1. Major Depressive Disorder and Diet: What Is the Relationship?
1.1. A General Perspective of Major Depressive Disorder
Major Depressive Disorder (MDD) is a leading disabling condition highly representative in our society, affecting nearly 280 million people worldwide
[1]. Currently, MDD is placed as the third cause of disability globally, just after headache and lower back pain; however, the World Health Organization (WHO) projects that by 2030, MDD will become the first cause of disability worldwide
[2][3][2,3]. Nowadays, the growing prevalence and annual incidence affected by pandemic times of the coronavirus disease 2019 (COVID-19) has made us reconsider the importance of mental health. In fact, according to current data, there has been a rise of 53.2 million MDD cases globally, representing an increase of 27.6% during the course of this pandemic
[4]. This condition affects people from all age ranges, sex, and cultures; although, being aged between 45 and 59, of the female sex, and from a high-income country are associated with a higher risk of suffering from MDD. In fact, prevalence is double in women than in men
[5][6][5,6]. In addition, it seems that the distribution of MDD can be different across regions. Thus, the lifetime prevalence of MDD can vary between 2 and 21% with the highest rates in some European countries and the lowest in certain Asian countries
[7].
The precise causes of this complex malady are not fully understood. It seems that MDD is the result of an interplay between genetic and environmental factors. The estimated role of genetics is about 37%; although, a single gene has not been identified as a causative factor of MDD
[8]. Instead, it seems that the inheritance of MDD is polygenic, the exposure to different environmental factors in certain critical periods being equally required
[9]. In this sense, early life stress (i.e., child abuse) is one of the most important environmental factors related to the development of MDD, leading to determinant changes in the brain structure and function
[10]. Similarly, chronic stress and frequent episodes of acute stress are also related to the onset of MDD
[11]. The presence of physical or mental comorbidities together with multiple social factors can be equally prominent contributors to those suffering from MDD. In this sense, ethnic minorities, marital status (separated/divorced), poor education and healthcare access/quality, neighborhood, built environment, intimate partner violence, and lower socioeconomic resources are the most important social determinants related to the onset of MDD
[7][12][13][7,12,13].
From a medical perspective, MDD can be considered a quite heterogeneous entity. Following the DSM-5 (Diagnostic and Statistical Manual of Mental Disorders, 5th Edition) criteria, MDD is clinically diagnosed by the presence of at least one of the two main criteria—depressed mood or anhedonia (the inability to feel pleasure or loss of interest) and ≥ 4 secondary symptoms during a minimum period of 2 weeks
[14]. Collectively, the secondary symptoms of MDD can be divided into somatic or non-somatic items. The former include sleep disturbances, appetite changes, poor concentration, fatigue, and psychomotor agitation/retardation, whereas non-somatic symptoms are related to psychobehavioral alterations such as feelings of worthless, and suicide thoughts
[15] The severity of MDD is generally assessed by the use of the Hamilton Depression Rating Scale (HAM-D), based on the use of specific punctuation marks for different clinical parameters
[16]. Thus, a rating scale between 0 and 7 is defined as no depression; 8 and 16 mild depression; 17 and 23 moderate depression; and ≥24 severe depression
[17][18][17,18]. The clinical management of these patients can be different according to the severity. Hence, for patients with mild depression, physical activity, or non-medical treatments may be encouraged. However, for patients with moderate or severe depression, the use of antidepressants, alone or in combination with psychotherapy and psychoeducation, is generally the most frequent approach
[19].
On the other hand, notwithstanding an important percentage of patients responding to the therapy received, approximately 30% of patients suffer from resistance, which is known as treatment-resistant depression, representing an important medical and socioeconomic challenge
[20]. In addition, patients with severe depression often require more intervention visits, more months of antidepressant treatment, and more antidepressant trials, but they tend to present worse clinical outcomes
[21]. In this context, the socioeconomic burden of MDD is notably high, and not only are direct costs derived from their clinical management, but also indirect costs related to their lack of productivity or work absence must be considered. In the United States alone, the economic burden of MDD was USD 326.2 billion between 2010 and 2018, with a substantial rise in the indirect costs during this period
[22].
