Plastic waste recycling refers to the waste management process that collects plastic waste materials and turns them into raw materials reused to produce other valuable products. Recycling is not only a method for disposing of plastic waste, but it is also an effective process to minimize the need for virgin plastics, which can help lessen global warming. According to the ASTM Standard D5033, plastic recycling can be categorized as primary, secondary, tertiary, and quaternary recycling. Based on the mechanism of the methods, plastic waste recycling can be classified as mechanical, chemical, and biological recycling. Chemical recycling, such as catalytic and thermal processes, can convert plastic waste into value-added chemicals/fuels. This process is a potential method to reduce plastic waste as a primary source of environmental issues.
- polypropylene
- polystyrene
- polyvinyl chloride
- high-density polyethylene
- low-density polyethylene
- polyurethanes
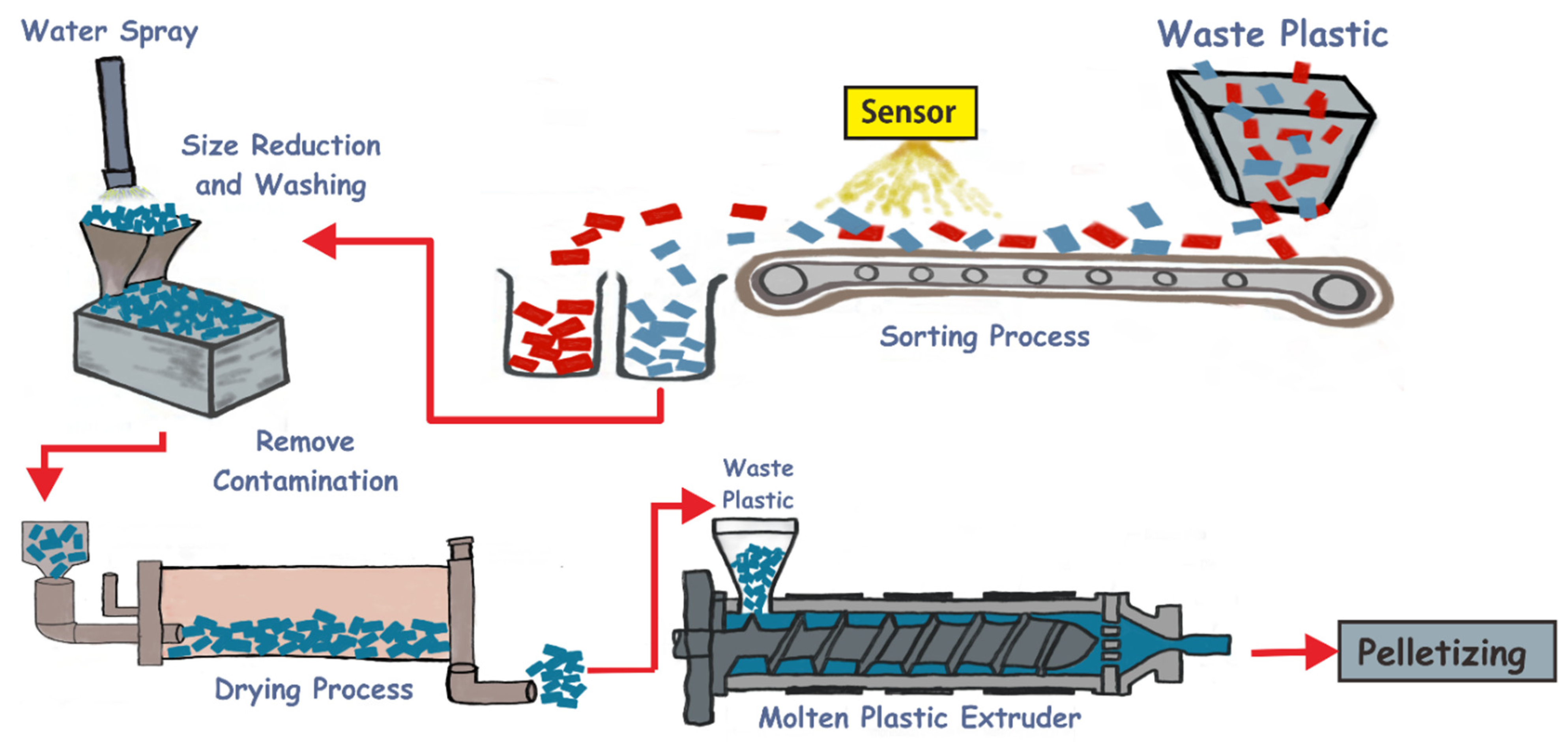
- chemical–mechanical recycling
1. Introduction
- (a)
-
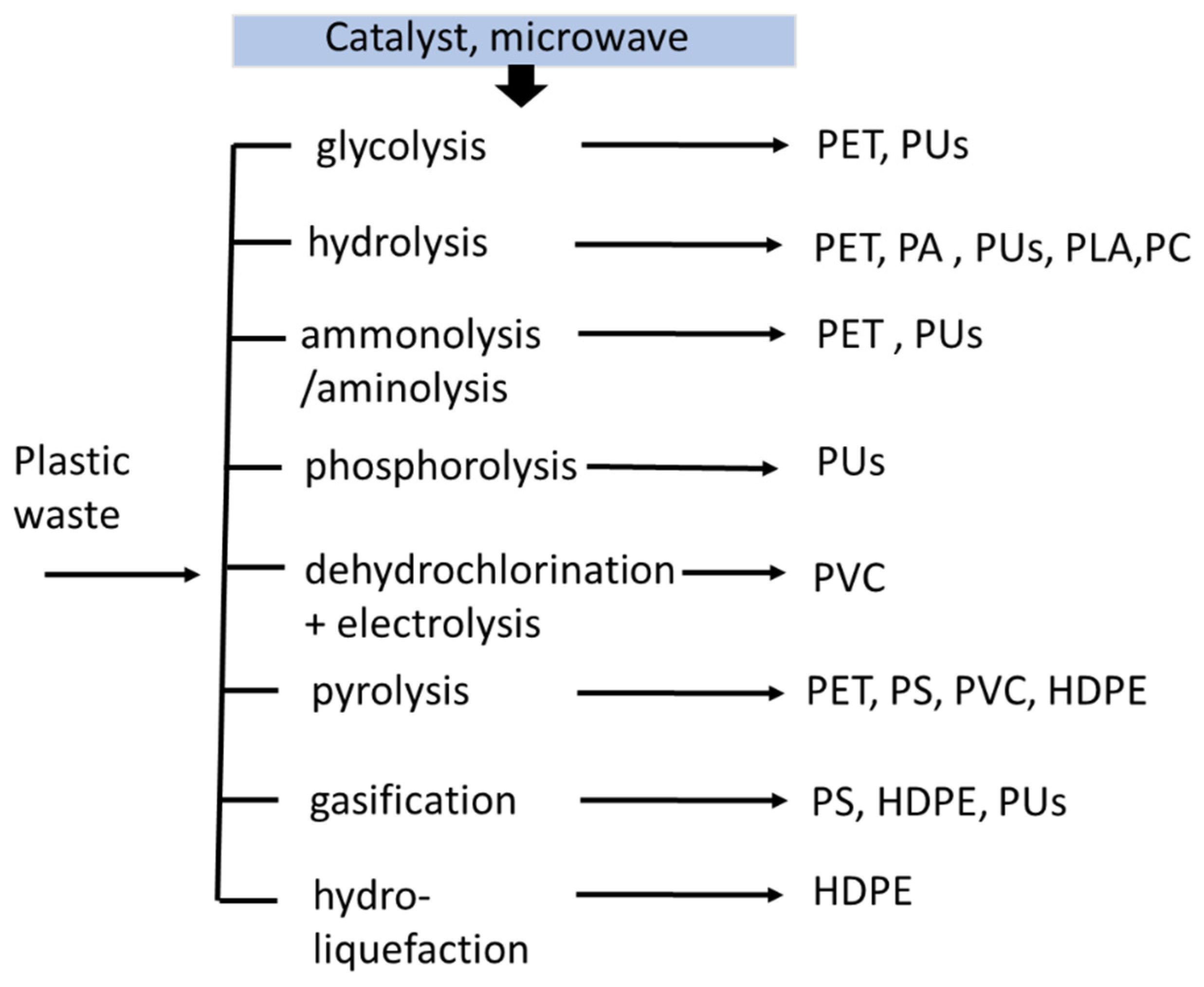
Gasification: the polymer is utilized as a refuse-derived fuel using high temperature. It is converted to syngas with an H2/CO molar ratio of 2:1 in a gasifier; the syngas produced depend on the various polymers.
- (b)
-
Pyrolysis: the plastic waste is converted to pyrolytic oil, which is equivalent to diesel oil. In this chemical recycling, the calorific value of the polymer affects the energy content of the diesel [26][12].
- (c)
-
