle?. Skin Pharmacol Physiol 2014, 27, 201, 10.1159/000357222.
- Araviiskaia, E.; Berardesca, E.; Bieber, T.; Gontijo, G.; Sanchez Viera, M.; Marrot, L.; Chuberre, B.; Dreno, B.; The Impact of Airborne Pollution on Skin. . J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1496, 10.1111/jdv.15583.
- Kim, K.E.; Cho, D.; Park, H.J.; Air Pollution and Skin Diseases: Adverse Effects of Airborne Particulate Matter on Various Skin Diseases.. Life Sci. 2016, 152, 126, 10.1016/j.lfs.2016.03.039.
- Burke, K.E.; Protection from Environmental Skin Damage with Topical Antioxidants.. Clin. Pharmacol. Ther. 2019, 105, 36, 10.1002/cpt.1235.
- Darvin, M.E.; Gersonde, I.; Albrecht, H.; Sterry, W.; Lademann, J.; In Vivo Raman Spectroscopic Analysis of the Influence of UV Radiation on Carotenoid Antioxidant Substance Degradation of the Human Skin.. Laser Phys 2006, 16, 833, 10.1134/S1054660x06050148.
- Darvin, M.E.; Richter, H.; Ahlberg, S.; Haag, S.F.; Meinke, M.C.; Le Quintrec, D.; Doucet, O.; Lademann, J.; Influence of Sun Exposure on the Cutaneous Collagen/Elastin Fibers and Carotenoids: Negative Effects Can Be Reduced by Application of Sunscreen.. J. Biophotonics 2014, 7, 735, 10.1002/jbio.201300171.
- Rabinovich, L.; Kazlouskaya, V.; Herbal Sun Protection Agents: Human Studies.. Clin. Dermatol. 2018, 36, 369, 10.1016/j.clindermatol.2018.03.014.
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L.; Natural Antioxidants: Multiple Mechanisms to Protect Skin from Solar Radiation.. Front. Pharmacol. 2018, 9, 392, 10.3389/fphar.2018.00392.
- Souza, C.; Maia Campos, P.; Schanzer, S.; Albrecht, S.; Lohan, S.B.; Lademann, J.; Darvin, M.E.; Meinke, M.C.; Radical-Scavenging Activity of a Sunscreen Enriched by Antioxidants Providing Protection in the Whole Solar Spectral Range.. Skin Pharmacol. Physiol. 2017, 30, 81, 10.1159/000458158.
- Guan, L.L.; Lim, H.W.; Mohammad, T.F.; Sunscreens and Photoaging: A Review of Current Literature.. Am J Clin Dermatol 2021, 22, 819, 10.1007/s40257-021-00632-5.
- Garbe, B.; Kockott, D.; Werner, M.; Theek, C.; Heinrich, U.; Braun, N.; The Influence of Short-Wave and Long-Wave Radiation Spectrum on the Photostability of Sunscreens.. Skin Pharmacol. Physiol. 2020, 33, 77, 10.1159/000505218.
- Vandersee, S.; Beyer, M.; Lademann, J.; Darvin, M.E.; Blue-Violet Light Irradiation Dose Dependently Decreases Carotenoids in Human Skin, Which Indicates the Generation of Free Radicals.. Oxid. Med. Cell Longev. 2015, 2015, 579675, 10.1155/2015/579675.
- Lohan, S.B.; Muller, R.; Albrecht, S.; Mink, K.; Tscherch, K.; Ismaeel, F.; Lademann, J.; Rohn, S.; Meinke, M.C.; Free Radicals Induced by Sunlight in Different Spectral Regions - in Vivo versus Ex Vivo Study.. Exp. Dermatol. 2016, 25, 380, 10.1111/exd.12987.
- Pourang, A.; Tisack, A.; Ezekwe, N.; Torres, A.E.; Kohli, I.; Hamzavi, I.H.; Lim, H.W.; Effects of Visible Light on Mechanisms of Skin Photoaging.. Photoderm Photoimm Photomed 2022, 38, 191, 10.1111/phpp.12736.