Therefore, MDD is a challenging and growing public health concern that has a devastating impact on the individuals who suffer from this condition and the entire society. It is imperative to find novel approaches that facilitate the prevention and clinical management of this neuropsychiatric disorder, and nutritional intervention may represent a promising key to achieve these objectives.
1.2. Nutritional Status of the Patient with MDD
Malnutrition in form of undernutrition, overnutrition, or an imbalance of specific nutrients is a global community concern
[23]. In general, children suffer more frequently from undernutrition, whereas adults tend to present overnutrition, especially in low- or middle-income countries and the poor population
[24]. Likewise, malnutrition is remarkably higher in elder subjects, particularly with specific nutrient deficiencies
[25]. It seems that there is a bidirectional link between malnutrition and MDD. On the one hand, malnutrition can drive several biological changes that can lead to the onset and progression of MDD. On the other hand, MDD can drive to eating disorders and lifestyle behaviors that may promote malnutrition
[26]. However, there is an important association between malnutrition and MDD that can be observed in these patients.
Nutritional questionnaires such as the Mini Nutritional Assessment (MNA) have been useful to understand the connection between both entities. MNA represents a useful tool for monitoring patients of any age who are at risk of malnutrition. In this sense, rating as “malnourished” or “at risk of malnutrition” is associated with a higher risk of MDD, especially in elder individuals
[27][28][27,28]. Moreover, some studies have found that patients with MDD are more likely to be at risk of undernutrition rather than overnutrition
[29].
Simultaneously, the introduction of anthropometric variables has also aided in further insights being gained into the relationship between malnutrition and MDD. For instance, higher median levels of body weight, waist circumference, hip circumference, and waist-to-hip ratio were found in patients with MDD
[30]. In this line, the waist-to-hip ratio appears to be inversely related to suicidality and severity of depression in women with postnatal depression, denoting the relevance of anthropometric measures in the clinical management of MDD
[31].
When combining biochemical plus anthropometric variables, some studies have found that women with MDD have higher triglyceride, aspartate aminotransferase (AST), blood urea nitrogen (BUN), and creatinine levels and lower high-density lipoprotein cholesterol (HDL-C), hematocrit, and red blood cell counts; while depressed men were more likely to have higher triglyceride levels, lower hematocrit, and BUN and, in general, they present with less height and weight than their control group
[32]. Self-perception can also be different between women and men. Women are more prone to perceive their body as larger than it really is, whereas, in men, the perception is just the opposite, idealizing a larger body
[33].
Regarding the lack or excessive intake of specific nutrients, it is common that patients with MDD have an aberrant distribution of macronutrients (carbohydrates, proteins, and fats) and a deficiency of critical micronutrients (vitamins and minerals). In this context, prior works have associated high consumption of carbohydrates and low intake of protein with MDD
[34]. Refined carbohydrates are one of the most deleterious nutrients included in westernized diets, being associated with multiple disease conditions
[35]. Likewise, a direct relationship between trans unsaturated fatty acids and depression risk has been demonstrated
[36], and a high dietary intake of saturated fats is sufficient to promote depressive-like behaviors in animal models
[37]. Conversely, low high-quality fat intake, such as omega 3 polyunsaturated fatty acids (ꞷ-3 PUFA), has been associated with a higher risk of MDD
[38]. Thus, patients with MDD often present with an insufficient protein and high-quality fat intake and with overconsumption of refined carbohydrates and poor-quality fats. In this situation, deficiencies in multiple vitamins and minerals are importantly linked to MDD. Particularly, a low intake of vitamin D and from the B complex—prominently thiamine (B1), niacin (B3), pyridoxin (B6), folate (B9), and cyanocobalamin (B12)—are observed in patients with MDD
[39][40][39,40] Regarding minerals, low calcium, magnesium, iron, and zinc consumption has been associated with MDD
[41], whereas low potassium, phosphorus, and copper intake can also be related to depressive symptoms for women, but not for men
[42].