Glycolysis: the ethylene glycol and waste plastic are added in the presence of a catalyst. The long polymer chain is degraded into building blocks, which can be recycled to produce new polymers.
- (d)
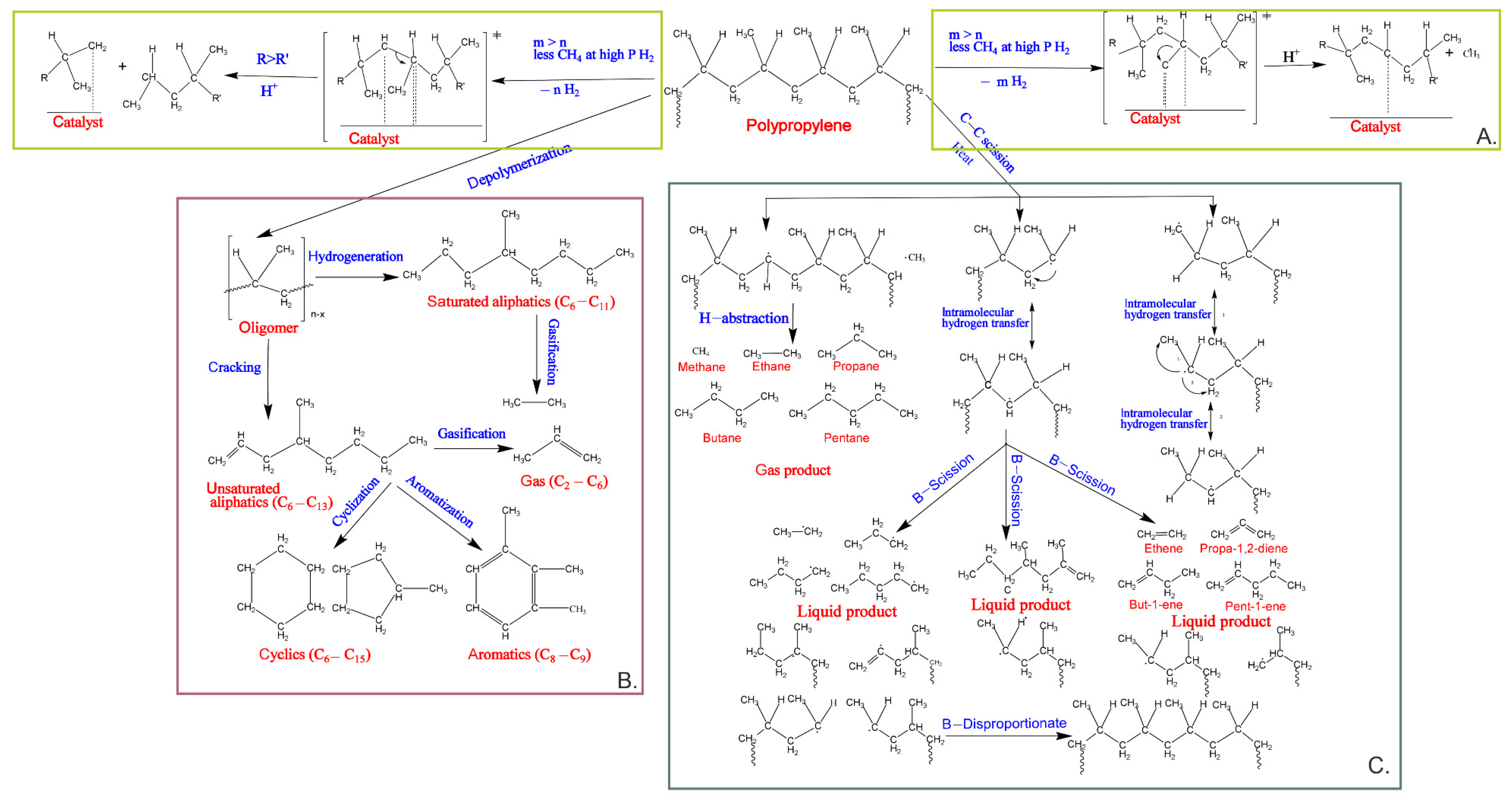
2. Recycling Polypropylene
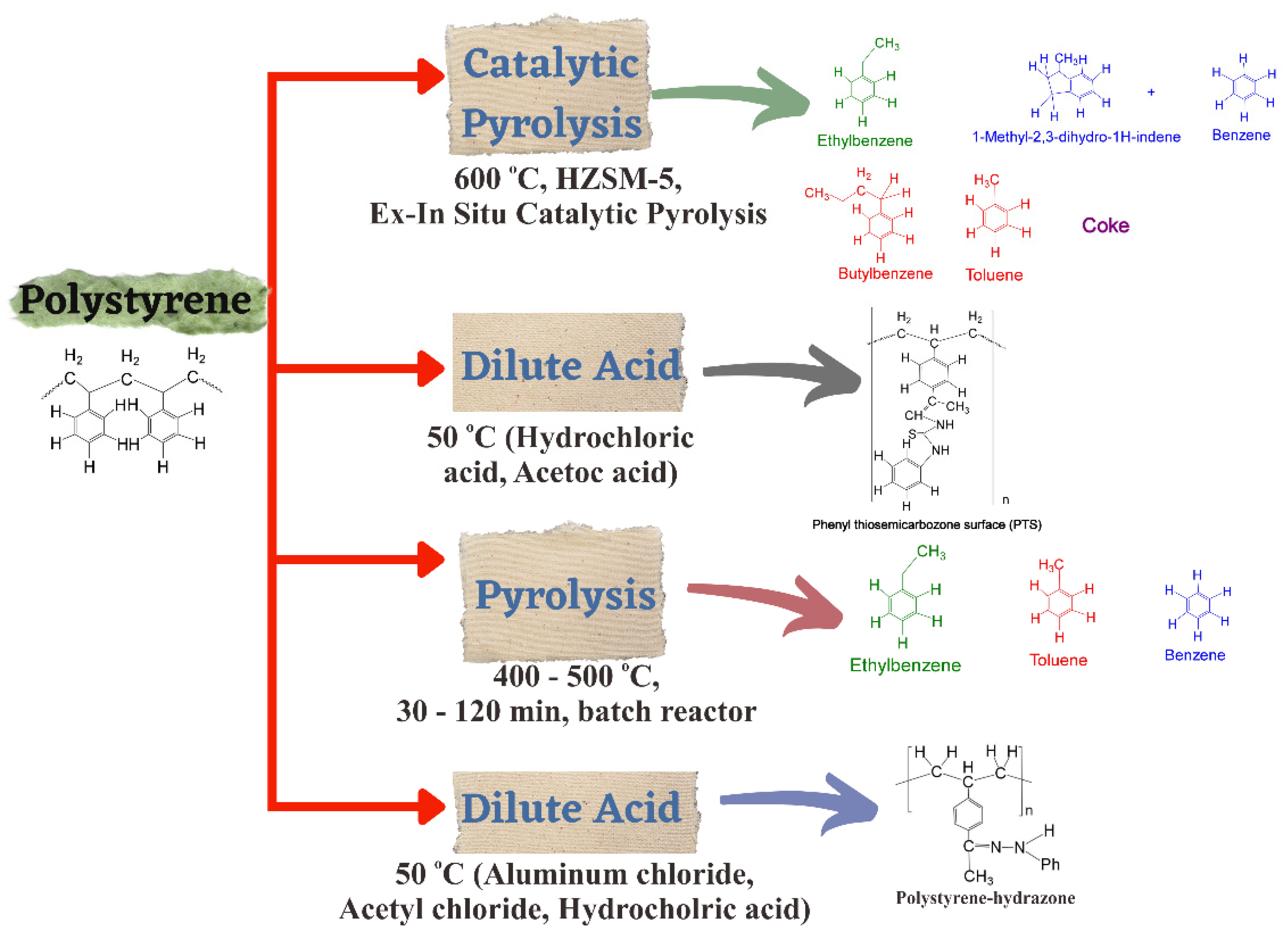
3. Recycling Polystyrene
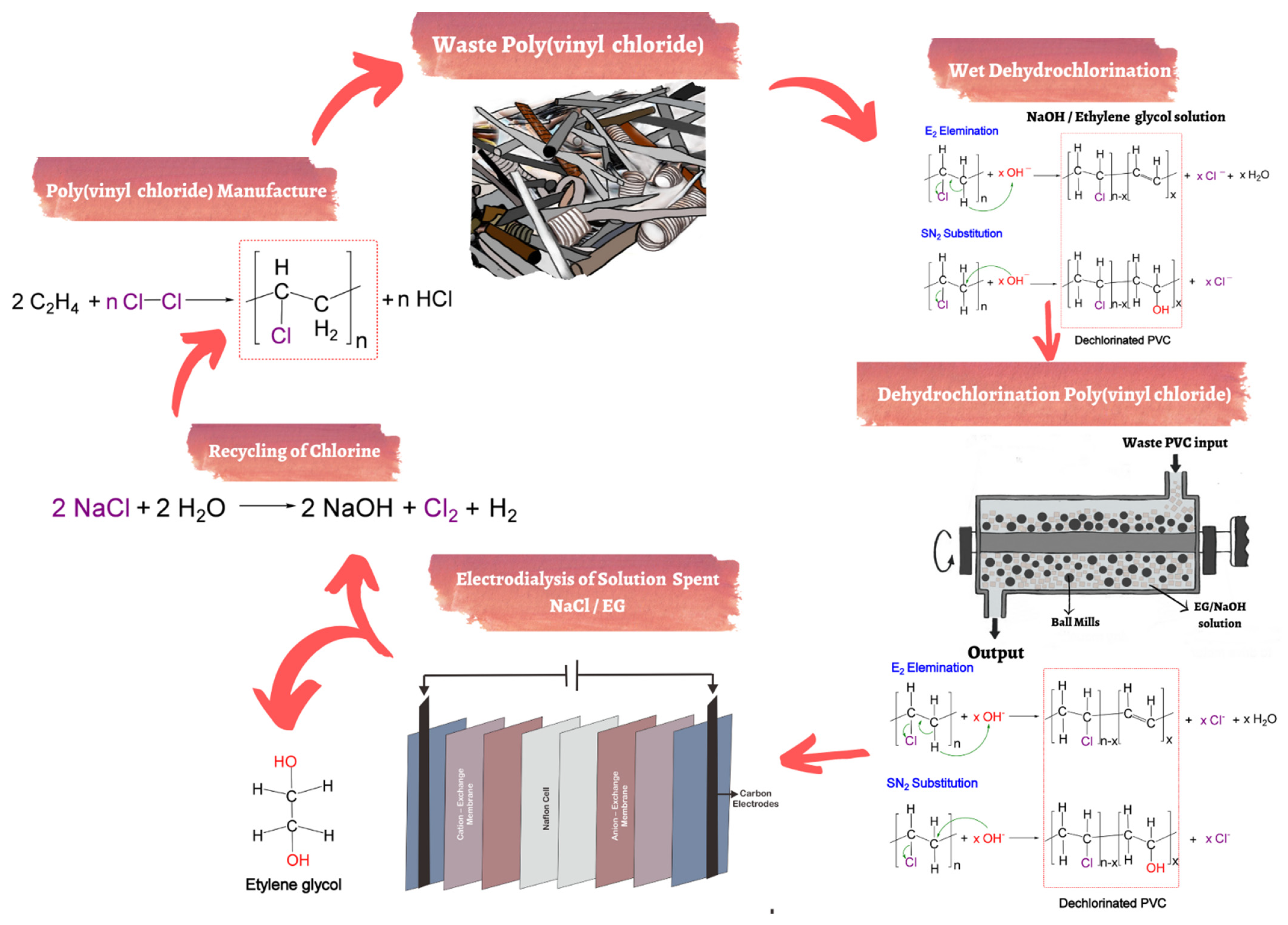
4. Recycling of Polyvinyl Chloride
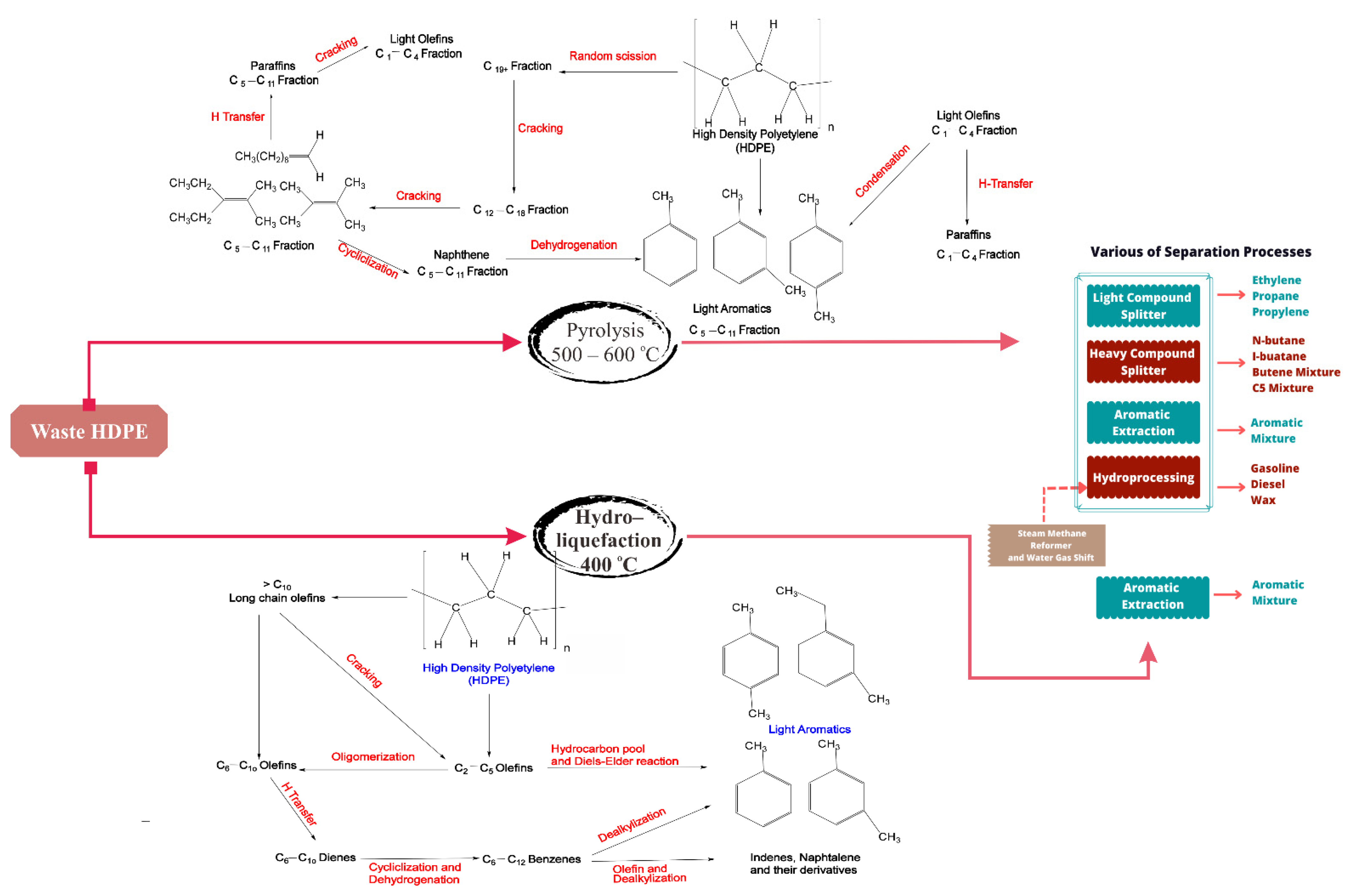
5. Recycling of High-Density Polyethylene and Low-Density Polyethylene
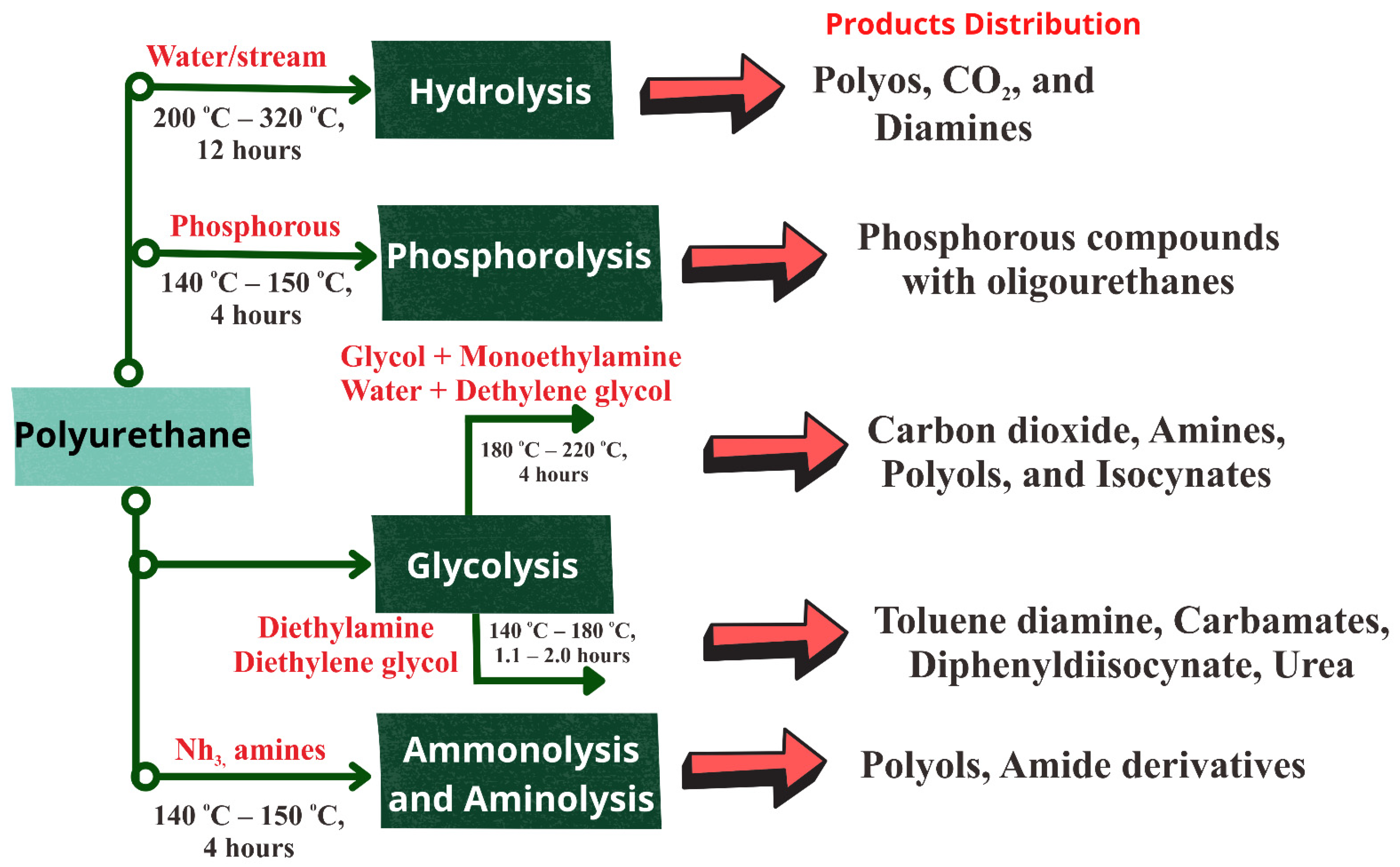
6. Recycling of Polyurethanes Waste
7. Recycling of Polyethylene Terephthalate
References
- Davidson, M.G.; Furlong, R.A.; McManus, M.C. Developments in the life cycle assessment of chemical recycling of plastic waste—A review. J. Clean. Prod. 2021, 293, 126163.
