You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Shaochuan Shi.
Floral scents possess high ornamental and economic values to rose production in the floricultural industry. IThe floral scent is on the past two decades, me of the most important traits in plants, which is essential to the fertilization of angiosperm plants by attracting and guiding pollinators. Molecular bases of floral scent production have been studied in the rose as well as their genetic inheritance. Some significant achievements have been acquired, such as the comprehensive rose genome and the finding of a novel geraniol synthase in plants.
- Rosa
- floral scents
- terpenoids
- phenylpropanoids
- benzenoids
- molecular breeding
1. Introduction
The floral scent is one of the most important traits in plants, which is essential to the fertilization of angiosperm plants by attracting and guiding pollinators [1]. Some volatile compounds in floral scents play important roles in plant defense against detrimental animals [2,3][2][3]. To humans, floral scent is an important flower trait; it brings mental pleasure. In addition, floral scent provides essential flavor to the food and perfume industries [4,5][4][5].
Most floral scent chemicals are produced by three general metabolic pathways, i.e., terpenoids, phenylpropanoids/benzenoids, and fatty-acid derivatives [1,6][1][6]. In plants, terpenes are the largest group of floral scent compounds, which are synthesized by two divergent pathways. One is the 2-C-Methyl-D-Erythritol-4-Phosphate (MEP) pathway, which is mainly located in plastids [7] and is responsible for the production of mono- and diterpenes [8]. The other is the mevalonate (MVA) pathway, which is mainly located in the cytosol, endoplasmic reticulum, and peroxisomes [9,10][9][10], and is responsible for the production of volatile sesquiterpenes. Phenylpropanoids/benzenoids represent the second-largest class of floral scent compounds [11], which are exclusively derived from L-phenylalanine (L-Phe). Most phenylpropanoids are not volatile unless they are further acylated or methylated at the C9 position, while benzenoids are volatile, which are synthesized through a branch of the phenylpropanoid pathway, i.e., the cinnamic acid pathway [12]. Fatty acid derivatives constitute the third largest class of flower volatiles, including low-molecular-weight alcohols, aldehydes, and lipids. Biosynthesis of volatile fatty acid derivatives is initiated by stereo-specific oxygenation of unsaturated C18 fatty acids, linolenic, and linoleic, and subsequently catalyzed by the lipoxygenase (LOX) pathway [13,14][13][14].
For centuries, roses have been one of the most important crops in the floriculture industry [15], and are highly popular worldwide as garden ornamental plants and cut flowers. Floral scent, as an important characteristic, not only improves the ornamental value of roses, but provides essential fragrances and flavorings for spices, perfumes, and cosmetics in related industries. Additionally, rose essential oil, which is composed of floral scent compounds, can be used as an analgesic or antispasmodic [16,17][16][17]. However, the development of fragrances in roses has been at a disadvantage in the breeding program, which has focused on the longevity and visual attributes of cut flowers for centuries [18]. The cause for the loss of fragrance in these flowers remains unknown, but it does not seem to conflict with the increase in the vase life [19]. Unraveling the molecular bases of floral scents is not only a fascinating topic in rose biology, but is also helpful for improving the yield of roses in the floriculture industry.
2. Scent Composition of Modern Roses
The evolution of the floral scent is a complex matter, which is influenced by various factors, including the dynamics between biosynthetic pathways and the balanced selection between pollinators and florivores, which may result in relatively rapid evolution [11]. Roses have been cultivated as early as 3000 BC in China, western Asia, and northern Africa [20]. Since the 14th century, when Chinese roses were first introduced to Europe, the Chinese and European roses began to hybridize extensively, forming the genetic basis of the ‘modern rose cultivars’ Rosa hybrida [21]. Although Rosa comprises approximately 200 species, only 8~20 contribute to the genetic make-up of modern roses, including Chinese rose R. chinensis, R. multiflora, and R. gigantea, and European rose R. moschata, R. gallica, R. canina, and R. Phoenicia [22,23][22][23]. Chinese and European roses differ greatly in scent composition [24,25,26,27,28,29,30][24][25][26][27][28][29][30]. Chinese roses principally produce lipid-derived alcohols and esters (such as hexenol and hexenyl acetate) and aromatic compounds (such as 3,5-dimethoxytoluene (DMT) and 1,3,5-trimethoxybenzene (TMB)), whereas the major scent components of European roses are 2-phenylethanol (2-PE) and a number of monoterpenes (such as rose oxide, geraniol, and nerol) (Table 1).Table 1.
Major components of rose floral scents.