Collectively, there is compelling evidence that patients with MDD are commonly malnourished, or at least at risk of malnutrition. Thus, as it will be subsequently discussed, patients with MDD can benefit from addressing their nutritional status, as these dietary changes may favorably influence the intricate biology of depression.
1.3. Biology of Depression: Is There a Role for Diet?
The numerous difficulties related to the clinical management of MDD may be a consequence of the complex pathophysiological signature of this neuropsychiatric condition. Traditionally, the most widely known biological mechanism implicated in the pathophysiology of MDD has been aberrant neurotransmitter functioning and, particularly, decreased monoamine levels. Monoamines are represented by serotonin, dopamine, and norepinephrine, acting in critical brain regions and mediating several perceptions and behaviors in the brain
[43]. However, currently, it is widely accepted that monoamines only represent a single mechanism of a really intricate picture
[44]. Apart from monoamines, abnormal functioning of other neurotransmitters, such as gamma-aminobutyric acid (GABA), glutamate, and acetylcholine, has been reported in patients with MDD
[45]. The available literature also considers the relevance of altered neuropeptides in the pathophysiology of MDD, including galanin (GAL), cholecystokinin (CCK), neuropeptide Y (NPY), oxytocin (OXT), vasopressin (VP), neuropeptide S (NPS), and melanin-concentrating hormone (MCH)
[46]. Likewise, alterations in the neuropeptide melatonin and circadian disruption are similarly reported in patients with MDD
[47], collectively supporting that there are many neurotransmitters and neuropeptides that are not working properly in the brain of depressed patients.
Furthermore, impaired neurogenesis and neuroplasticity have also been observed in the brain of depressed patients, having been proposed as a major pathophysiological signature of MDD
[48]. For instance, alterations in neurotrophic factors such as brain-derived neurotrophic factor (BDNF) or glial-derived neurotrophic factor (GDNF) are critically involved in these changes, affecting several cellular processes
[49][50][49,50]. The aberrant neurogenesis and neuroplasticity are closely linked to the effects of psychological stress in the patient with MDD. In this sense, it is widely accepted that this condition is associated with severe dysfunction of the hypothalamus–pituitary–adrenal (HPA) axis, characterized by altered levels of cortisol and their regulators, cortisol release factor (CRF) and the adrenocorticotropin hormone (ACTH), entailing detrimental consequences for the brain and the entire organism
[51].
Moreover, consistent damage to neuronal and glial cells has been described in the brain of patients with MDD, with an enhanced mitochondrial dysfunction, apoptosis, oxidative stress, and excitotoxicity
[52][53][52,53] All these changes lead to structural, functional, and connectivity abnormalities in specific regions of the central nervous system (CNS), such as the hippocampus, amygdala, dorsomedial thalamus, dorsal and medial prefrontal cortex, the dorsal and ventral anterior cingulate cortex, the orbital frontal cortex, the insula, and the striatum and raphe nucleus
[54], which ultimately are responsible for the behavioral and mood dysfunctions related to MDD.
Not only local, but also extra-brain affections are observed in the patient with MDD. The immune system is a pivotal player in the pathophysiology of this condition and an aberrant inflammatory response can be observed either in the brain (neuroinflammation), or at systemic levels
[55][56][57][58][55,56,57,58]. Metabolic and endocrine alterations (insulin resistance, type 2 diabetes, obesity
, and so onetc.) are closely related to immune dysfunction and can influence at the onset and development of MDD
[59]. Indeed, an exacerbated inflammatory response frequently co-occurs with endocrine, neural, and psychiatric alterations such as in MDD, which is studied in the field of psychoneuroimmunoendocrinology, explaining the biological link between MDD and systemic diseases
[60]. Moreover, gut dysbiosis and an altered microbiota–gut–brain (MGB) axis are tightly linked with inflammation and the above-mentioned mechanisms, also representing a potential etiopathogenic mechanism of MDD
[61][62][61,62]. Thereby, the pathophysiology of MDD is undoubtedly complex, explaining the clinical and translational difficulties of this condition.