- Al-Sabagh, A.; Yehia, F.; Eshaq, G.; Rabie, A.; ElMetwally, A. Greener routes for recycling of polyethylene terephthalate. Egypt. J. Pet. 2016, 25, 53–64.
- Lerici, L.C.; Renzini, M.S.; Pierella, L.B. Chemical catalyzed recycling of polymers: Catalytic conversion of PE, PP and PS into fuels and chemicals over HY. Procedia Mater. Sci. 2015, 8, 297–303.
- Mazhandu, Z.S.; Muzenda, E.; Mamvura, T.A.; Belaid, M. Integrated and consolidated review of plastic waste management and bio-based biodegradable plastics: Challenges and opportunities. Sustainability 2020, 12, 8360.
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58.
- Damayanti; Wu, H.S. Strategic possibility routes of recycled PET. Polymers 2021, 13, 1475.
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782.
- de Camargo, R.V.; Saron, C. Mechanical–chemical recycling of low-density polyethylene waste with polypropylene. J. Polym. Environ. 2020, 28, 794–802.
- Serranti, S.; Bonifazi, G. Techniques for separation of plastic wastes. In Use of Recycled Plastics in Eco-Efficient Concrete; Elsevier: Amsterdam, The Netherlands, 2019; pp. 9–37.
- Quienne, B.; Cuminet, F.; Pinaud, J.; Semsarilar, M.; Cot, D.; Ladmiral, V.; Caillol, S. Upcycling Biobased Polyurethane Foams into Thermosets: Toward the Closing of the Loop. ACS Sustain. Chem. Eng. 2022, 10, 7041–7049.
- Garcia, J.M.; Robertson, M.L. The future of plastics recycling. Science 2017, 358, 870–872.
- Fulgencio-Medrano, L.; Garcia-Fernandez, S.; Asueta, A.; Lopez-Urionabarrenechea, A.; Perez-Martinez, B.B.; Arandes, J.M. Oil Production by Pyrolysis of Real Plastic Waste. Polymers 2022, 14, 553.
