Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Frédérique Blanc-Béguin and Version 2 by Lindsay Dong.
Lung ventilation/perfusion (V/Q) positron emission tomography-computed tomography (PET/CT) is a promising imaging modality for regional lung function assessment. The same carrier molecules as a conventional V/Q scan (i.e., carbon nanoparticles for ventilation and macro aggregated albumin particles for perfusion) are used, but they are labeled with gallium-68 (68Ga) instead of technetium-99m (99mTc).
- V/Q PET/CT
- [68Ga]Ga-MAA
- 68Ga-labelled carbon nanoparticles
1. Introduction
Lung ventilation-perfusion (V/Q) scintigraphy allows the regional lung function distribution of the two major components of gas exchanges, namely ventilation and perfusion, to be assessed [1]. Regional lung ventilation can be imaged after inhaling inert gases or radiolabelled aerosols that reach alveoli or terminal bronchioles. Regional lung perfusion can be assessed after intravenous injection of radiolabelled macroaggregated albumin (MAA) particles trapped during the first pass in the terminal pulmonary arterioles [2][3][2,3].
Pulmonary embolism (PE) diagnosis is the main clinical indication of lung V/Q scintigraphy in pulmonary embolism (PE) diagnosis. V/Q scanning was the first non-invasive test validated for PE diagnosis. The technique was then further improved with the introduction of single-photon emission computed tomography (SPECT) and, more recently, SPECT/computed tomography (CT) imaging [4]. There are many other clinical situations in which an accurate assessment of regional lung function may improve patient management besides PE diagnosis. This includes predicting post-operative pulmonary function in patients with lung cancer, radiotherapy planning to minimize the dose to the lung parenchyma with preserved function and reduce radiation-induced lung toxicities, or pre-surgical assessment of patients with severe emphysema undergoing a lung volume reduction surgery. However, although lung scintigraphy should play a central role in these clinical scenarios, its use has not been widely implemented in daily clinical practice [5]. One of the likely explanations could be the inherent technical limitations of SPECT imaging for the accurate delineation and quantification of regional ventilation and perfusion function [4].
Lung V/Q positron emission tomography (PET)/CT is a novel promising imaging modality for regional lung function assessment [6][7][6,7]. The technique has shown promising results in various clinical scenarios, including PE diagnosis [8], radiotherapy planning [9], or pre-surgical evaluation of lung cancer patients [10]. Several large prospective clinical trials are underway, such as (NCT04179539, NCT03569072, NCT04942275, and NCT05103670). The rationale is simple [5]. PET/CT uses the same carrier molecules as conventional V/Q scanning, i.e., carbon nanoparticles for ventilation imaging and MAA particles for perfusion imaging. Similar physiological processes are therefore assessed with SPECT or PET imaging. However, carrier molecules are labelled with gallium-68 (68Ga) instead of technetium-99m (99mTc), allowing the acquisition of images with PET technology. PET has technical advantages compared with SPECT, including higher sensitivity, higher spatial and temporal resolution, superior quantitative capability and much greater access to respiratory-gated acquisition [11].
2. Challenges of the Transition from 99mTc- to 68Ga-Labelled Radiopharmaceuticals for Lung Imaging
The first challenge of the switch from 99mTc- to 68Ga-labelled radiopharmaceuticals for lung V/Q imaging is to maintain the pharmacological properties of V and Q tracers. Both MAA and carbon nanoparticles labelled with 99mTc have been largely studied. They have been shown to have a biodistribution throughout the lungs that allow an accurate assessment of regional lung perfusion and ventilation function. The principle of lung V/Q PET/CT imaging is to assess similar physiological processes than with conventional V/Q scan, but with greater technology for image acquisition. The technique needs to be easy to implement in nuclear medicine facilities to enable routine use. The preparation should be fast, simple, GMP-compliant and safe for the operators. Furthermore, radiopharmaceutical production should use unmodified commercially available kits of carrier molecules and similar equipment and devices as much as possible as those used for conventional V/Q scans.3. Lung Perfusion Imaging
3.1. [
99m
Tc]Tc-MAA
3.1.1. Chemical Aspects of [99mTc]Tc-MAA Particles