Diet has pleiotropic effects in the organism. Depending on the content of specific nutrients and foods, as well as the whole dietary context, they may promote health or facilitate the onset and progression of multiple pathological conditions
[35]. In the field of MDD, an umbrella review using 28 meta-analyses has defined the potential of nutrients, foods, beverages, and dietary patterns in the amelioration or progression of MDD; although, the quality of evidence is still moderate or low
[63]. This means that further studies are warranted before drawing any definitive conclusion about the potential of diet and MDD. Indeed, some authors have claimed that narrative reviews tend to overestimate the impact of diet as preventive or as adjunctive support in this field
[64], and
thwe
re is an agreement agree that is critical to be objective and put in context the promising but still inconclusive role of diet in MDD. What the evidence seems to indicate is that dietary interventions can aid in the alleviation of depressive symptoms, but most of these results have been obtained in patients without a clinical diagnosis of depression
[65], so once again it should be understood that MDD is a complex and multifactorial disorder in which plenty biological, cultural, and psychosocial factors are interacting, being a huge challenge for healthcare professionals (
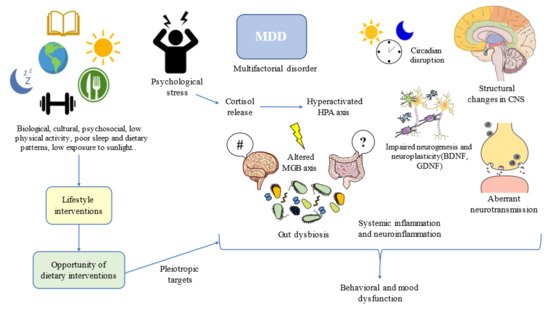
Figure 1).
Figure 1. Lifestyle interventions such as dietary interventions suppose an opportunity for addressing multiple targets in the complex network of depression. MDD: Major Depressive Disorder; HPA: hypothalamic–pituitary–adrenal; CNS: central nervous system; MGB: microbiota–gut–brain; BDNF: brain-derived neurotrophic factor; GDNF: glial-derived neurotrophic factor. #/?: Aberrant gut brain crosstalk due to an impaired MGB axis.
One of the most plausible explanations for the high prevalence of MDD and its relationship with diet can be perceived from an evolutionary context. Following the words of Theodosius Dobzhansky, “nothing in the biological aspects of medicine makes sense except in the light of evolution”. Evolutionary-based approaches have brought great advances in the understanding and clinical management of MDD
[66]. MDD can be considered a disease that emerged as the result of low physical activity levels, sleep and circadian disturbances, low exposure to sunlight, social isolation, poor contact with nature, and, of course, due to poor dietary patterns
[67]. Thus, lifestyle interventions following this evolutionary background have led to notable improvements in physical and mental health, while aiding to prevent the onset of mood disorders
[68]. Moreover, addressing the entire picture from an evolutionary perspective, in combination with antidepressants and/or psychosocial support, along with additional medical therapy, if needed, can bring maximum benefits to patients with MDD (
Figure 1).
2. Translational Opportunities: Clinical Management of MDD through Diet
2.1. Foods and Nutrients of Interest
Many efforts have been placed to find potential foods and dietary supplements with antidepressant effects as well as to prevent the onset of depression. These foods contain a wide variety of nutrients that act synergically to exert their benefits. Many times, these effects are different than those played by the nutrient alone. This concept is defined under the term food matrix, having numerous consequences for the global effect of foods
[69][71]. Dietary supplements consist of an isolated nutrient with some interesting properties and both foods and supplements with potential preventive and supportive antidepressant activity can be defined as nutraceuticals, a growing area of research in the field of MDD
[70][72]. In the field and clinical management of MDD, it is equally important to limit or avoid the consumption of ultra-processed foods, sugar-sweetened drinks, alcohol, as well as red and processed meats consumption, as they increase the risk of suffering from MDD
[63]. In this part,
thwe
will focus
is on different food sources and supplements with potential preventive and antidepressant effects.
