Fluorinated polymers are renowned for their chemical inertness and thus poor wettability and adhesion of various coatings. Apart from chemical methods employing somewhat toxic primers, gaseous plasma treatment is a popular method for the modification of surface properties. Different authors have used different plasmas, and the resultant surface finish spans between super-hydrophobic and super-hydrophilic character.
- gaseous plasma
- surface wettability
- fluorinated polymers
- polytetrafluoroethylene
- discharge parameters
1. Introduction
The surface properties of polymers depend on numerous parameters including composition, structure and morphology, as well as properties of any foreign material that might have been adsorbed. The surface properties may not always be adequate, so they have to be modified for an appropriate adhesion of various coatings, including glues and inks. A traditional method for tailoring the surface properties of polymers is the application of primers. These chemicals stick to the surface of a polymer and assure the appropriate adhesion of the desired coating. The primers are not always ecologically benign, so there is a trend of suppressing their applications. The most popular alternative is the application of gaseous plasma, in particular non-equilibrium gaseous plasma sustained in different gases or gas mixtures at different pressures. This technique works well for a range of polymers, excluding fluorinated ones. A comprehensive review of new developments in surface functionalization and the nanostructuring of fluorine-free polymers using controlled plasma treatments has been published in several papers, including [1][2][3][4][5][6][7][1,2,3,4,5,6,7].
Gaseous plasma is a source of charged particles (particularly free electrons and positively charged ions, sometimes also negatively charged ions), neutral reactive particles (usually molecular fragments including free atoms), and radiation. The surface finish depends on the fluxes or fluences of all plasma constituents onto the surface of the polymer. The complete characterization of gaseous plasma is not a trivial task, so most authors who have used gaseous plasma for tailoring the surface properties of polymer materials skip the influence of plasma parameters on the surface finish and prefer reporting discharge parameters. The discharge power (or power density), type of discharge, gas pressure and/or flow, as well as treatment time, are often reported. Plasma parameters depend on discharge parameters, but the dependence is not always straightforward. Many authors found large differences in plasma parameters upon a slight change of discharge parameters due to non-linear effects, and mainly due to the influence of gaseous impurities on the plasma parameters. The interaction of gaseous plasma with polymer materials has been a subject of numerous scientific studies, and the results were found useful in the optimization of industrial-size reactors [8][9][10][11][8,9,10,11].
Gaseous plasma glows, so it is a source of radiation in the visible range of wavelengths. Usually, there is also significant radiation in the invisible part of the light spectrum. Of particular importance is radiation in the ultraviolet (UV) and vacuum ultraviolet (VUV) ranges of wavelengths. Such radiation is capable of breaking bonds in the polymers, so its contribution to the polymer chemistry is by far more important than the contribution of visible radiation. Furthermore, the radiation in the invisible range (UV and VUV) may be orders of magnitude more intensive than visible radiation. The penetration depth of photons in the UV and VUV range of wavelengths increases with increasing wavelength. For example, the penetration depth of soft UV radiation useful for polymer cross-linking is in the sub-millimeter range. In contrast, the radiation at the major line of a low-pressure mercury lamp (254 nm) penetrates only a few micrometers into many polymer materials. The penetration depth of VUV radiation may be only a few 10 nm. Depending on the predominant radiation type, polymers are modified over the surface layer spanning from a few 10 nm to almost a millimeter. The influence of VUV radiation on the surface properties of a model polymer was elaborated recently in [12].
The neutral radicals will not penetrate deep into a polymer upon plasma treatment. Their kinetic temperature is usually close to room temperature (RT), so any polymer modification by neutral reactive particles is limited to the very surface unless chain reactions are triggered by the interaction between a radical and the polymer. The same applies to charged particles. Their kinetic energy depends enormously on the type of discharge adopted for plasma generation. The positively charged ions impinging polymer'’s surface may have various kinetic energy anywhere between a few eV and several 1000 eV. The interaction of energetic particles with polymers causes radiation damage and thus the partial or complete destruction of the surface layer. The etching is also pronounced, either by kinetic effects (sputtering) or a combination of kinetic and chemical effects (reactive ion etching).
Gaseous plasma is preferably sustained in a selected gas of high purity. In practice, this is not always feasible. For example, a vacuum system usually contains a specific concentration of water vapor that is slowly released from the surface of any vacuum component, thus contributing to the impurities in the vacuum system. Furthermore, polymer samples themselves may serve as a source of impurities. The desorption is less pronounced at atmospheric pressure, but there are very few papers on plasma characterization revealing the concentration of H, OH, and O impurities below the detection limit. Usually, the concentration of such reactive species is high enough even in a plasma sustained in noble gases, so one should take them into account at any attempt to interpret the results of plasma-treated polymers.
Although often neglected, gaseous plasma treatment may cause residual surface stress or strain [13][14][15][13,14,15]. This effect usually leads to modifications of the surface morphology.