| Compound Variety | Compounds | Odor | References |
|---|---|---|---|
| terpenes | β-cubebene | citrus, fruity, radish | [25,31][25][31] |
| β-elemene | herbal, waxy, fresh | [26,31][26][31] | |
| δ-cadinene | thyme, herbal, woody | [27] | |
| germacrene D | woody, spice | [31] | |
| geraniol | rose-like, sweet | [28,31,32][28][31][32] | |
| citronellol | fresh rosy | [27,28,31][27][28][31] | |
| nerol | lemon-like, floral | [27,28,32][27][28][32] | |
| linalool | citrus and floral | [27,32][27][32] | |
| farnesyl acetate | green-floral rose | [27] | |
| geranyl acetate | lavender | [27,[2731]][31] | |
| citronellyl acetate | fresh, rose, fruity odor | [25,31][25][31] | |
| neryl acetate | rose and lavender-like | [25,31][25][31] | |
| citral | citrus and lemon | [25,27][25][27] | |
| dihydro-β-ionone | violet-like and earthy | [27] | |
| rose oxide | herbal, green floral, earthy | [30] | |
| Phenylpropanoids/ benzenoids |
2-phenylethanol | honey-like | [28,30,31][28][30][31] |
| 2-phenylethyl acetate | sweet, honey, rosy, with a slight yeasty honey note | [27,30,31,32][27][30][31][32] | |
| 1,3,5-Trimethoxybenzene (TMB) | phenolic spicy, earthy note | [28,33,34][28][33][34] | |
| dimethoxytoluene (DMT) | fresh, earthy, phenolic spicy | [28,33][28][33] | |
| benzyl acetate | floral, fruity, sweet, fresh | [27,32][27][32] | |
| eugenol | clove, carnation | [28,35][28][35] | |
| methyl eugenol | clove, carnation | [27,34][27][34] | |
| methyl isoeugenol | clove, carnation, woody | [27,34][27][34] | |
| fatty-acid derivatives | cis-3-hexenyl-1-alcohol | fresh and leafy green | [25,28,32][25][28][32] |
| 2-hexenyl acetate | fresh, fruity green | [27,31,32][27][31][32] | |
| cis-3-hexenyl acetate | fresh and leafy green | [27,31][27][31] |
3. Molecular Research Progress on Rose Scent Biosynthesis