2.2. Dietary Strategies to Implement in Patients with MDD
Not only specific nutrients and foods, but also the entire dietary context are important approaches to consider in the clinical management and prevention of MDD. Indeed, unhealthy dietary patterns clearly influence the development and aggravation of MDD. For instance, it is well-known that excessive ultra-processed food consumption in westernized and proinflammatory diets is associated with pro-depressant effects
[71][72][198,199]. On the other hand, different dietary approaches have been emerging since the last century in order to improve depressive symptoms. Some of the best-known strategies studied to support the prevention and treatment of MDD include the Mediterranean diet (MDiet), low-carbohydrate diet (LCD), and plant-based diet (vegetarianism and veganism). Here, it must be understood that the type of dietary strategy is not the most important point to consider, but the adherence to a healthy dietary pattern and the inclusion of a wide variety of food and nutrients is what really matters.
The MDiet is a type of dietary pattern mainly characterized by a richness of highly complex carbohydrates in fiber (cereals, legumes, vegetables, and fruits), polyunsaturated fatty acids with antiatherogenic and anti-inflammatory properties (i.e., fish, extra virgin olive oil, and nuts), and a plethora of bioactive compounds with antioxidative properties such as flavonoids, phytosterols, terpenes, and polyphenols
[35]. This strategy has been widely explored in the prevention and management of non-communicable diseases (NCDs), especially those associated with aging
[73][200] In this sense, some authors have described the relevance of the MDiet and some of their most representative elements on a differential epigenetic profile, which may explain the benefits of this diet in different disorders
[74][201]. MDD is an intricate and multifactorial disorder and different results have been obtained regarding the inclusion of the MDiet. Some of the most important studies regarding the role of the MDiet in the prevention of different NCDs are PREDIMED (Prevención con Dieta Mediterránea) trials. One study described that moderate fish and long-chain ꞷ-3 PUFA consumption integrated in an MDiet was associated with lower odds of depression in a U-shaped way
[75][202]. Likewise, one systematic review and meta-analysis found that adherence to the MDiet was prominently related to a reduced incidence of depression
[76][203]. More detailly, another meta-analysis also found benefits from high and moderate MDiet adherence with a reduced risk of depression. Nevertheless, the positive association of moderate adherence seemed to fade with age
[77][204]. Despite these conclusions, there are also other systematic reviews and meta-analyses that have failed to find significant associations between high adherence to the MDiet and the risk of depression
[76][78][203,205], suggesting promising but still inconsistent evidence of the benefits of the MDiet in the prevention of depression. In the context of clinical management of patients with MDD, the SMILES (Supporting the Modification of lifestyle in Lowered Emotional States) trial should be highlighted. A total of 67 individuals were studied, 33 with a modified MDiet intervention and 34 without any nutritional but social support. Of them, 55 were also receiving some type of therapy, pharmacology, psychotherapy, or a combination of both. A total of 31 of the dietary support group and 25 of the controls completed the study at 12 weeks and after further analysis, the study concluded that this type of nutritional intervention helped in the clinical management of MDD, measured with the Montgomery–Åsberg Depression Rating Scale (MADRS)
[79][206]. The dietary protocol for these patients is described in
[80][207]. Extra virgin olive oil (EVOO), one of the most representative elements of the MDiet has recently proven antidepressant effects in patients with severe MDD, but not in those with mild to moderate
[81][208]. All in all, the growing number of studies focusing on the preventive and therapeutic support of the MDiet has opened an interesting field of research. Further efforts are warranted to unravel the mechanisms and optimum protocols to obtain the greatest benefits from this type of dietary intervention.
