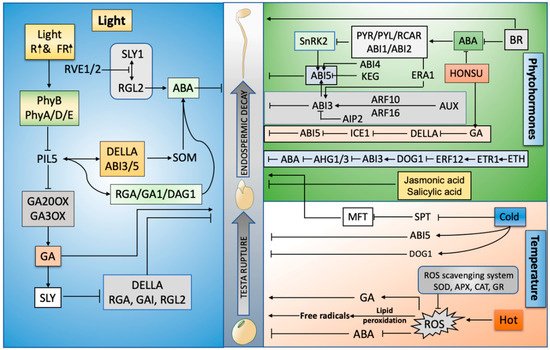
With the burgeoning population of the world, the successful germination of seeds to achieve maximum crop production is very important. Seed germination is a precise balance of phytohormones, light, and temperature that induces endosperm decay. Abscisic acid and gibberellins—mainly with auxins, ethylene, and jasmonic and salicylic acid through interdependent molecular pathways—lead to the rupture of the seed testa, after which the radicle protrudes out and the endosperm provides nutrients according to its growing energy demand. The incident light wavelength and low and supra-optimal temperature modulates phytohormone signaling pathways that induce the synthesis of ROS, which results in the maintenance of seed dormancy and germination.
1. Introduction
The germination of seeds plays a significant role in crop production, and it is an intricate process that occurs due to the precise optimization of endogenous (phytohormones, endosperm decay) and exogenous factors (light and temperature)
[1]. The transition from dormancy to germination begins when the dry seed comes in contact with water and ends when the radicle has emerged through all the coats of the developing embryo
[2]. This encounter activates the internal metabolic process, involving the careful equilibrium phytohormones
[3] in the presence of optimum light and temperature to overcome the seed’s dormancy
[4] Nevertheless, a deeper and sequential understanding of the interplay of intrinsic and extrinsic factors for seed germination is a prerequisite for the improvement of seed germination potential in various crops.
Abscisic acid (ABA) and gibberellic acid (GA) play a key role in a number of physiological processes during seed germination
[5]. ABA induces dormancy, while GA plays a key role in the release of dormancy and germination. A high ABA:GA ratio maintains dormancy, while dormancy release involves a net shift to increased biosynthesis of GA and ABA degradation resulting in a low ABA:GA ratio. These two hormones may also act in an antagonistic manner in the promotion of testa and endosperm rupture
[6].
The degree of seed dormancy is established during seed maturation and governs the behavior of the seed after shedding or even while still attached to the mother plant
[7]. The initiation of seed dormancy is coordinated in zygotic tissues by environmental factors that also perform overlapping roles in the control of embryonic identity, storage reserve accumulation, and onset of desiccation tolerance. In addition to the metabolic pathways triggering under the influence of environmental cues, for the seed to rupture, it must go through a series of checks in a sequential manner that leads to testa and endosperm rupture. In many species, seed covering layers impose a physical constraint to radical protrusion, which must be overcome by the growth potential of the embryo
[8].
Numerous extrinsic factors can prolong or terminate seed dormancy and promote seed germination and development. Light
[9], temperature
[10], and soil conditions
[11] are major signals that can be perceived by seeds to regulate the timing of germination. The regulatory effect of light on seed germination depends on its spectrum
[12]. Blue light activates ABA and delays seed germination, whereas red or far-red light plays a key role in the activation of seed germination via the activation of GA biosynthesis and restricting the production of ABA
[13]. Seed germination is dependent on the surrounding temperature, which can delay or expedite the germination process after sowing. Most species germinate in the presence of temperatures between 15–30 °C
[14]. Weakening of the endosperm is a prerequisite for the initiation of seed germination and is driven by various internal and external factors. The decay of the endosperm is directly linked to the production of ROS in response to the availability of external environmental signals
[15]. This
article
ntry summarizes the roles of environmental factors (temperature and light), phytohormones, and endosperm decay in seed germination (
Figure 1).