- Vollmer, I.; Jenks, M.J.; Roelands, M.C.; White, R.J.; van Harmelen, T.; de Wild, P.; van Der Laan, G.P.; Meirer, F.; Keurentjes, J.T.; Weckhuysen, B.M. Beyond mechanical recycling: Giving new life to plastic waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423.
- Schwarz, A.; Ligthart, T.; Bizarro, D.G.; De Wild, P.; Vreugdenhil, B.; van Harmelen, T. Plastic recycling in a circular economy; determining environmental performance through an LCA matrix model approach. Waste Manag. 2021, 121, 331–342.
- Siddiqui, M.N. Conversion of hazardous plastic wastes into useful chemical products. J. Hazard. Mater. 2009, 167, 728–735.
- Harussani, M.; Sapuan, S.; Rashid, U.; Khalina, A.; Ilyas, R. Pyrolysis of polypropylene plastic waste into carbonaceous char: Priority of plastic waste management amidst COVID-19 pandemic. Sci. Total Environ. 2021, 803, 149911.
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364.
- Mark, L.O.; Cendejas, M.C.; Hermans, I. Cover Feature: The Use of Heterogeneous Catalysis in the Chemical Valorization of Plastic Waste (ChemSusChem 22/2020). ChemSusChem 2020, 13, 5773.
- Ellis, L.D.; Rorrer, N.A.; Sullivan, K.P.; Otto, M.; McGeehan, J.E.; Román-Leshkov, Y.; Wierckx, N.; Beckham, G.T. Chemical and biological catalysis for plastics recycling and upcycling. Nat. Catal. 2021, 4, 539–556.
- Chen, L.; Zhu, Y.; Meyer, L.C.; Hale, L.V.; Le, T.T.; Karkamkar, A.; Lercher, J.A.; Gutiérrez, O.Y.; Szanyi, J. Effect of reaction conditions on the hydrogenolysis of polypropylene and polyethylene into gas and liquid alkanes. React. Chem. Eng. 2022, 7, 844–854.
- Miao, Y.; von Jouanne, A.; Yokochi, A. Current Technologies in Depolymerization Process and the Road Ahead. Polymers 2021, 13, 449.
- Yan, G.; Jing, X.; Wen, H.; Xiang, S. Thermal Cracking of Virgin and Waste Plastics of PP and LDPE in a Semibatch Reactor under Atmospheric Pressure. Energy Fuels 2015, 29, 2289–2298.
- Chaudhary, A.K.; Vijayakumar, R. Studies on biological degradation of polystyrene by pure fungal cultures. Environ. Dev. Sustain. 2020, 22, 4495–4508.
- Chaudhary, A.; Dave, M.; Upadhyay, D.S. Value-added products from waste plastics using dissolution technique. Mater. Today: Proc. 2022, 57, 1730–1737.
- Terashima, T. Polystyrene (PSt). In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 2077–2091.
- Gebre, S.H.; Sendeku, M.G.; Bahri, M. Recent Trends in the Pyrolysis of Non-Degradable Waste Plastics. ChemistryOpen 2021, 10, 1202–1226.
- Siyal, A.N.; Memon, S.Q.; Khuhawar, M. Recycling of styrofoam waste: Synthesis, characterization and application of novel phenyl thiosemicarbazone surface. Pol. J. Chem. Technol. 2012, 14, 11–18.
- Cella, R.F.; Mumbach, G.D.; Andrade, K.L.; Oliveira, P.; Marangoni, C.; Bolzan, A.; Bernard, S.; Machado, R.A.F. Polystyrene recycling processes by dissolution in ethyl acetate. J. Appl. Polym. Sci. 2018, 135, 46208.
- García, M.T.; Gracia, I.; Duque, G.; de Lucas, A.; Rodríguez, J.F. Study of the solubility and stability of polystyrene wastes in a dissolution recycling process. Waste Manag. 2009, 29, 1814–1818.
- Zhao, Y.-B.; Lv, X.-D.; Ni, H.-G. Solvent-based separation and recycling of waste plastics: A review. Chemosphere 2018, 209, 707–720.
- Achilias, D.; Antonakou, E.; Koutsokosta, E.; Lappas, A. Chemical recycling of polymers from waste electric and electronic equipment. J. Appl. Polym. Sci. 2009, 114, 212–221.
- Gil-Jasso, N.D.; Segura-González, M.A.; Soriano-Giles, G.; Neri-Hipolito, J.; López, N.; Mas-Hernández, E.; Barrera-Díaz, C.E.; Varela-Guerrero, V.; Ballesteros-Rivas, M.F. Dissolution and recovery of waste expanded polystyrene using alternative essential oils. Fuel 2019, 239, 611–616.
- Mumbach, G.D.; Alves, J.L.F.; Da Silva, J.C.G.; De Sena, R.F.; Marangoni, C.; Machado, R.A.F.; Bolzan, A. Thermal investigation of plastic solid waste pyrolysis via the deconvolution technique using the asymmetric double sigmoidal function: Determination of the kinetic triplet, thermodynamic parameters, thermal lifetime and pyrolytic oil composition for clean energy recovery. Energy Convers. Manag. 2019, 200, 112031.
- Maafa, I.M. Pyrolysis of polystyrene waste: A review. Polymers 2021, 13, 225.
- Huang, J.; Cheng, X.; Meng, H.; Pan, G.; Wang, S.; Wang, D. Density functional theory study on the catalytic degradation mechanism of polystyrene. AIP Adv. 2020, 10, 085004.
- Cho, K.H.; Cho, D.R.; Kim, K.H.; Park, D.W. Catalytic degradation of polystyrene using albite and montmorillonite. Korean J. Chem. Eng. 2007, 24, 223–225.
- Anwar, J.; Munawar, M.A.; Waheed-uz-Zaman; Dar, A.; Tahira, U. Catalytic depolymerisation of polystyrene. Prog. Rubber Plast. Recycl. Technol. 2008, 24, 47–51.
- Miliute-Plepiene, J.; Fråne, A.; Almasi, A.M. Overview of polyvinyl chloride (PVC) waste management practices in the Nordic countries. Clean. Eng. Technol. 2021, 4, 100246.