Molecular and genetic approaches, including the candidate gene approach, transcriptomic analysis, and genetic mapping, have been used to identify scent-related genes and to unravel the gene expression network and floral scent inheritance in roses [31,33,42,43,44,45,46][31][33][42][43][44][45][46]. With these tools and approaches, dozens of scent-related genes have been identified and functionally validated, and many pathways of floral scent production have been uncovered in the rose (Figure 1).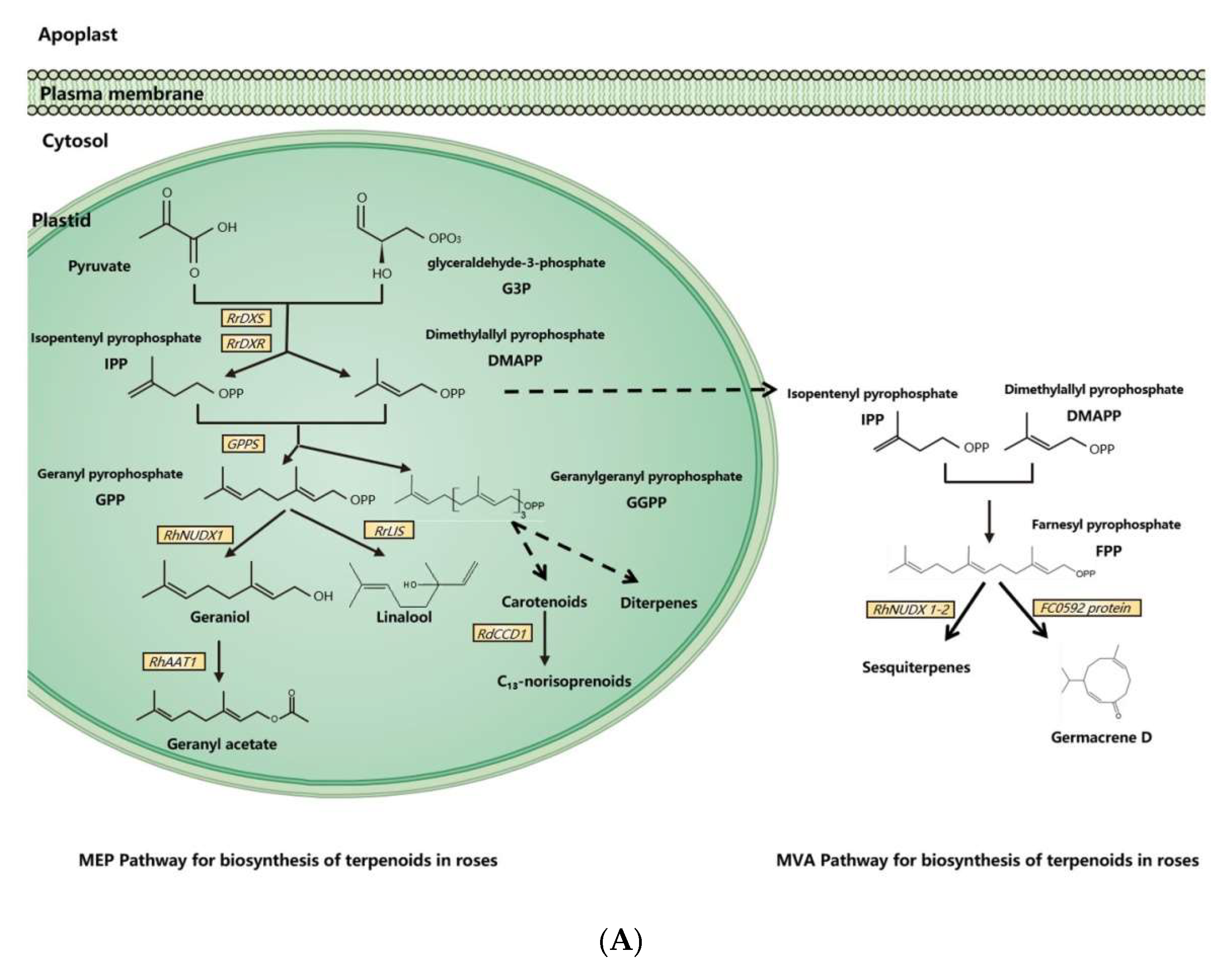
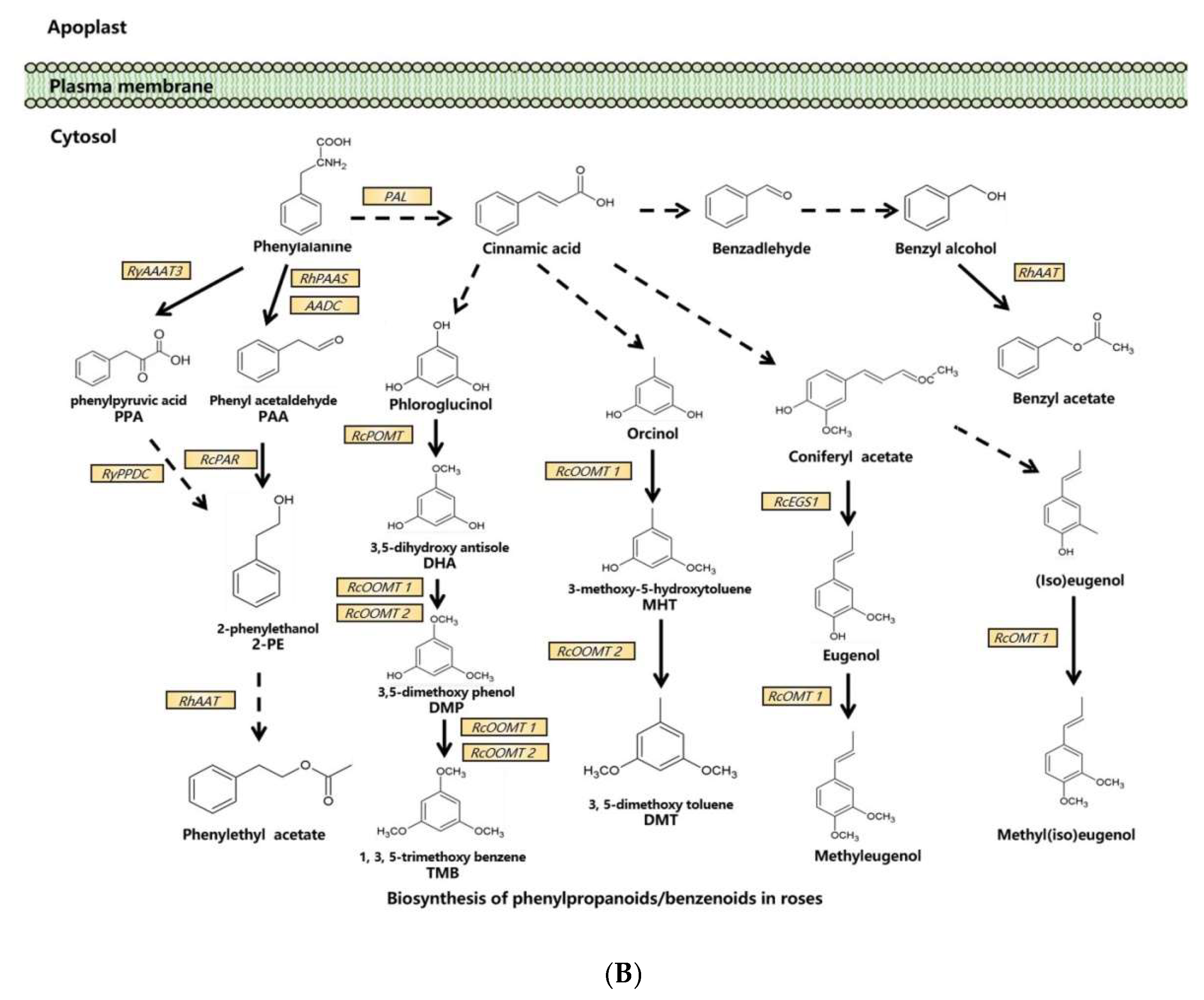
Figure 1. Synthesis pathways of floral scents in roses. (A) The biosynthesis pathway of terpenoids in roses. (B) Biosynthesis pathway of phenylpropanoids/benzenoids in roses. Solid lines indicate established biochemical reactions, and broken lines indicate possible steps. G3P: glyceraldehyde-3-phosphate; DXS: 1-deoxy-D-xylulose-5-phosphate synthase; DXR: 1-deoxy-D-xylulose-5-phosphate reductoisomerase; IPP: isopentenyl pyrophosphate; DMAPP: dimethylallyl pyrophosphate; GPP: geranyl pyrophosphate; GPPS: GPP synthase; GGPP, geranylgeranyl pyrophosphate; NUDX: nudix hydrolase; LIS: linalool synthase; AAT: alcohol acetyltransferase; CCD: carotenoid cleavage (di-)oxygenase; FPP: farnesyl pyrophosphate; PAL: phenylalanine ammonia lyase; PAAS: phenylacetaldehyde synthase; AADC: aromatic amino acid decarboxylase; AAAT: aromatic amino acid aminotransferase; PPA: phenylpyruvic acid; PAA: phenyl acetaldehyde; PAR: phenyl acetaldehyde reductase; PPDC: phenylpyruvate decarboxylase; 2-PE: 2-phenylethanol; POMT: phloroglucinol O-methyltransferase; OMT: O-methyltransferases; OOMT: orcinol O-methyltransferases; MHT: 3-methoxy-5-hydroxytoluene; DMT: 3,5-dimethoxy toluene; DHA: 3,5-dihydroxy antisole; DMP: 3,5-dimethoxy phenol; TMB: 1,3,5-trimethoxy benzene.