Figure 1. Graphical abstract: driving forces of seed germination: phytohormones, high temperature, light, and endosperm decay. R (Red), FR (Far-red), Sleepy (SLY), ABA (Abscisic acid), GA (Gibberellins), Reactive oxygen species (ROS), Reduced Dormancy 5 (RD05), RGA-LIKE2 (RGL2), SPINDLY (SPY), Teosinte branches 1/cycloidea/proliferating cell factor (TCP), Ethylene (ETH), Indole-3-acetic acid (IAA), Salicylic acid (SA), PHY-INTERACTING FACTORS (PIF), Delay of Germination (DOG), Brassinosteroids (BRs), ABA HYPERSENSITIVE GERMINATION1 (AHG1), Super oxidase dismutase (SOD), Ascorbate peroxidase (APX), Catalase (CAT), Glutathione reductase (GR), MOTHER OF FT and TFL1 (MFT), ABA-insensitive (ABI), Phytochrome (Phy), Serine palmitoyltransferase (SPT), auxin-responsive factors (ARF), Ethylene responsive transcription factor (ERF), ICE1 (Inducer of CBF Expression 1), Diacylglycerol (DAG), Auxin (AUX), Repressor of ga1-3 (RGA), ABI3-interacting protein (AIP), ETHYLENE RESPONSE1 (ETR1), SOMNUS (SOM, a set of 98 genes), Resolvin E1 (RVE1).
2. Phytohormone Regulation of Dormancy and Germination
Seed germination and dormancy are strictly regulated by hormones, particularly ABA and GA, which have antagonistic effects. ABA biosynthesis takes place mainly due to carotenoid cleavage dioxygenase gene 6 (NCED 6), NCED 9, and other genes, such as ABA-deficient (ABA2) and abscisic aldehyde oxidase 3 (AAO3) during seed development to maintain seed dormancy. ABA-insensitive (ABI) genes, i.e., ABI3, ABI4, and ABI5 are more plentiful in dormant seeds than in seeds with reduced seed dormancy levels. Among ABI genes, ABI3 expressed in the developing seeds also regulates the accumulation of chlorophyll, anthocyanins, and storage proteins together with two other seed-related regulators, FUSCA3 (FUS3) and leafy cotyledon 1 (LEC1). ABA levels in seeds depend on the degradation process. The catabolism of ABA is mediated by ABA8 hydroxylase encoded by P450 (CYP707A) genes and is induced by imbibition and stratification; the concentration of ABA declines and that of GA increases
[16]. ABA-dependent pathways are also very important in the regulation of seed germination. ABA signaling must be terminated by a process in which the membrane-associated transcription factor peptidases S1P (Site-1 Protease) and S2P translocate the bZIP17 protein from the endoplasmic reticulum (ER) to the Golgi apparatus and then to the nucleus. There, activated bZIP17 regulates the transcription of downstream negative regulators of ABA signaling
[17]. ABA acts through the PYR/PYL/-RCAR-PP2C-SnRKs signaling cascade. The PP2C proteins ABI1 and ABI2 bind to ABA receptors to inhibit signaling
[18][19][18,19]. Their dominant negative mutants, abi1-1 and abi2-2, show reduced dormancy due to the impaired interaction between the mutated proteins and their receptors. The other PP2C HONSU (HON) protein also influences seed germination by downregulating ABA signaling and upregulating GA signaling
[20]. In addition, another PP2C gene, RD05 (reduced dormancy 5), has a positive role in the reduction of seed dormancy
[21]. Genetic and bioinformatic analysis showed that RD05 controls seed dormancy by mediating transcription of the PUF family RNA-binding genes APUM9 (Arabidopsis PUMILI09) and APUM11
[21]. However, RD05 appears to function independently of the ABA pathway, and further research is needed to accurately delineate its role in seed germination processes.
GA biosynthesis mainly occurs in the radicle of the embryo, which in turn ensures germination progression. However, high exogenous concentrations of GA can negatively influence the germination process
[22]. The activation of GA-responsive genes induces cell-wall-remodeling enzymes, such as endo-β mannase, xyloglucan endotransglycolase, expansin, and β 1,3-mannase. Their activity leads to the weakening of the surrounding embryo layers. GA breaks dormancy by antagonistically suppressing ABA-triggered seed dormancy. This process appears to involve the secretion of hydrolytic enzymes gibberellin 3-oxidase 1 (GA3ox1), GA20ox3, and ENT-kaurene oxidase 1 (KO1) to weaken the seed testa, although detailed and precise information about this mechanism is lacking. GA-deficient mutants, such as ga1 and ga2, show strong dormancy and cannot germinate without external GA application
[22]. Mutations in DELLA genes, including RGL2 (RGA-LIKE2) and SPY (SPINDLY), two negative regulators of the GA signaling pathway, can rescue the non-germination phenotype of ga1. DELLAs also maintain the seed in a quiescent state of cell cycle progression by repressing the activities of TCP14 (Teosinte branches 1/cycloidea/proliferating cell factor) and TCP15
[23]. The sleepy1 (SLY1) is an F-box protein which enables 26S-proteasome-mediated degradation of DELLA proteins in the presence of active GA.
sly1 mutants show reduced germination even after the application of exogenous GA
[24].