- Lim, H.W.; Kohli, I.; Ruvolo, E.; Kolbe, L.; Hamzavi, I.H.; Impact of Visible Light on Skin Health: The Role of Antioxidants and Free Radical Quenchers in Skin Protection.. J Am Acad Dermatol 2022, 86, S27, 10.1016/j.jaad.2021.12.024.
- Chamayou-Robert, C.; DiGiorgio, C.; Brack, O.; Doucet, O.; Blue Light Induces DNA Damage in Normal Human Skin Keratinocytes.. Photodermatology, Photoimmunology & Photomedicine 2022, 38, 69, 10.1111/phpp.12718.
- Lyons, A.B.; Trullas, C.; Kohli, I.; Hamzavi, I.H.; Lim, H.W.; Photoprotection beyond Ultraviolet Radiation: A Review of Tinted Sunscreens.. Journal of the American Academy of Dermatology 2021, 84, 1393, 10.1016/j.jaad.2020.04.079.
- Mann, T.; Eggers, K.; Rippke, F.; Tesch, M.; Buerger, A.; Darvin, M.E.; Schanzer, S.; Meinke, M.C.; Lademann, J.; Kolbe, L.; et al. High-Energy Visible Light at Ambient Doses and Intensities Induces Oxidative Stress of Skin-Protective Effects of the Antioxidant and Nrf2 Inducer Licochalcone A in Vitro and in Vivo.. Photodermatol. Photoimmunol. Photomed. 2020, 36, 135, 10.1111/phpp.12523.
- Darvin, M.E.; Gersonde, I.; Albrecht, H.; Zastrow, L.; Sterry, W.; Lademann, J.; In Vivo Raman Spectroscopic Analysis of the Influence of IR Radiation on the Carotenoid Antioxidant Substances Beta-Carotene and Lycopene in the Human Skin. Formation of Free Radicals.. Laser Phys Lett 2007, 4, 318, 10.1002/lapl.200610113.
- Darvin, M.E.; Haag, S.; Meinke, M.; Zastrow, L.; Sterry, W.; Lademann, J.; Radical Production by Infrared A Irradiation in Human Tissue.. Skin Pharmacol. Physiol. 2010, 23, 40, 10.1159/000257262.
- Zastrow, L.; Groth, N.; Klein, F.; Kockott, D.; Lademann, J.; Renneberg, R.; Ferrero, L.; The Missing Link--Light-Induced (280-1,600 Nm) Free Radical Formation in Human Skin. . Skin Pharmacol. Physiol. 2009, 22, 31, 10.1159/000188083.
- Darvin, M.E.; Patzelt, A.; Meinke, M.; Sterry, W.; Lademann, J.; Influence of Two Different IR Radiators on the Antioxidative Potential of the Human Skin.. Laser Phys Lett 2009, 6, 229, 10.1002/lapl.200810124.
- Lohan, S.B.; Lauer, A.C.; Arndt, S.; Friedrich, A.; Tscherch, K.; Haag, S.F.; Darvin, M.E.; Vollert, H.; Kleemann, A.; Gersonde, I.; et al.et al. Determination of the Antioxidant Status of the Skin by in Vivo - Electron Paramagnetic Resonance (EPR) Spectroscopy.. Cosmetics 2015, 2, 286, 10.3390/cosmetics2030286.
- Schroeder, P.; Lademann, J.; Darvin, M.E.; Stege, H.; Marks, C.; Bruhnke, S.; Krutmann, J.; Infrared Radiation-Induced Matrix Metalloproteinase in Human Skin: Implications for Protection.. J. Invest. Dermatol. 2008, 128, 2491, 10.1038/jid.2008.116.
- Darvin, M.E.; Fluhr, J.W.; Meinke, M.C.; Zastrow, L.; Sterry, W.; Lademann, J.; Topical Beta-Carotene Protects against Infra-Red-Light-Induced Free Radicals.. Exp. Dermatol. 2011, 20, 125, 10.1111/j.1600-0625.2010.01191.x.