3.1. Biosynthesis of Terpenoids in Rose Floral Scents
Dimethylallyl pyrophosphate (DMAPP) and isopentenyl pyrophosphate (IPP) are synthesized from glyceraldehyde-3-phosphate (G3P) and pyruvate by a series of enzymes in plasmids [11]. As an enzyme for the synthesis of DMAPP and IPP, 1-deoxy-D-xylulose-5-phosphate reductoisomerase (RrDXR) is shown to play a key role in the production of volatile monoterpenes in R. rugosa [50][47]. Geranyl pyrophosphate (GPP) is synthesized from DMAPP and IPP by the MEP pathway in plastids [60][48]. GPP is a precursor of monoterpenes in plants, from which various monoterpenes are produced by a series of monoterpene synthases. In roses, a novel monoterpene synthase, designated as RhNUDX1, is responsible for geraniol synthesis. Recombinant RhNUDX1 only shows the diphosphohydrolase activity by transforming GPP to geranyl monophosphate (GP) in vitro. However, in a cytosolic context, RhNUDX1 is equivalent to geraniol synthase (GES), which is found in other plants responsible for geraniol production [18]. In rose cultivar Rosa× wichurana, another NUDX1 gene RwNUDX1-2 is involved in the biosynthesis of a group of sesquiterpenoids, especially E,E-farnesol. It is proposed that roses utilize different NUDX1 protein complexes to generate different derivatives [61][49]. Monoterpene alcohols are normally transformed to acetate esters by alcohol acetyltransferase (AAT), by which the acetyl moiety is transferred from acetyl-CoA to the alcoholic substrate [62,63][50][51]. In the rose, an AAT, RhAAT 1, is isolated, which shows limited substrate specificity in vitro. Its preferred substrate is geraniol, while it can also accept other alcohols as substrates, including citronellol, nerol, 1-octanol, 2-PE, and cis-3-hexen-1-ol [32]. Despite the fact that the preferred substrate is geraniol in vitro, the transgenic petunia flower of RhAAT1 mainly produces phenylethyl acetate and phenylmethyl acetate using 2-PE and benzyl alcohol as the substrates. When fed with geraniol or octanol, the transgenic flowers also produce acetates, suggesting its dependence on substrate availability in planta [51][52]. As a sesquiterpene, germacrene is a common ingredient of rose floral scents and an intermediate in the biosynthesis of other sesquiterpenes [64][53]. In R. hybrida, a gene (Clone FC0592) for sesquiterpenes has been identified and confirmed to produce germacrene D in an in vitro assay with farnesyl pyrophosphate (FPP) as the substrate [31]. Carotenoids are one class of tetraterpenoids, from which C13-norisoprenoids (such as monoterpenes β-damascenone, α-ionone, and β-ionone) are generated by degradation. A carotenoid cleavage (di-)oxygenase (CCD) gene RdCCD1 was confirmed to be responsible for the accumulation of C13-norisoprenoids in flowers of R. damascene and the cleavage of a variety of carotenoids in in vitro assays [48][54]. However, as the subclass 4 of CCD genes, RdCCD4 cannot utilize β-carotene as a substrate in in vivo assays, indicating that it is not involved in the production of β-ionone in R. damascene [49][55].3.2. Biosynthesis of Phenylpropanoids/Benzenoids in Rose Floral Scents
In one branch pathway of phenylpropanoid synthesis, L-Phe is the direct precursor of 2-PE and its β-D-glucopyranoside (2-PEG) [41]. In roses, L-Phe can be converted into phenyl acetaldehyde (PAA) by both aromatic amino acid decarboxylase (AADC) and phenyl acetaldehyde synthase (PAAS) [52[56][57],65], and PAA is subsequently converted to 2-PE by phenyl acetaldehyde reductase (PAR) [53,58][58][59]. Another 2-PE biosynthetic pathway via phenylpyruvic acid (PPA) from L-Phe has been reported in roses. The aromatic amino acid aminotransferase (AAAT) catalyzes the production of PPA from L-Phe, and RNAi suppression of the AAAT gene RyAAAT3 decreases 2-PE production in rose protoplasts [54][60]. In this pathway, phenylpyruvate decarboxylase (RyPPDC) is also shown to be responsible for 2-PE production. However, RyPPDC, as a heat adaptation in the summer, is likely to participate in an alternative principal pathway for rose floral scent production [55][61]. Multiple branching pathways are involved in the synthesis of rose benzenoids. In the branching pathway for TMB, phloroglucinol O-methyltransferase (POMT) catalyzes the first methylation step of phloroglucinol (PLG) to 3,5-dihdroxyanisole (DHA) in R. chinensis [56][62]. DHA is subsequently converted to TMB by two orcinol O-methyltransferases, OOMT1 and OOMT2, through two final methylation reactions [28,35][28][35]. OOMT1 and OOMT2 are also responsible for DMT synthesis with orcinol as the initial substrate in the first and the second methylation steps, respectively [28,35][28][35]. Despite sharing a 96.5% similarity at the amino acid level, OOMT1 and OOMT2 exhibit different substrate specificities in PME biosynthesis. The main sequence difference is the single amino acid polymorphism in the phenolic substrate binding site. OOMT1 is mainly found in Chinese roses instead of European roses. It is speculated that OOMT1 may have evolved from an OOMT2-like gene, and its emergence is a critical step in the evolution of scent production in Chinese roses [36]. OOMTs also catalyze the production of methyleugenol and methyl(iso)eugenol by efficiently methylating eugenol and (iso)eugenol in R. chinensis [34]. In general, the rose floral scent is dominated by terpenoids, phenylpropanoids, and benzenoids. Only a few fatty acid derivatives are involved in rose floral scents. However, no related enzymes or genes have been isolated or characterized from roses to date.3.3. Transcriptional Regulation of Rose Floral Scent Synthesis
Transcription factors (TFs) have been shown to participate in the coordinated regulation of the scent biosynthetic network [66,67][63][64]. Some TFs have been identified for the regulation of floral scents of phenylpropanoids/benzenoids in petunia, including activators of ODO1 [68][65], EOBI [69[66][67],70], EOBII [70[67][68][69],71,72], PH4 [73][70], and the repressor of PhMYB4 [74][71]. Recently, only three TFs have been isolated for transcriptional regulation of floral terpenoid production, including GaWRKY1 in Gossypium arboretum [75][72], MYC2 in Arabidopsis thaliana [76][73], and PbbHLH4 in Phalaenopsis bellina [77][74]. Interestingly, some TFs may act upstream of multiple metabolic pathways across terpenoids and phenylpropanoids/benzenoids. When the Arabidopsis transcription factor production of anthocyanin pigment 1 (PAP1) is introduced into the petunia and rose, phenylpropanoid- and terpenoid-derived scent compounds show elevated expressions compared to the control flowers [78,79][75][76]. In roses, only one R-type MYB TF, RhMYB1, may play a role in floral scent production, but its function has not been validated [59][77]. Sun found the expression of NUDX1 is transcriptionally regulated between scented R. chinensis ‘Old blush’ and unscented R.×wichurana, but failed to identify the functionary TFs in the regulation of NUDX1 [80][78]. In rose petals, the miR156-SPL9 regulatory hub is proposed to orchestrate the production of both colored anthocyanins and certain terpenes, by permitting the complexation of preexisting MYB-bHLH-WD40 proteins [15]. The maximum expression of GDS, which encodes the enzyme catalyzing germacrene D synthesis, is correlated with miR156 activation and with SPL9 downregulation. The miR156-SPL9 regulatory hub might also regulate the expression of terpene synthase genes directly. The absence of the expression of nerolidol synthase (NES) genes is correlated with the downregulation of SPL9 through activation of miR156.References
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902.