Genes in hormone signaling pathways also play an important role in regulating seed germination. The expression levels of numerous genes are up- and downregulated to mediate seed dormancy and germination (
Table 1). The APETALA 2 (AP2) domain containing transcription factor ABI4 plays a significant role in seed dormancy regulation. ABI4 controls many signaling pathways, including responses to ABA, glucose, sucrose, ethylene (ET), and salt stress. ABI4 positively regulates ABA catabolism genes and negatively regulates GA biogenesis genes. The loss of ABI4 function increases the expression of GA biosynthesis genes but decreases the expression of GA inactivation genes; together, these changes lead to decreased primary seed dormancy in the abi4 mutant
[25]. Furthermore, ABI4 binds to the promoters of CYP707A1 and CYP707A2, which mediate ABA catabolism, inhibiting their transcription and thereby promoting the accumulation of ABA. However, no direct role of ABI4 in GA metabolism has been identified, and ABI4 may not directly bind to the promoters of GA biosynthesis genes. Instead, it may recruit additional seed-specific transcription factors to repress the transcription of GA metabolism genes
[26]. Clearly, the AP2 domain plays an important role in the dual regulation of ABA and GA biosynthesis to optimize seed dormancy and germination.
Table 1.
Genes involved in seed dormancy and germination.
| Name of Gene |
Mutant Dormancy Level |
General Description of Gene |
References |
| ABI3 |
Decreased |
Positively regulates ABA signaling and represses seed germination |
[27] |
| ABI4 |
Decreased |
Positively regulates ABA signaling and represses seed germination |
[28][29][28,29] |
| ABI5 |
Not Changed |
Positively regulates ABA signaling and represses seed germination |
[30][31][30,31] |
| NCED5 |
Decreased |
ABA-biosynthesis gene; the ABA content is decreased |
[32] |
| CYP707A1/2 |
Enhanced |
ABI4 negatively regulates its transcription |
[33] |
| GAI/2 |
Enhanced |
GA-biosynthesis genes; GA content is decreased in mutants |
[23] |
| GA2oxs |
Decreased |
GA-inactivate genes; GA content is upregulated in mutants |
[34] |
| RGL2/SPY |
Enhanced |
GA signaling is blocked in mutants |
[35] |
| MYB96 |
Decreased |
Decreases transcription of ABI4 and some ABA biogenesis genes |
[36][37][36,37] |
| DOG1 |
Enhanced |
ABA sensitivity of dog1 seeds is unchanged |
[38][39][38,39] |
| SUVH4/SUVH5 |
Enhanced |
Repress DOG1 and ABI3 transcription |
[40] |
| LDL1/LDL2 |
Enhanced |
Repress seed dormancy by negatively regulating DOG1 |
[17] |
| WRKY41 |
Decreased |
WRKY41 directly promotes ABI3 transcription |
[41] |
| RAF10/RAF11 |
Decreased |
Directly enhances ABI3 transcription |
[42] |
| DEP |
Decreased |
Promotes ABI3 transcription |
[43] |
| SPT |
Decreased in Ler but enhanced in Col background |
Opposite roles in Ler and Col ecotypes |
[44][45][44,45] |
| ARF10/ARF16 |
Decreased |
ARF10/ARF16 directly promote ABI3 transcription |
[46] |
| BIN2 |
Not mentioned |
Phosphorylates and stabilizes ABI5 to enhance ABA signaling |
[47] |
| PKS5 |
Not mentioned |
Phosphorylates ABI5 (Ser42) and controls transcription of ABA-responsive genes |
[48] |
| HONSU |
Enhanced |
A PP2C protein that impairs ABA signaling |
[20] |
| RDO5 |
Enhanced |
Its ABA sensitivity and content remain unchanged |
[21] |
| ABI1/2 |
Decreased |
Dominant-negative mutants; the mutated proteins cannot interact with ABA receptors |
[49] |
| CHO1 |