- Darvin, M.E.; Sterry, W.; Lademann, J.; Patzelt, A.; Alcohol Consumption Decreases the Protection Efficiency of the Antioxidant Network and Increases the Risk of Sunburn in Human Skin. . Skin Pharmacol. Physiol. 2013, 26, 45, 10.1159/000343908.
- Vierck, H.B.; Darvin, M.E.; Lademann, J.; Reisshauer, A.; Baack, A.; Sterry, W.; Patzelt, A.; The Influence of Endurance Exercise on the Antioxidative Status of Human Skin.. Eur. J. Appl. Physiol. 2012, 112, 3361, 10.1007/s00421-011-2296-2.
- Jung, S.; Darvin, M.E.; Chung, H.S.; Jung, B.; Lee, S.H.; Lenz, K.; Chung, W.S.; Yu, R.X.; Patzelt, A.; Lee, B.N.; et al.et al. Antioxidants in Asian-Korean and Caucasian Skin: The Influence of Nutrition and Stress.. Skin Pharmacol. Physiol. 2014, 27, 293, 10.1159/000361053.
- Maeter, H.; Briese, V.; Gerber, B.; Darvin, M.E.; Lademann, J.; Olbertz, D.M.; Case Study: In Vivo Stress Diagnostics by Spectroscopic Determination of the Cutaneous Carotenoid Antioxidant Concentration in Midwives Depending on Shift Work.. Laser Phys Lett 2013, 10, 105701, Artn 105701 10.1088/1612-2011/10/10/105701.
- Lademann, H.; Gerber, B.; Olbertz, D.M.; Darvin, M.E.; Stauf, L.; Ueberholz, K.; Heinrich, V.; Lademann, J.; Briese, V.; Non-Invasive Spectroscopic Determination of the Antioxidative Status of Gravidae and Neonates.. Skin Pharmacol. Physiol. 2015, 28, 189, 10.1159/000365520.
- Lohan, S.B.; Bühring, K.; Lauer, A.-C.; Friedrich, A.; Lademann, J.; Buss, A.; Sabat, R.; Wolk, K.; Meinke, M.C.; Analysis of the Status of the Cutaneous Endogenous and Exogenous Antioxidative System of Smokers and the Short-Term Effect of Defined Smoking Thereon.. Antioxidants 2020, 9, 537, 10.3390/antiox9060537.
- Meinke, M.C.; Friedrich, A.; Tscherch, K.; Haag, S.F.; Darvin, M.E.; Vollert, H.; Groth, N.; Lademann, J.; Rohn, S.; Influence of Dietary Carotenoids on Radical Scavenging Capacity of the Skin and Skin Lipids.. Eur. J. Pharm. Biopharm. 2013, 84, 365, 10.1016/j.ejpb.2012.11.012.
- Meinke, M.C.; Lauer, A.C.; Taskoparan, B.; Gersonde, I.; Lademann, J.; Darvin, M.E.; Influence on the Carotenoid Levels of Skin Arising from Age, Gender, Body Mass Index in Smoking/Non-Smoking Individuals.. Free Radic. Antiox. 2011, 1, 14.
- Mayne, S.T.; Cartmel, B.; Scarmo, S.; Lin, H.Q.; Leffell, D.J.; Welch, E.; Ermakov, I.; Bhosale, P.; Bernstein, P.S.; Gellermann, W.; et al. Noninvasive Assessment of Dermal Carotenoids as a Biomarker of Fruit and Vegetable Intake. . Am J Clin Nutr 2010, 92, 794, 10.3945/ajcn.2010.29707.
- Mayne, S.T.; Cartmel, B.; Scarmo, S.; Jahns, L.; Ermakov, I.V.; Gellermann, W.; Resonance Raman Spectroscopic Evaluation of Skin Carotenoids as a Biomarker of Carotenoid Status for Human Studies.. Arch Biochem Biophys 2013, 539, 163, 10.1016/j.abb.2013.06.007.
- Obana, A.; Gohto, Y.; Gellermann, W.; Ermakov, I.V.; Sasano, H.; Seto, T.; Bernstein, P.S.; Skin Carotenoid Index in a Large Japanese Population Sample.. Sci. Rep. 2019, 9, 9318, 10.1038/s41598-019-45751-6.