- Caruso, C.M.; Parachnowitsch, A.L. Do Plsants Eavesdrop on Floral Scent Signals? Trends Plant Sci. 2016, 21, 9–15.
- Picazo-Aragonés, J.; Terrab, A.; Balao, F. Plant Volatile Organic Compounds Evolution: Transcriptional Regulation, Epigenetics and Polyploidy. Int. J. Mol. Sci. 2020, 21, 8956.
- Grammer, K.; Fink, B.; Møller, A.P.; Thornhill, R. Darwinian aesthetics: Sexual selection and the biology of beauty. Biol. Control 2003, 78, 385–407.
- Jianwu, R.; Lina, Y.; Yan, W.; Hongjun, Y. Chemical profile of floral scent at different flower developmental stages of rose de rescht (Rosa damascena Mill.) cultivated in Beijing. J. Essent. Oil Bear. Plants 2016, 19, 433–443.
- Croteau, R.; Kutchan, T.; Lewis, N. Natural Products (Secondary Metabolites). In Biochemistry & Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2000; pp. 1251–1318.
- Hsieh, M.-H.; Chang, C.-Y.; Hsu, S.-J.; Chen, J.-J. Chloroplast localization of methylerythritol 4-phosphate pathway enzymes and regulation of mitochondrial genes in ispD and ispE albino mutants in Arabidopsis. Plant Mol. Biol. 2008, 66, 663–673.
- Knudsen, J.T.; Gershenzon, J. The chemical diversity of floral scent. In Biology of PLANT volatiles; Jette, T., Knudsen, J.G., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 57–78.
- Pulido, P.; Perello, C.; Rodriguez-Concepcion, M. New insights into plant isoprenoid metabolism. Mol. Plant 2012, 5, 964–967.
- Simkin, A.J.; Guirimand, G.; Papon, N.; Courdavault, V.; Thabet, I.; Ginis, O.; Bouzid, S.; Giglioli-Guivarc’h, N.; Clastre, M. Peroxisomal localisation of the final steps of the mevalonic acid pathway in planta. Planta 2011, 234, 903.
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120.
- Boatright, J.; Negre, F.; Chen, X.; Kish, C.M.; Wood, B.; Peel, G.; Orlova, I.; Gang, D.; Rhodes, D.; Dudareva, N.; et al. Understanding in vivo benzenoid metabolism in petunia petal tissue. Plant Physiol. 2004, 135, 1993–2011.
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297.
- Schaller, F. Enzymes of the biosynthesis of octadecanoid-derived signalling molecules. J. Exp. Bot. 2001, 52, 11–23.
- Raymond, O.; Gouzy, J.; Just, J.; Badouin, H.; Verdenaud, M.; Lemainque, A.; Vergne, P.; Moja, S.; Choisne, N.; Pont, C. The Rosa genome provides new insights into the domestication of modern roses. Nat. Genet. 2018, 50, 772–777.
- Basim, E.; Basim, H. Antibacterial activity of Rosa damascena essential oil. Fitoterapia 2003, 74, 394–396.
- Elliott, J. Delmar’s Integrated Herb Guide for Nurses. Northeast. Nat. 2002, 9, 360.
- Magnard, J.-L.; Roccia, A.; Caissard, J.-C.; Vergne, P.; Sun, P.; Hecquet, R.; Dubois, A.; Hibrand-Saint Oyant, L.; Jullien, F.; Nicolè, F. Biosynthesis of monoterpene scent compounds in roses. Science 2015, 349, 81–83.
- Borda, A.M.; Clark, D.G.; Huber, D.J.; Welt, B.A.; Nell, T.A. Effects of ethylene on volatile emission and fragrance in cut roses: The relationship between fragrance and vase life. Postharvest. Biol. Technol. 2011, 59, 245–252.
- Bendahmane, M.; Dubois, A.; Raymond, O.; Bris, M.L. Genetics and genomics of flower initiation and development in roses. J. Exp. Bot. 2013, 64, 847–857.
- Raymond, O. Domestication et Sélection Dirigée Chez le Rosier: Analyse Historique via les Phénotypes Morphologique, Chimique et Biochimique. Ph.D. Thesis, Université Claude Bernard Lyon 1, Lyon, France, 1999.