Decreased |
Acts upstream on ABI4 genetically |
[50] |
| OsAP2-39 |
Decreased |
Promotes OsNCEDI and OsEUI, thereby enhancing ABA biogenesis and impairing GA accumulation |
[51] |
| DDF1 |
Decreased |
Directly promotes GAox7 and thus decreases GA content |
[52] |
Auxin is involved in all stages of plant development and in the response to a multitude of environmental cues. Exogenous auxin application triggers seed dormancy under salt stress, indicating its role in seed germination
[53]. IAA (indole-3-acetic acid) delays seed germination and inhibits preharvest sprouting in wheat
[54]. Seed dormancy and germination are controlled by auxin-related genes. Biochemical studies have shown that when auxin levels are low due to the suppression of auxin-responsive factors 10 (ARF10) and ARF16 by AXR2/3, the expression of AB13 is not activated and seed dormancy is released. Contrarily, when auxin levels are high, ARF10 and ARF16 are released to activate AB13 transcription and seed dormancy is maintained
[55]. Increases in the biosynthesis of auxins can be linked to the release of dormancy in monocots. Furthermore, TaAuxin-resistant 1 (TaAXR1), TaUbiquitin-related protein 1 (TaRUB1), and TaARF@ were upregulated in the ripened wheat seeds. TaAXR1 is associated with AUX/IAA-proteasome-mediated degradation, whereas TaRUB1 is related to ubiquitin action. The higher expression of TaAXR1 and TaRUB1 can exert a negative impact on auxin signaling
[46].
Ethylene (ET) breaks seed dormancy and enhances seed germination by reducing the effects of ABA. Changes in positive regulators of the ET signaling pathway cause severe dormancy, whereas mutations in the negative ET regulator Ctr1 (Constitutive Triple Response 1) lead to rapid or early seed germination
[56]. Brassinosteroids (BRs) have been reported to act in opposition to ABA to improve seed germination, partly through an MFT (MOTHER OF FT and TFL1)-mediated pathway that generates a negative feedback loop to modulate ABA signaling. The ABA response of BR mutants or BR-deficient plants is stronger than that of wild-type seeds, indicating that BR overcomes the inhibitory effect of ABA on germination
[57]. Salicylic acid (SA) controls seed germination by inhibiting the expression of GA-induced α-amylase genes under normal growth conditions. Moreover, SA has been found to promote seed germination under salt stress through another signaling pathway that reduces oxidative damage
[58]. Cytokinins (CTKs) improve seed germination by reducing the impact of ABA, specifically by the downregulation of AB15 transcription. A recent study has shown that AB15 plays an important role in ABA and CTK signaling at both the mRNA and protein levels
[59]. Jasmonic acid (JA) has an antagonistic relationship with ABA: it not only suppresses ABA biosynthesis genes but also inhibits ABA inactivating genes (
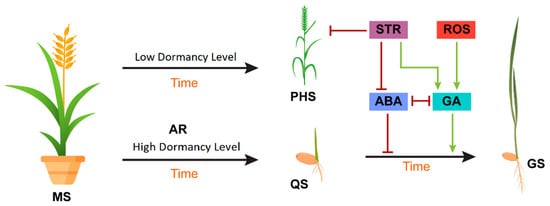
Figure 2)
[60].
Figure 2. Model showing the effects of extrinsic and intrinsic factors on seed dormancy and germination. During the maturation of the seed (MS), intrinsic ABA is upregulated, and GA is downregulated to inhibit preharvest sprouting (PHS) on the mother plant. After harvest, stratification (STR) and reactive oxygen species (ROS) increase GA biosynthesis and repress ABA biosynthesis, turning the quiescent seed (QS) into a germinating seed (GS). Red bars indicate an inhibition effect, whereas green arrows indicate a promotion effect.