2. Interaction Mechanisms
It is possible to distinguish between two types of plasma treatments that enable a different surface finish: (i) treatment with plasma rich in reactive oxygen species, and (ii) treatment with plasma that contains a minute quantity of oxygen species but is rich in reactants that cause bond-scission and thus the depletion of fluorine from the surface film. The first one is illustrated in Figure 1. In cases where the gaseous plasma is rich in oxygen, the radiation that causes bond breaking between C and F atoms in PTFE arises practically only from excited neutral oxygen atoms. The radiation appears at the wavelength around 130 nm [12]. The radiation is not extensive enough to cause significant bond breakage, but the reactive oxygen species readily interact chemically with the PTFE surface causing oxidation. The result is a formation of unstable fragments containing carbon, fluorine and oxygen. Known fragments of such type include oxy (x = 1) and peroxy (x = 2) radicals of formulae CF3Ox, FC(O)Ox, CF3C(O)Ox and CF3OC(O)Ox. Such moieties desorb from the surface, which results in etching. The etching outcome is increased roughness, which in turn results in the super-hydrophobic surface finish. A typical example of such a mechanism was elaborated by Ryu et al. [16][19].
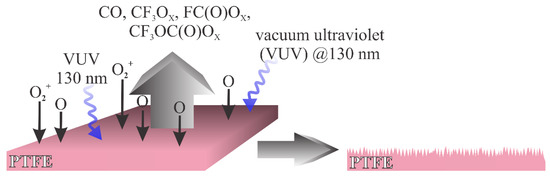
Figure 1. Schematic of the interaction between oxygen plasma and PTFE.
Another type of plasma used in a modification of PTFE is plasma rich in VUV radiation. Such plasma was used by Nguyen et al. [17][34], and more recently by Lojen et al. [18][38]. The interaction of such plasma with the PTFE surface is illustrated in Figure 2. Here, the flux of oxygen plasma species is marginal compared to the flux of the radiation arising from hydrogen molecules and atoms. The PTFE surface is thus exposed to the VUV radiation that causes the C–F bond scission. Simultaneously, the PTFE surface is exposed to radicals that may interact with F atoms on the surface, for example, NH3+, as proposed by Nguyen et al. [17][34], and H atoms as elaborated by Lojen et al. [18][38]. The PTFE surface layer is thus depleted of fluorine, and the oxidation of the dangling bonds occurs by interaction with OH radicals that are likely to be presented in a low-pressure plasma reactor in minute quantities. An alternative may be surface oxidation upon venting the plasma reactor. Namely, the oxidation of polymer samples treated by VUV radiation in the absence of plasma conditions occurs spontaneously as the sample is exposed to ambient conditions [19][39].
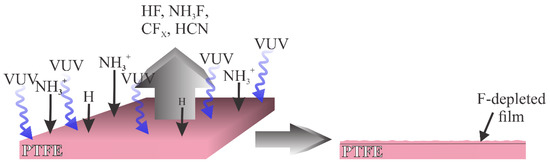
Figure 2. Schematic of the interaction between plasma rich in VUV radiation and PTFE.
3. Conclusions and Roadmap
The mechanisms governing the surface finish of PTFE upon plasma treatment are still far from being well understood. At least a moderate concentration of oxygen functional groups on the PTFE surface is essential for improved wettability, the same as for other polymers. A trivial solution for the activation of PTFE materials is the deposition of a foreign material onto the polymer surface. The deposition is achievable by using gaseous plasma sustained with a discharge that enables the high kinetic energy of ions impinging an electrode. Widely used is capacitively coupled high-frequency (possibly RF) discharge. The smaller electrode of such a discharge is self biased to a relatively high negative potential that attracts positively charged ions from gaseous plasma. The ions cause sputtering of the electrode material and thus, the deposition of a material with high surface energy. The sputtering is most efficient when the sheath between the plasma and electrode is almost collisionless, therefore at low pressure. Such a solution, however, does not enable the good adhesion of any coating since there is a weak chemical interaction between PTFE and the deposited material.
A non-trivial method for achieving the good wettability of PTFE is treatment with a gaseous plasma sustained in a gas mixture with a minimal content of oxygen or oxygen-containing gas. Radiation in the invisible range (UV and in particular VUV) causes bond scission and thus, fluorine depletion on the polymer surface. The radiation depends enormously on the type of discharge and gaseous impurities. Especially impurities tend to quench metastables and cause a decrease in the electron temperature, so the radiation in the deep UV range is suppressed. Furthermore, the VUV radiation is intensively absorbed in ambient gas, so it is better to place the polymer samples close to the source of radiation, i.e., in the region of most luminous plasma. Both noble and some molecular gases are rich sources of VUV radiation arising from transitions of highly-excited atoms or molecules. At atmospheric pressure, the most intensive radiation occurs from excited Ar or He dimers, whereas at low pressures, the radiation from atoms is often as intensive, if not more so.
The massive scattering of results reported by different authors suggests that the processing parameters such as the treatment time, type of gas, discharge power, or frequency are not the most appropriate for studying the evolution of the surface wettability or surface functionalization with oxygen functional groups. A better choice of parameters would include fluences (or fluxes) of reactive gaseous species and invisible radiation. Such parameters are not trivial to determine, so the complete characterization of gaseous plasma represents a scientific challenge that will enable understanding the complex surface mechanisms involved upon the plasma treatment of polytetrafluoroethylene.
The VUV radiation is rarely measured upon the treatment of polymers with gaseous plasma. Since the ratio between VUV and reactive oxygen species seems to be the decisive factor for the surface wettability, a trend in the modification of PTFE polymer materials in plasma conditions is the estimation of the fluxes and/or fluences of such radiation, for example, using a technique as elaborated by Fanz et al. [20][36]. The desired surface finish is obtained by a proper choice of the type of plasma radicals and fluences. From a scientific point of view, the best method for explaining the surface finish would be by adjusting the fluxes of VUV radiation and chemically reactive plasma species independently, as demonstrated recently by Lojen et al. [18][38].