- De Vries, D.; Dubois, L.A. Rose breeding: Past, present, prospects. In Proceedings of the II International Rose Symposium 424, Antibes, France, 20–24 February 1995.
- Reynders-Aloisi, S.; Bollereau, P. Characterisation of genetic diversity in genus Rosa by randomly amplified polymorphic DNA. In Proceedings of the II International Rose Symposium 424, Antibes, France, 20–24 February 1995; ISHS Acta: Antibes, France, 1995.
- Flament, I.; Debonneville, C.; Furrer, A. Volatile compounds of roses: Characterization of cultivars based on the headspace analysis of living flower emissions. In ACS Symposium Series (USA); AGRIS: San Francisco, CA, USA, 1993.
- Gang, D.R. Evolution of flavors and scents. Annu. Rev. Plant Biol. 2005, 56, 301–325.
- Jirovetz, L.; Buchbauer, G.; Stoyanova, A.; Balinova, A.; Guangjiun, Z.; Xihan, M. Solid phase microextraction/gas chromatographic and olfactory analysis of the scent and fixative properties of the essential oil of Rosa damascena L. from China. Flavour Fragr. J. 2005, 20, 7–12.
- Joichi, A.; Yomogida, K.; Awano, K.i.; Ueda, Y. Volatile components of tea-scented modern roses and ancient Chinese roses. Flavour Fragr. J. 2005, 20, 152–157.
- Scalliet, G.; Journot, N.; Jullien, F.; Baudino, S.; Magnard, J.-L.; Channelière, S.; Vergne, P.; Dumas, C.; Bendahmane, M.; Cock, J.M. Biosynthesis of the major scent components 3,5-dimethoxytoluene and 1,3,5-trimethoxybenzene by novel rose O-methyltransferases. FEBS Lett. 2002, 523, 113–118.
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Singh, A.; Yadav, A.K. Volatile constituents of essential oil and rose water of damask rose (Rosa damascena Mill.) cultivars from North Indian hills. Nat. Prod. Res. 2011, 25, 1577–1584.
- Watanabe, N.; Washio, H.; Straubinger, M.; Knapp, H.; Winterhalter, P. Occurrence of a glucosidic progenitor of rose oxide in rose flowers, Rosa damascena Mill. Nat. Prod. Lett. 1998, 12, 5–10.
- Guterman, I. Rose Scent: Genomics Approach to Discovering Novel Floral Fragrance-Related Genes. Plant Cell 2002, 14, 2325–2338.
- Shalit, M.; Guterman, I.; Volpin, H.; Bar, E.; Tamari, T.; Menda, N.; Adam, Z.; Zamir, D.; Vainstein, A.; Weiss, D. Volatile ester formation in roses. Identification of an acetyl-coenzyme A. Geraniol/Citronellol acetyltransferase in developing rose petals. Plant Physiol. 2003, 131, 1868–1876.
- Scalliet, G.; Lionnet, C.; Le Bechec, M.; Dutron, L.; Magnard, J.-L.; Baudino, S.; Bergougnoux, V.; Jullien, F.; Chambrier, P.; Vergne, P. Role of petal-specific orcinol O-methyltransferases in the evolution of rose scent. Plant Physiol. 2006, 140, 18–29.
- Wu, S.; Watanabe, N.; Mita, S.; Ueda, Y.; Shibuya, M.; Ebizuka, Y. Two O-Methyltransferases isolated from flower petals of Rosa chinensis var. spontanea involved in scent biosynthesis. J. Biosci. Bioeng. 2003, 96, 119–128.
- Lavid, N.; Wang, J.; Shalit, M.; Guterman, I.; Bar, E.; Beuerle, T.; Menda, N.; Shafir, S.; Zamir, D.; Adam, Z. O-methyltransferases involved in the biosynthesis of volatile phenolic derivatives in rose petals. Plant Physiol. 2002, 129, 1899–1907.
- Scalliet, G.; Piola, F.; Douady, C.J.; Réty, S.; Raymond, O.; Baudino, S.; Bordji, K.; Bendahmane, M.; Dumas, C.; Cock, J.M. Scent evolution in Chinese roses. Proc. Natl. Acad. Sci. USA 2008, 105, 5927–5932.
- Babaei, A.; Tabaei-Aghdaei, S.R.; Khosh-Khui, M.; Omidbaigi, R.; Naghavi, M.R.; Esselink, G.D.; Smulders, M.J. Microsatellite analysis of Damask rose (Rosa damascena Mill.) accessions from various regions in Iran reveals multiple genotypes. BMC Plant Biol. 2007, 7, 12.
- Baydar, N.G.; Baydar, H.; Debener, T. Analysis of genetic relationships among Rosa damascena plants grown in Turkey by using AFLP and microsatellite markers. J. Biotechnol. 2004, 111, 263–267.
- Kiani, M.; Zamani, Z.; Khalighi, A.; Fatahi, R.; Byrne, D.H. Wide genetic diversity of Rosa damascena Mill. germplasm in Iran as revealed by RAPD analysis. Sci. Hortic. 2008, 115, 386–392.
- Rusanov, K.; Kovacheva, N.; Vosman, B.; Zhang, L.; Rajapakse, S.; Atanassov, A.; Atanassov, I. Microsatellite analysis of Rosa damascena Mill. accessions reveals genetic similarity between genotypes used for rose oil production and old Damask rose varieties. Theor. Appl. Genet. 2005, 111, 804–809.