Among the various type of human serum albumin (HSA) available for radionuclide labeling, MAA is the most commonly used form in nuclear medicine facilities. The nature of the complex [99mTc]Tc-MAA has not been fully elucidated. It was hypothesized that the labelling of proteins with 99mTcO4− involved reduction of the anionic Tc(VII) to a cationic Tc by the tin Sn(II) contained in the commercial kit, which was then complexed with electron-donating groups [12][13][14][15,16,17]. Some authors have assumed that 99mTcO4− reduced by the Sn(II)- albumin aggregates probably formed a (Tc = O)3+ complex with the aggregates [12][15]. More recently, high positive cooperativity was shown between 99mTc and MAA, although MAA particles did not seem to have binding pockets [15][16][18,19].3.1.2. Technical Aspects: [99mTc]Tc-MAA Preparation
[99mTc]Tc-MAA particles are manually prepared by introducing a 99mTc solution in a commercially available MAA kit. The 99mTc is obtained from a 99Mo/99mTc generator as sodium pertechnetate (99mTcO4−, Na+). The MAA labelling with 99mTc, which occurred at pH 6, is a simple and fast (about 15 min) process, which allows the production of GMP [99mTc]Tc-MAA without heating step [15][18].3.1.3. Pharmacological Aspects
In a [99mTc]Tc-MAA suspension, the average particle size is 20–40 µm, and 90% have a size between 10 and 90 µm. There should be no particles larger than 150 µm [17][21]. [99mTc]Tc-MAA particles reach the lung via the pulmonary arterial circulation. Due to the size of the alveolar capillaries (5.5 µm on average), the [99mTc]Tc-MAA does not reach the alveolar capillaries but largely accumulates in the terminal pulmonary arterioles. Particles inferior to 10 µm may pass through the lungs and then phagocytose by the reticuloendothelial system [17][21]. According to the requirement of the MAA suppliers, the number of MAA particles injected should range from 60,000 to 700,000 to obtain uniform distribution of activity reflecting regional perfusion (for over 280 billion pulmonary capillaries and 300 million pre-capillary arterioles) [18][22].3.2. [
68
Ga]Ga-MAA
3.2.1. Chemical Aspects of [68Ga]Ga-MAA Particles
MAA labelling with 68Ga has been proposed using bifunctional chelators such as EDTA or DTPA, forming quite stable and inert chelates [19][20][24,25]. However, direct labelling was performed by most groups. Direct labelling uses a co-precipitation of 68Ga(III) and albumin particles [21][22][26,27]. Mathias et al. hypothesized that 68Ga was adsorbed to the surface of the MAA particles after hydrolysis to insoluble gallium hydroxide without excluding specific interactions of Ga(III) ion with ion pairs exposed at the particle surface [23][28]. As Ga is present as Ga(OH)4− at a basic pH, 68Ga does not bind to MAA at a pH above 7. The MAA behavior matches with solvent-exposed glutamate and aspartate amino acids, which should be binding sites for multivalent cations with low affinity and low cation specificity [15][18].3.2.2. Technical Aspects: [68Ga]Ga-MAA Preparation
[99mTc]Tc-MAA preparation is a manual and simple process involving only 2 steps: generator elution and mixing the eluate with the MAA. In contrast, because of the chemical properties of 68Ga, at least four steps are required to label MAA particles with 68Ga: 68Ge/68Ga generator elution, mixing the 68Ga eluate with the MAA, heating the reaction medium and the purification of the [68Ga]Ga-MAA. The key steps of MAA labelling with 68Ga are presented in Figure 1.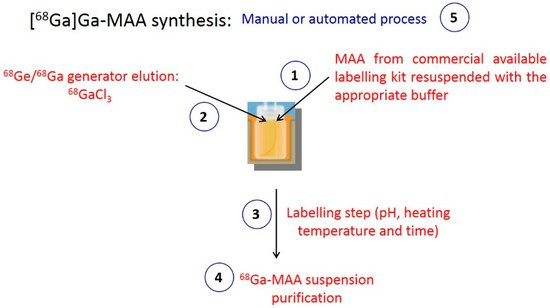
Figure 1.
Key points of MAA labelling with
68
Ga.
3.2.3. Pharmacological Aspects
An important challenge of the switch from 99m Tc- to 68Ga-labelled MAA is maintaining the pharmacological properties of particles to ensure similar biodistribution throughout the terminal pulmonary arterioles. Accordingly, the key parameter is the particle size, which should range between 10.0 and 90.0 µm, with no particles size superior to 150.0 µm. On the other hand, particles should not be inferior to 10.0 µm because the target organs would be the reticuloendothelial system and the bones instead of the lungs [18][22]. Most of the literature data reported a mean diameter ranging from 10 to 90 µm (15.0–75.0 µm for Blanc-Béguin et al., 52.9 ± 15.2 for Jain et al. and 43.0–51.0 for Canziani et al.) [15][24][25][18,29,39].
Hence, [99mTc]Tc-MAA and [68Ga]Ga-MAA particles have similar sizes and structures. The number of [68Ga]Ga-MAA particles injected should range from 60,000 to 700,000, no differently from [99mTc]Tc-MAA, to obtain uniform distribution of activity reflecting regional perfusion.