- Kameda, T.; Fukushima, S.; Shoji, C.; Grause, G.; Yoshioka, T. Electrodialysis for NaCl/EG solution using ion-exchange membranes. J. Mater. Cycles Waste Manag. 2013, 15, 111–114.
- Qi, Y.; He, J.; Li, Y.; Yu, X.; Xiu, F.-R.; Deng, Y.; Gao, X. A novel treatment method of PVC-medical waste by near-critical methanol: Dechlorination and additives recovery. Waste Manag. 2018, 80, 1–9.
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314.
- Zakharyan, E.; Petrukhina, N.; Maksimov, A. Pathways of Chemical Recycling of Polyvinyl Chloride: Part 1. Russ. J. Appl. Chem. 2020, 93, 1271–1313.
- Pan, J.; Jiang, H.; Qing, T.; Zhang, J.; Tian, K. Transformation and kinetics of chlorine-containing products during pyrolysis of plastic wastes. Chemosphere 2021, 284, 131348.
- Zhou, J.; Liu, G.; Wang, S.; Zhang, H.; Xu, F. TG-FTIR and Py-GC/MS study of the pyrolysis mechanism and composition of volatiles from flash pyrolysis of PVC. J. Energy Inst. 2020, 93, 2362–2370.
- Liu, H.; Wang, C.; Zhang, J.; Zhao, W.; Fan, M. Pyrolysis kinetics and thermodynamics of typical plastic waste. Energy Fuels 2020, 34, 2385–2390.
- Zhou, X.-L.; He, P.-J.; Peng, W.; Yi, S.-X.; Lü, F.; Shao, L.-M.; Zhang, H. Upcycling waste polyvinyl chloride: One-pot synthesis of valuable carbon materials and pipeline-quality syngas via pyrolysis in a closed reactor. J. Hazard. Mater. 2022, 427, 128210.
- Kumagai, S.; Lu, J.; Fukushima, Y.; Ohno, H.; Kameda, T.; Yoshioka, T. Diagnosing chlorine industrial metabolism by evaluating the potential of chlorine recovery from polyvinyl chloride wastes—A case study in Japan. Resour. Conserv. Recycl. 2018, 133, 354–361.
- Takeshita, Y.; Kato, K.; Takahashi, K.; Sato, Y.; Nishi, S. Basic study on treatment of waste polyvinyl chloride plastics by hydrothermal decomposition in subcritical and supercritical regions. J. Supercrit. Fluids 2004, 31, 185–193.
- Enomoto, H. Dechlorination Treatment of Poly (vinyl chloride). J. Jpn. Soc. Waste Mgmt. Exp. 1995, 6, 16–22.
- Zhao, P.; Li, T.; Yan, W.; Yuan, L. Dechlorination of PVC wastes by hydrothermal treatment using alkaline additives. Environ. Technol. 2018, 39, 977–985.
- Sogancioglu, M.; Yel, E.; Ahmetli, G. Pyrolysis of waste high density polyethylene (HDPE) and low density polyethylene (LDPE) plastics and production of epoxy composites with their pyrolysis chars. J. Clean. Prod. 2017, 165, 369–381.
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596.
- Marcilla, A.; Gomez, A.; Reyes-Labarta, J.; Giner, A.; Hernández, F. Kinetic study of polypropylene pyrolysis using ZSM-5 and an equilibrium fluid catalytic cracking catalyst. J. Anal. Appl. Pyrolysis 2003, 68, 467–480.
- Miranda, R.; Pakdel, H.; Roy, C.; Vasile, C. Vacuum pyrolysis of commingled plastics containing PVC II. Product analysis. Polym. Degrad. Stab. 2001, 73, 47–67.
- Singh, M.V. Pyrolysis of Waste Polyolefins into Liquid Petrochemicals Using Metal Carbonate Catalyst. Eng. Sci. 2022, 19, 285–291.
- Marcilly, C. Evolution of refining and petrochemicals. What is the place of zeolites. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2001; Volume 135, pp. 37–60.
- Degnan Jr, T.F. The implications of the fundamentals of shape selectivity for the development of catalysts for the petroleum and petrochemical industries. J. Catal. 2003, 216, 32–46.
- del Remedio Hernández, M.; Gómez, A.; García, Á.N.; Agulló, J.; Marcilla, A. Effect of the temperature in the nature and extension of the primary and secondary reactions in the thermal and HZSM-5 catalytic pyrolysis of HDPE. Appl. Catal. A Gen. 2007, 317, 183–194.
- del Remedio Hernández, M.; García, Á.N.; Marcilla, A. Catalytic flash pyrolysis of HDPE in a fluidized bed reactor for recovery of fuel-like hydrocarbons. J. Anal. Appl. Pyrolysis 2007, 78, 272–281.
- Yang, M.; Tian, X.; You, F. Manufacturing ethylene from wet shale gas and biomass: Comparative technoeconomic analysis and environmental life cycle assessment. Ind. Eng. Chem. Res. 2018, 57, 5980–5998.
- Murali, A.; Berrouk, A.S.; Dara, S.; AlWahedi, Y.F.; Adegunju, S.; Abdulla, H.S.; Das, A.K.; Yousif, N.; Hosani, M.A. Efficiency enhancement of a commercial natural gas liquid recovery plant: A MINLP optimization analysis. Sep. Sci. Technol. 2020, 55, 955–966.
- Buekens, A.G.; Froment, G.F. Thermal cracking of propane. Kinetics and product distributions. Ind. Eng. Chem. Process Des. Dev. 1968, 7, 435–447.
- Haribal, V.P.; Chen, Y.; Neal, L.; Li, F. Intensification of ethylene production from naphtha via a redox oxy-cracking scheme: Process simulations and analysis. Engineering 2018, 4, 714–721.
- Qyyum, M.A.; Naquash, A.; Haider, J.; Al-Sobhi, S.A.; Lee, M. State-of-the-art assessment of natural gas liquids recovery processes: Techno-economic evaluation, policy implications, open issues, and the way forward. Energy 2022, 238, 121684.
- Ahmad, S.; Tanwar, R.; Gupta, R.; Khanna, A. Interaction parameters for multi-component aromatic extraction with sulfolane. Fluid Phase Equilibria 2004, 220, 189–198.