LCDs such as the ketogenic or Atkins diet are mainly characterized by a reduced carbohydrate and high fat intake. Both aim to induce nutritional ketosis, and previous studies have found some evidence from the use of these strategies in the prevention and treatment of some neurological disorders such as epilepsy or Alzheimer’s disease
[82][209]. Ketone bodies are not only a fat-derived energy supply, but they also exert important epigenetic functions, especially through histone post-translational modifications
[83][210]. To date, there is some evidence of the antidepressant actions of ketone bodies in preclinical studies, case reports, and case series, but no clinical trials have been conducted in this field
[84][211]. The benefits seem to be related to their regulatory role on different neurotransmitter levels, mitigating oxidative stress, insulin dysfunction, and inflammation, while favoring mitochondrial function and neurotrophic factors
[84][211]. A negative point of following an LCD, however, may be related to negative long-term effects, as some authors have hypothesized that LCDs can induce metabolic depression, linked to reduced glycogen stores, and increased feelings of fatigue
[85][212]. Likewise, combining this kind of strategy with intermittent fasting can exert synergic effects in the amelioration or prevention of depressive symptoms, sharing some similarities with an evolutionary or Paleolithic lifestyle; although, there are no clinical studies conducted with intermittent fasting in psychiatric populations yet
[86][87][213,214]. Further efforts are required in this area before establishing the adequacy of this dietary context in MDD.
Plant-based diets also have different dietary patterns that have gained popularity in the last years, mainly aiming to reduce the environmental footprint of the diet and promote human health and animal welfare
[88][215]. In general terms, plant-based diets are prominently represented by non-animal products; although, there is a broad spectrum of possibilities: including low meat but all food items (omnivore), all except meat (pesco-vegetarian), all except meat and fish (ovo-lacto-vegetarian), or plant-based items (vegan). Despite some evidence arising regarding the actions of a plant-based diet in the brain, the benefits and underlying mechanisms remain to be explored
[89][216]. In this line, a possible epigenetic role of different bioactive components found in plants, especially by modulating DNA methylation, has been described
[90][217]. There is some evidence that vegetarians/vegans may be at a higher risk of suffering from depression
[91][92][218,219]. It is inconclusive if these associations can be mediated by a proper diet or if the vegans/vegetarians’ dietary pattern is a result of an increased susceptibility to depression. A potential explanation for this fact is that the vegetarian/vegan diet may not be properly balanced, being associated with the lack of some critical dietary components
[93][220]. However, some authors argue that when well-designed and supplemented with vitamin B12, plant-based diets could bring potential benefits for overall health
[94][221]. Once again, the quality of the diet may be a determinant factor. A cross-sectional study provided evidence that high-quality plant-based products (avoiding ultra-processed products and ensuring an adequate intake of different nutrients) can be protective for vegans/vegetarians without depression; although, individuals affected by depression did not show that benefit
[95][222]. Similarly, avoiding the consumption of animal-based products such as meat, eggs, or dairy products did not show an improvement in mental health or MDD, according to some studies and meta-analyses
[96][97][223,224]. This is attributed to the fact that these natural foods are rich in the different above-mentioned nutrients with potential benefits for the body
[98][225]; although, vegetarianism/veganism if properly balanced, is also a laudable choice. Overall, it must be added that the actual studies in this area are quite heterogeneous. Currently, for patients with MDD, it seems that limiting or avoiding animal products may not be the appropriate approach. However, further research is needed, especially on well-designed and balanced vegetarian/vegan diets, before any final conclusions can be drawn.
Collectively, different studies regarding the implementation of a dietary pattern to prevent and ameliorate depression have been conducted. The MDiet has been the most widely studied strategy, providing many promising results; although, the level of evidence of its benefits is still insufficient. There are few studies on LCDs in depression; although, they may have some short-term benefits from depression due to the multiple regulatory benefits of ketone bodies. Finally, it seems that there is a negative association between a plant-based diet and depression; however, there is great heterogeneity in the current literature. Moreover, it is not well-understood if vegetarianism/veganism can be a consequence of, or contributes to, some pathophysiological mechanisms. The most important points to consider are both the adherence to a healthy dietary pattern and, more prominently, the inclusion of high-quality foods, avoiding ultra-processed products, and ensuring a complete nutritional content, which will bring the most benefits for the prevention and, in an adequate context, for the clinical management of MDD.