3. Light Controls Seed Germination and Dormancy
Light is indispensable for germination, although the exact functions of light in seed germination require additional study. Light regulates various plant physiological processes, such as seed germination and dormancy, photomorphogenesis, phototropism, and flowering. There are many factors involved in light regulation of seed dormancy and germination. At least five kinds of photoreceptors in plants that monitor surrounding light signals have been reported
[14]. Blue light (320–500 nm wavelength) is absorbed by photosensory receptors, including the cryptochromes (CRYs), FLAVIN-BINDING KELCH REPEAT F-BOX1/ ZEITLUPE /LOV KELCH PROTEIN2, and phototropins. Numerous genetics studies have indicated that alterations in these photoreceptors can cause changes in seed germination and agronomical traits. Blue light has been identified to play a role in seed germination inhibition. Cryptochrome 1 mediates this inhibition by downregulating CRY1a and CRY1b products in barley through an RNA interference (RNAi) approach that results in reduced blue light inhibition of grain germination, suggesting the specific role of cry1 in promoting seed dormancy in this monocot. This effect is due to the induction of ABA biosynthetic gene 9-CIS-EPOXYCAROTENOID DIOXYGENASE 1 (NCED1) with consequent ABA biosynthesis. Blue light does not induce NCED 1 in germinating CRY1a/CRY1b RNAi seed, whereas ABA-catabolic gene ABA8′OH-1 is upregulated during early phases of germination
[61][62][61,62]. The blue light receptor cryptochrome circadian regulator 1 (CrY1) mediates the stimulatory effects of blue light on the expression of NCED1, which increases ABA content and inhibits seed germination in dormant barley
[61][63][61,63]. Blue light has also been reported to inhibit seed germination in Brachypodium distachyon
[14]. Previous studies have suggested that blue light represses seed germination by enhancing the transcription of ABA biosynthesis genes and repressing the expression of ABA catabolism genes
[60].
Phytochromes are necessary for the light-induced promotion of seed germination. PhyB occupies the most important position. PhyB mediates the red/far-red photo-reversible response (LFR) to induce the early stages of seed germination. In response to long nights and imbibition, phyA mediates the very low fluence response (VLFR) to different light spectra (UV-A-FR) and the R/FR high irradiance response (R/FR-HIR) to accelerate seed germination in the absence of active phyB
[14]. PhyA- and phyB-dependent germination induction are spatially separated and occur in the endosperm and embryo, respectively
[64][86]. PhyE is required for germination in the presence of continuous far-red light
[65][87]. PhyE and phyD stimulate germination at very low red/far-red ratios, whereas phyC antagonizes the promotion of germination by light
[66][65].
Basic helix–loop–helix (bHLH) transcription factors from the PHY-INTERACTING FACTORS (PIF) family negatively regulate the phytochrome-mediated light-signaling pathway
[67][84]. The Arabidopsis genome encodes eight PIFs: PIF1, PIF2/PIL1, and PIF3–PIF8. The interaction between the phytochromes and the PIFs depends mainly on short domains located in the amino termini of the PIFs: APB for Pfr phyB binding and APA for Pfr phyA binding. Light-activated Phys modulate the functions of PIFs through different mechanisms. For example, the phyB-PIF interaction lowers the DNA-binding capacity of PIF1, PIF3, and PIF4
[68][88]. However, there is still much to learn about the regulation of seed germination by light of different wavelengths in order to devise appropriate strategies for individual plant species.
Red or far-red light (600–750 nm) is perceived by the phytochromes
[69][89], and UV-B light (280–320 nm) is perceived by UV RESISTANCE LOCUS8 (UVR8). These photoreceptors mediate light signals to remodel global transcriptional programs by selectively interacting with transcription factors or E3 ubiquitin ligases that regulate the stability of transcription factors
[70][90]. Studies have demonstrated that red and far-red light modulates seed germination through interactions between phys and PIF1, which in turn controls ABA and GA pathways
[71][91]. Phytochromes were first identified in lettuce as regulators of seed germination. Red light can induce seed germination, whereas far-red light has an inhibitory effect
[72][92]. These effects have been confirmed repeatedly in multiple plant species. Both the inactive form (Pr) and the active form (Pfr) of phytochromes are present in plants. Pr is converted into Pfr by the absorption of red light and promotes seed germination, whereas Pfr is converted into Pr in the presence of far-red light. Pfr translocates to the nucleus, where it controls the transcription of GA- and ABA-related genes by altering the stability or mRNA abundance of several transcription factors
[73][93].
In summary, the germination of a seed is dependent on the precise balance of ABA and GA. ABA is a key player for the entrance and the establishment of seed dormancy and is necessary for the quiescent stage of the seeds, whereas the GA-mediated pathway is an important regulator for the promotion of seed germination in favorable conditions. Many components of the ABA and GA pathways, i.e., ABI3, ABI4, ABI5, RGL2, MFT, and DOG1, effectively control the germination of the seed. Moreover, auxin, jasmonic acid, brassinosteroids, and ethylene modulate the ABA pathway in seeds, which indicates that seed germination is an extensive process which occurs due to the crosstalk of phytohormones.