- Rerksuppaphol, S.; Rerksuppaphol, L.; Effect of Fruit and Vegetable Intake on Skin Carotenoid Detected by Non-Invasive Raman Spectroscopy.. J. Med. Assoc. Thai. 2006, 89, 1206.
- Pham-Huy, L.A.; He, H.; Pham-Huy, C.; Free Radicals, Antioxidants in Disease and Health. . Int. J. Biomed. Sci. 2008, 4, 89.
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J.; Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. . Int. J. Biochem. Cell Biol. 2007, 39, 44, 10.1016/j.biocel.2006.07.001.
- Matschke, V.; Theiss, C.; Matschke, J.; Oxidative Stress: The Lowest Common Denominator of Multiple Diseases.. Neural Regen Res 2019, 14, 238, 10.4103/1673-5374.244780.
- Avila-Roman, J.; Garcia-Gil, S.; Rodriguez-Luna, A.; Motilva, V.; Talero, E.; Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids. . Mar Drugs 2021, 19, 531, ARTN 531 10.3390/md19100531.
- Amengual, J.; Bioactive Properties of Carotenoids in Human Health.. Nutrients 2019, 11, 2388, ARTN 2388 10.3390/nu11102388.
- Donaldson, M.S.; Nutrition and Cancer: A Review of the Evidence for an Anti-Cancer Diet.. Nutr J 2004, 3, 19, Artn 19 10.1186/1475-2891-3-19.
- Voutilainen, S.; Nurmi, T.; Mursu, J.; Rissanen, T.H.; Carotenoids and Cardiovascular Health.. Am J Clin Nutr 2006, 83, 1265.
- Toh, D.W.K.; Sutanto, C.N.; Loh, W.W.; Lee, W.Y.; Yao, Y.; Ong, C.N.; Kim, J.E.; Skin Carotenoids Status as a Potential Surrogate Marker for Cardiovascular Disease Risk Determination in Middle-Aged and Older Adults.. Nutrition, Metabolism and Cardiovascular Diseases 2021, 31, 592, 10.1016/j.numecd.2020.10.016.
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M.; A Mechanistic Review of Beta-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. . Antioxidants (Basel) 2020, 9, 1046, 10.3390/antiox9111046.
- Takayanagi, Y.; Obana, A.; Muto, S.; Asaoka, R.; Tanito, M.; Ermakov, I.V.; Bernstein, P.S.; Gellermann, W.; Relationships between Skin Carotenoid Levels and Metabolic Syndrome.. Antioxidants 2022, 11, 14, 10.3390/antiox11010014.
- Fiedor, J.; Burda, K.; Potential Role of Carotenoids as Antioxidants in Human Health and Disease.. Nutrients 2014, 6, 466, 10.3390/nu6020466.
- Jung, S.; Darvin, M.E.; Schleusener, J.; Thiede, G.; Lademann, J.; Braune, M.; Maiwald, M.; Sumpf, B.; Trankle, G.; Kutzer, D.; et al.et al. In Vivo Detection of Changes in Cutaneous Carotenoids after Chemotherapy Using Shifted Excitation Resonance Raman Difference and Fluorescence Spectroscopy. . Skin Res. Technol. 2020, 26, 301, 10.1111/srt.12800.
- Li, D.G.; LeCompte, G.; Golod, L.; Cecchi, G.; Irwin, D.; Harken, A.; Matecki, A.; Dermal Carotenoid Measurement Is Inversely Related to Anxiety in Patients with Breast Cancer.. J Invest Med 2018, 66, 329, 10.1136/jim-2017-000546.
- Lee, B.N.; Jung, S.; Darvin, M.E.; Eucker, J.; Kuhnhardt, D.; Sehouli, J.; Chekerov, R.; Patzelt, A.; Fuss, H.; Yu, R.X.; et al.et al. Influence of Chemotherapy on the Antioxidant Status of Human Skin.. Anticancer Res. 2016, 36, 4089.