- Watanabe, S.; Hayashi, K.; Yagi, K.; Asai, T.; MacTavish, H.; Picone, J.; Turnbull, C.; Watanabe, N. Biogenesis of 2-phenylethanol in rose flowers: Incorporation of L-phenylalanine into 2-phenylethanol and its beta-D-glucopyranoside during the flower opening of Rosa ’Hoh-Jun’ and Rosa damascena Mill. Biosci. Biotechnol. Biochem. 2002, 66, 943–947.
- Dubois, A.; Carrere, S.; Raymond, O.; Pouvreau, B.; Cottret, L.; Roccia, A.; Onesto, J.-P.; Sakr, S.; Atanassova, R.; Baudino, S. Transcriptome database resource and gene expression atlas for the rose. BMC Genom. 2012, 13, 638.
- Dubois, A.; Raymond, O.; Maene, M.; Baudino, S.; Langlade, N.B.; Boltz, V.; Vergne, P.; Bendahmane, M. Tinkering with the C-function: A molecular frame for the selection of double flowers in cultivated roses. PLoS ONE 2010, 5, e9288.
- Ma, N.; Xue, J.; Li, Y.; Liu, X.; Dai, F.; Jia, W.; Luo, Y.; Gao, J. Rh-PIP2; 1, a rose aquaporin gene, is involved in ethylene-regulated petal expansion. Plant Physiol. 2008, 148, 894–907.
- Nakamura, N.; Fukuchi-Mizutani, M.; Katsumoto, Y.; Togami, J.; Senior, M.; Matsuda, Y.; Furuichi, K.; Yoshimoto, M.; Matsunaga, A.; Ishiguro, K.; et al. Environmental risk assessment and field performance of rose (Rosa × hybrida) genetically modified for delphinidin production. Plant Biotechnol. 2011, 28, 251–261.
- Spiller, M.; Berger, R.G.; Debener, T. Genetic dissection of scent metabolic profiles in diploid rose populations. Theor. Appl. Genet. 2010, 120, 1461–1471.
- Feng, L.; Chen, C.; Li, T.; Wang, M.; Tao, J.; Zhao, D.; Sheng, L. Flowery odor formation revealed by differential expression of monoterpene biosynthetic genes and monoterpene accumulation in rose (Rosa rugosa Thunb.). Plant Physiol. Biochem. 2014, 75, 80–88.
- Rodríguez-Concepción, M.; Boronat, A. Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol. 2002, 130, 1079–1089.
- Sun, P.; Dégut, C.; Réty, S.; Caissard, J.C.; Hibrand-Saint Oyant, L.; Bony, A.; Paramita, S.N.; Conart, C.; Magnard, J.L.; Jeauffre, J. Functional diversification in the Nudix hydrolase gene family drives sesquiterpene biosynthesis in Rosa× wichurana. Plant J. 2020, 104, 185–199.
- Aharoni, A.; Keizer, L.C.; Bouwmeester, H.J.; Sun, Z.; Alvarez-Huerta, M.; Verhoeven, H.A.; Blaas, J.; van Houwelingen, A.M.; De Vos, R.C.; van der Voet, H. Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays. Plant Cell 2000, 12, 647–661.
- Shalit, M.; Katzir, N.; Tadmor, Y.; Larkov, O.; Burger, Y.; Shalekhet, F.; Lastochkin, E.; Ravid, U.; Amar, O.; Edelstein, M. Acetyl-CoA: Alcohol acetyltransferase activity and aroma formation in ripening melon fruits. J. Agric. Food Chem. 2001, 49, 794–799.
- Guterman, I.; Masci, T.; Chen, X.; Negre, F.; Pichersky, E.; Dudareva, N.; Weiss, D.; Vainstein, A. Generation of phenylpropanoid pathway-derived volatiles in transgenic plants: Rose alcohol acetyltransferase produces phenylethyl acetate and benzyl acetate in petunia flowers. Plant Mol. Biol. 2006, 60, 555–563.
- Colby, S.M.; Crock, J.; Dowdle-Rizzo, B.; Lemaux, P.G.; Croteau, R. Germacrene C synthase from Lycopersicon esculentum cv. VFNT Cherry tomato: cDNA isolation, characterization, and bacterial expression of the multiple product sesquiterpene cyclase. Proc. Natl. Acad. Sci. USA 1998, 95, 2216–2221.
- Huang, F.-C.; Horváth, G.; Molnár, P.; Turcsi, E.; Deli, J.; Schrader, J.; Sandmann, G.; Schmidt, H.; Schwab, W. Substrate promiscuity of RdCCD1, a carotenoid cleavage oxygenase from Rosa damascena. Phytochemistry 2009, 70, 457–464.
- Huang, F.-C.; Molnár, P.; Schwab, W. Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. J. Exp. Bot. 2009, 60, 3011–3022.
- Farhi, M.; Lavie, O.; Masci, T.; Hendel-Rahmanim, K.; Weiss, D.; Abeliovich, H.; Vainstein, A. Identification of rose phenylacetaldehyde synthase by functional complementation in yeast. Plant Mol. Biol. 2010, 72, 235–245.
- Sakai, M.; Saori, T.; Hiroshi, H.; Tatsuo, A.; Hideo, D.; Masakazu, H.; Naoharu, W. Purification and Characterization of β-Glucosidase Involved in the Emission of 2-Phenylethanol from Rose Flowers. Biosci. Biotechnol. Biochem. 2008, 72, 219–221.
- Chen, X.M.; Kobayashi, H.; Sakai, M.; Hirata, H.; Asai, T.; Ohnishi, T.; Baldermann, S.; Watanabe, N. Functional characterization of rose phenylacetaldehyde reductase (PAR), an enzyme involved in the biosynthesis of the scent compound 2-phenylethanol. J. Plant Physiol. 2011, 168, 88–95.