- Choi, Y.J.; Cho, K.W.; Cho, B.W.; Yeo, Y.-K. Optimization of the sulfolane extraction plant based on modeling and simulation. Ind. Eng. Chem. Res. 2002, 41, 5504–5509.
- Gaile, A.; Erzhenkov, A.; Semenov, L.; Varshavskii, O.; Zalishchevskii, G.; Somov, V.; Marusina, N. Extraction of aromatic hydrocarbons with triethylene glycol-sulfolane mixed extractant. Russ. J. Appl. Chem. 2001, 74, 1668–1671.
- Gaile, A.; Zalishchevskii, G.; Erzhenkov, A.; Kayfadzhyan, E.; Koldobskaya, L. Extraction of aromatic hydrocarbons from reformates with mixtures of triethylene glycol and sulfolane. Russ. J. Appl. Chem. 2007, 80, 591–594.
- Gaile, A.; Zalishchevskii, G.; Erzhenkov, A.; Koldobskaya, L. Benzene separation from the benzene fraction of reformer naphtha by extractive rectification with N-methylpyrrolidone-sulfolane mixtures. Russ. J. Appl. Chem. 2008, 81, 1375–1381.
- Lei, Z.; Li, C.; Li, J.; Chen, B. Suspension catalytic distillation of simultaneous alkylation and transalkylation for producing cumene. Sep. Purif. Technol. 2004, 34, 265–271.
- Kelly, M.F.; Uitti, K.D. Extractive Distillation of Aromatics with a Sulfolane Solvent. US3551327A, 12 March 1969.
- Wang, Q.; Zhang, B.; He, C.; He, C.; Chen, Q. Optimal design of a new aromatic extractive distillation process aided by a co-solvent mixture. Energy Procedia 2017, 105, 4927–4934.
- Zhao, X.; You, F. Waste high-density polyethylene recycling process systems for mitigating plastic pollution through a sustainable design and synthesis paradigm. AIChE J. 2021, 67, e17127.
- Pan, Z.; Xue, X.; Zhang, C.; Wang, D.; Xie, Y.; Zhang, R. Production of aromatic hydrocarbons by hydro-liquefaction of high-density polyethylene (HDPE) over Ni/HZSM-5. J. Anal. Appl. Pyrolysis 2018, 136, 208–214.
- Orozco, S.; Artetxe, M.; Lopez, G.; Suarez, M.; Bilbao, J.; Olazar, M. Conversion of HDPE into Value Products by Fast Pyrolysis Using FCC Spent Catalysts in a Fountain Confined Conical Spouted Bed Reactor. ChemSusChem 2021, 14, 4291–4300.
- Farooqi, A.S.; Yusuf, M.; Zabidi, N.A.M.; Saidur, R.; Sanaullah, K.; Farooqi, A.S.; Khan, A.; Abdullah, B. A comprehensive review on improving the production of rich-hydrogen via combined steam and CO2 reforming of methane over Ni-based catalysts. Int. J. Hydrogen Energy 2021, 46, 31024–31040.
- Wang, B.; Gebreslassie, B.H.; You, F. Sustainable design and synthesis of hydrocarbon biorefinery via gasification pathway: Integrated life cycle assessment and technoeconomic analysis with multiobjective superstructure optimization. Comput. Chem. Eng. 2013, 52, 55–76.
- Yang, H.; Zhang, C.; Gao, P.; Wang, H.; Li, X.; Zhong, L.; Wei, W.; Sun, Y. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598.
- Zia, K.M.; Bhatti, H.N.; Bhatti, I.A. Methods for polyurethane and polyurethane composites, recycling and recovery: A review. React. Funct. Polym. 2007, 67, 675–692.
- Zahedifar, P.; Pazdur, L.; Vande Velde, C.M.; Billen, P. Multistage chemical recycling of polyurethanes and dicarbamates: A glycolysis–hydrolysis demonstration. Sustainability 2021, 13, 3583.
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane foams: Past, present, and future. Materials 2018, 11, 1841.
- Sheel, A.; Pant, D. Chemical depolymerization of polyurethane foams via glycolysis and hydrolysis. In Recycling of Polyurethane Foams; Elsevier: Amsterdam, The Netherlands, 2018; pp. 67–75.
- Greene, T.W.; Wuts, P.G. Protective Groups in Organic Synthesis; Wiley: Hoboken, NJ, USA, 1991.
- Nikje, M.M.A.; Garmarudi, A.B.; Idris, A.B. Polyurethane waste reduction and recycling: From bench to pilot scales. Des. Monomers Polym. 2011, 14, 395–421.
- Motokucho, S.; Nakayama, Y.; Morikawa, H.; Nakatani, H. Environment-friendly chemical recycling of aliphatic polyurethanes by hydrolysis in a CO2-water system. J. Appl. Polym. Sci. 2018, 135, 45897.
- Kemona, A.; Piotrowska, M. Polyurethane recycling and disposal: Methods and prospects. Polymers 2020, 12, 1752.
- Simón, D.; Borreguero, A.; De Lucas, A.; Rodríguez, J. Recycling of polyurethanes from laboratory to industry, a journey towards the sustainability. Waste Manag. 2018, 76, 147–171.
- Akhmetova, F.; Aubakirov, Y.A.; Tashmukhambetova, Z.H.; Sassykova, L.R.; Arbag, H.; Kurmangaliyeva, A. Recycling of waste plastics to liquid fuel mixture over composite zeolites catalysts. Chem. Bull. Kazakh Natl. Univ. 2021, 101, 12–18.
- Maddodi, B.S.; Lathashri, U.A.; Devesh, S.; Rao, A.U.; Shenoy, G.B.; Wijerathne, H.T.; Sooriyaperkasam, N.; Kumar M, P. Repurposing Plastic Wastes in Non-conventional Engineered Wood Building Bricks for Constructional Application—A Mechanical Characterization using Experimental and Statistical Analysis. Eng. Sci. 2022, 18, 329–336.
- Martín-Lara, M.; Moreno, J.; Garcia-Garcia, G.; Arjandas, S.; Calero, M. Life cycle assessment of mechanical recycling of post-consumer polyethylene flexible films based on a real case in Spain. J. Clean. Prod. 2022, 365, 132625.