- Lademann, J.; Martschick, A.; Jacobi, U.; Richter, H.; Darvin, M.; Sehouli, J.; Oskay-Oezcelik, G.; Blohmer, J.U.; Lichtenegger, W.; Sterry, W.; et al. Investigation of Doxorubicin an the Skin: A Spectroscopic Study to Understand the Pathogenesis of PPE. . J Clin Oncol 2005, 23, 477s.
- Sangeetha, P.; Das, U.N.; Koratkar, R.; Suryaprabha, P.; Increase in Free-Radical Generation and Lipid-Peroxidation Following Chemotherapy in Patients with Cancer.. Free Radical Bio Med 1990, 8, 15, 10.1016/0891-5849(90)90139-A.
- Korac, B.; Buzadzic, B.; Doxorubicin Toxicity to the Skin: Possibility of Protection with Antioxidants Enriched Yeast.. J. Dermatol. Sci. 2001, 25, 45, 10.1016/s0923-1811(00)00106-7.
- Meinke, M.C.; Schanzer, S.; Lohan, S.B.; Shchatsinin, I.; Darvin, M.E.; Vollert, H.; Magnussen, B.; Kocher, W.; Helfmann, J.; Lademann, J.; et al. Comparison of Different Cutaneous Carotenoid Sensors and Influence of Age, Skin Type, and Kinetic Changes Subsequent to Intake of a Vegetable Extract. . J. Biomed. Opt. 2016, 21, 107002, 10.1117/1.JBO.21.10.107002.
- Darvin, M.E.; Fluhr, J.W.; Schanzer, S.; Richter, H.; Patzelt, A.; Meinke, M.C.; Zastrow, L.; Golz, K.; Doucet, O.; Sterry, W.; et al.et al. Dermal Carotenoid Level and Kinetics after Topical and Systemic Administration of Antioxidants: Enrichment Strategies in a Controlled in Vivo Study. . J. Dermatol. Sci. 2011, 64, 53, 10.1016/j.jdermsci.2011.06.009.
- Meinke, M.C.; Darvin, M.E.; Vollert, H.; Lademann, J.; Bioavailability of Natural Carotenoids in Human Skin Compared to Blood.. Eur. J. Pharm. Biopharm. 2010, 76, 269, 10.1016/j.ejpb.2010.06.004.
- Meinke, M.C.; Lohan, S.B.; Kocher, W.; Magnussen, B.; Darvin, M.E.; Lademann, J.; Multiple Spatially Resolved Reflection Spectroscopy to Monitor Cutaneous Carotenoids during Supplementation of Fruit and Vegetable Extracts in Vivo. . Skin Res. Technol. 2017, 23, 459, 10.1111/srt.12356.
- Darvin, M.E.; Gersonde, I.; Albrecht, H.; Sterry, W.; Lademann, J.; Resonance Raman Spectroscopy for the Detection of Carotenolds in Foodstuffs. Influence of the Nutrition on the Antioxidative Potential of the Skin.. Laser Phys Lett 2007, 4, 452, 10.1002/lapl.200710004.
- Scarmo, S.; Cartmel, B.; Lin, H.Q.; Leffell, D.J.; Ermakov, I.V.; Gellermann, W.; Bernstein, P.S.; Mayne, S.T.; Single v. Multiple Measures of Skin Carotenoids by Resonance Raman Spectroscopy as a Biomarker of Usual Carotenoid Status. . Brit J Nutr 2013, 110, 911, 10.1017/S000711451200582x.
- Pitts, S.B.J.; Moran, N.E.; Wu, Q.; Harnack, L.; Craft, N.E.; Hanchard, N.; Bell, R.; Moe, S.G.; Johnson, N.; Obasohan, J.; et al.et al. Pressure-Mediated Reflection Spectroscopy Criterion Validity as a Biomarker of Fruit and Vegetable Intake: A 2-Site Cross-Sectional Study of 4 Racial or Ethnic Groups.. J Nutr 2022, 152, 107, 10.1093/jn/nxab349.
- Keller, J.E.; Taylor, M.K.; Smith, A.N.; Littrell, J.; Spaeth, K.; Boeckman, C.R.; Burns, J.M.; Sullivan, D.K.; Correlation of Skin Carotenoid Content with 3-Day Dietary Intake in Community Dwelling Older Adults. . Journal of Food Composition and Analysis 2022, 105, 104243, 10.1016/j.jfca.2021.104243.