- Sakai, M.; Hirata, H.; Sayama, H.; Sekiguchi, K.; Itano, H.; Asai, T.; Dohra, H.; Hara, M.; Watanabe, N. Production of 2-phenylethanol in roses as the dominant floral scent compound from L-phenylalanine by two key enzymes, a PLP-dependent decarboxylase and a phenylacetaldehyde reductase. Biosci. Biotechnol. Biochem. 2007, 71, 2408–2419.
- Hirata, H.; Ohnishi, T.; Ishida, H.; Tomida, K.; Sakai, M.; Hara, M.; Watanabe, N. Functional characterization of aromatic amino acid aminotransferase involved in 2-phenylethanol biosynthesis in isolated rose petal protoplasts. J. Plant Physiol. 2012, 169, 444–451.
- Hirata, H.; Ohnishi, T.; Tomida, K.; Ishida, H.; Kanda, M.; Sakai, M.; Yoshimura, J.; Suzuki, H.; Ishikawa, T.; Dohra, H. Seasonal induction of alternative principal pathway for rose flower scent. Sci. Rep. 2016, 6, 20234.
- Wu, S.; Watanabe, N.; Mita, S.; Dohra, H.; Ueda, Y.; Shibuya, M.; Ebizuka, Y. The key role of phloroglucinol O-methyltransferase in the biosynthesis of Rosa chinensis volatile 1, 3, 5-trimethoxybenzene. Plant Physiol. 2004, 135, 95–102.
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949.
- Nagegowda, D.A.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457.
- Verdonk, J.C.; Haring, M.A.; van Tunen, A.J.; Schuurink, R.C. ODORANT1 regulates fragrance biosynthesis in petunia flowers. Plant Cell 2005, 17, 1612–1624.
- Spitzer-Rimon, B.; Farhi, M.; Albo, B.; Cna’ani, A.; Zvi, M.M.B.; Masci, T.; Edelbaum, O.; Yu, Y.; Shklarman, E.; Ovadis, M. The R2R3-MYB–like regulatory factor EOBI, acting downstream of EOBII, regulates scent production by activating ODO1 and structural scent-related genes in petunia. Plant Cell 2012, 24, 5089–5105.
- Van Moerkercke, A.; Haring, M.A.; Schuurink, R.C. The transcription factor EMISSION OF BENZENOIDS II activates the MYB ODORANT1 promoter at a MYB binding site specific for fragrant petunias. Plant J. 2011, 67, 917–928.
- Colquhoun, T.A.; Schwieterman, M.L.; Wedde, A.E.; Schimmel, B.C.; Marciniak, D.M.; Verdonk, J.C.; Kim, J.Y.; Oh, Y.; Gális, I.; Baldwin, I.T. EOBII controls flower opening by functioning as a general transcriptomic switch. Plant Physiol. 2011, 156, 974–984.
- Spitzer-Rimon, B.; Marhevka, E.; Barkai, O.; Marton, I.; Edelbaum, O.; Masci, T.; Prathapani, N.-K.; Shklarman, E.; Ovadis, M.; Vainstein, A. EOBII, a gene encoding a flower-specific regulator of phenylpropanoid volatiles’ biosynthesis in petunia. Plant Cell 2010, 22, 1961–1976.
- Cna’ani, A.; Spitzer-Rimon, B.; Ravid, J.; Farhi, M.; Masci, T.; Aravena-Calvo, J.; Ovadis, M.; Vainstein, A. Two showy traits, scent emission and pigmentation, are finely coregulated by the MYB transcription factor PH4 in petunia flowers. New Phytol. 2015, 208, 708–714.
- Colquhoun, T.A.; Kim, J.Y.; Wedde, A.E.; Levin, L.A.; Schmitt, K.C.; Schuurink, R.C.; Clark, D.G. PhMYB4 fine-tunes the floral volatile signature of Petunia× hybrida through PhC4H. J. Exp. Bot. 2010, 62, 1133–1143.
- Xu, Y.-H.; Wang, J.-W.; Wang, S.; Wang, J.-Y.; Chen, X.-Y. Characterization of GaWRKY1, a cotton transcription factor that regulates the sesquiterpene synthase gene (+)-δ-cadinene synthase-A. Plant Physiol. 2004, 135, 507–515.
- Hong, G.J.; Xue, X.Y.; Mao, Y.B.; Wang, L.J.; Chen, X.Y. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 2012, 24, 2635–2648.
- Chuang, Y.C.; Hung, Y.C.; Tsai, W.C.; Chen, W.H.; Chen, H.H. PbbHLH4 regulates floral monoterpene biosynthesis in Phalaenopsis orchids. J. Exp. Bot. 2018, 69, 4363–4377.
- Zvi, M.M.B.; Shklarman, E.; Masci, T.; Kalev, H.; Debener, T.; Shafir, S.; Ovadis, M.; Vainstein, A. PAP1 transcription factor enhances production of phenylpropanoid and terpenoid scent compounds in rose flowers. New Phytol. 2012, 195, 335–345.
- Zvi, M.M.B.; Negre-Zakharov, F.; Masci, T.; Ovadis, M.; Shklarman, E.; Ben-Meir, H.; Tzfira, T.; Dudareva, N.; Vainstein, A. Interlinking showy traits: Co-engineering of scent and colour biosynthesis in flowers. Plant Biotechnol. J. 2008, 6, 403–415.
- Yan, H.; Zhang, H.; Wang, Q.; Jian, H.; Qiu, X.; Wang, J.; Tang, K. Isolation and identification of a putative scent-related gene RhMYB1 from rose. Mol. Biol. Rep. 2011, 38, 4475–4482.
- Sun, P. Molecular and biochemical studies of fragrance biosynthesis in rose. Ph.D. Thesis, Universiteit van Amsterdam, Amsterdam, The Netherlands, 2017.
More