- Tarshish, E.; Hermoni, K.; Sharoni, Y.; Muizzuddin, N.; Effect of Lumenato Oral Supplementation on Plasma Carotenoid Levels and Improvement of Visual and Experiential Skin Attributes.. Journal of Cosmetic Dermatology 2022, 0, 1, 10.1111/jocd.14724.
- Kadoh, Y.; Takayanagi, Y.; Sasaki, J.; Tanito, M.; Fingertip-Measured Skin Carotenoids and Advanced Glycation End Product Levels in Glaucoma. . Antioxidants 2022, 11, 1138, 10.3390/antiox11061138.
- Nguyen, L.M.; Scherr, R.E.; Linnell, J.D.; Ermakov, I.V.; Gellermann, W.; Jahns, L.; Keen, C.L.; Miyamoto, S.; Steinberg, F.M.; Young, H.M.; et al.et al. Evaluating the Relationship between Plasma and Skin Carotenoids and Reported Dietary Intake in Elementary School Children to Assess Fruit and Vegetable Intake.. Arch Biochem Biophys 2015, 572, 73, 10.1016/j.abb.2015.02.015.
- Jilcott Pitts, S.B.; Johnson, N.S.; Wu, Q.; Firnhaber, G.C.; Preet Kaur, A.; Obasohan,; A Meta-Analysis of Studies Examining Associations between Resonance Raman Spectroscopy-Assessed Skin Carotenoids and Plasma Carotenoids among Adults and Children. . J. Nutrition Reviews 2022, 80, 230, 10.1093/nutrit/nuab016.
- Toh, D.W.K.; Loh, W.W.; Sutanto, C.N.; Yao, Y.; Kim, J.E.; Skin Carotenoid Status and Plasma Carotenoids: Biomarkers of Dietary Carotenoids, Fruits and Vegetables for Middle-Aged and Older Singaporean Adults.. British Journal of Nutrition 2021, 126, 1398, 10.1017/S0007114521000143.
- Matsumoto, M.; Suganuma, H.; Shimizu, S.; Hayashi, H.; Sawada, K.; Tokuda, I.; Ihara, K.; Nakaji, S.; Skin Carotenoid Level as an Alternative Marker of Serum Total Carotenoid Concentration and Vegetable Intake Correlates with Biomarkers of Circulatory Diseases and Metabolic Syndrome.. Nutrients 2020, 12, 1825, 10.3390/nu12061825.
- Meinke, M.C.; Nowbary, C.K.; Schanzer, S.; Vollert, H.; Lademann, J.; Darvin, M.E.; Influences of Orally Taken Carotenoid-Rich Curly Kale Extract on Collagen I/Elastin Index of the Skin.. Nutrients 2017, 9, 775, 10.3390/nu9070775.
- Fitzpatrick, T.B.; The Validity and Practicality of Sun-Reactive Skin Types I through VI.. Arch. Dermatol. 1988, 124, 869, 10.1001/archderm.124.6.869.
- Heinrich, U.; Gartner, C.; Wiebusch, M.; Eichler, O.; Sies, H.; Tronnier, H.; Stahl, W.; Supplementation with Beta-Carotene or a Similar Amount of Mixed Carotenoids Protects Humans from UV-Induced Erythema. . J. Nutr. 2003, 133, 98, 10.1093/jn/133.1.98.
- Zerres, S.; Stahl, W.; Carotenoids in Human Skin. . Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1865, 158588, 10.1016/j.bbalip.2019.158588.
- Yu, R.X.; Kocher, W.; Darvin, M.E.; Buttner, M.; Jung, S.; Lee, B.N.; Klotter, C.; Hurrelmann, K.; Meinke, M.C.; Lademann, J.; et al. Spectroscopic Biofeedback on Cutaneous Carotenoids as Part of a Prevention Program Could Be Effective to Raise Health Awareness in Adolescents.. J. Biophotonics 2014, 7, 926, 10.1002/jbio.201300134.
