Biosensors are ubiquitous in a variety of disciplines, such as biochemical, electrochemical, agricultural, and biomedical areas. They can integrate various point-of-care applications, such as in the food, healthcare, environmental monitoring, water quality, forensics, drug development, and biological domains. Multiple strategies have been employed to develop and fabricate miniaturized biosensors, including design, optimization, characterization, and testing. In view of their interactions with high-affinity biomolecules, they find application in the sensitive detection of analytes, even in small sample volumes. Among the many developed techniques, microfluidics have been widely explored; these use fluid mechanics to operate miniaturized biosensors. The currently used commercial devices are bulky, slow in operation, expensive, and require human intervention; thus, it is difficult to automate, integrate, and miniaturize the existing conventional devices for multi-faceted applications. Microfluidic biosensors have the advantages of mobility, operational transparency, controllability, and stability with a small reaction volume for sensing.
1. Evolution of Biosensors
Biosensors were first reported by Leland Charles Clark Jr. in 1962, who conceived the idea of demonstrating the components of a biosensor along with a strategy to integrate a bioreceptor with a transducer device
[1][6].
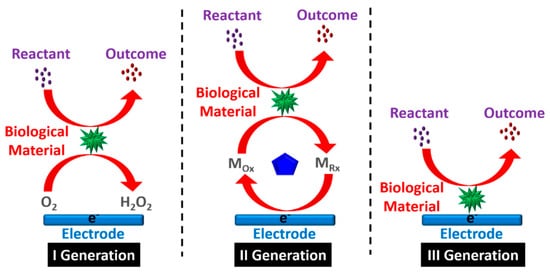
Figure 1 shows three generations of the development of biosensors, while
Table 1 summarizes the evolution pathway.
Figure 1.
Three generations of the biosensor process.
Table 1.
Evolution of the development of biosensors.
| Year |
Generation |
Development Phases of Biosensor |
| 1906 |
First |
M. Cramer noticed voltage difference generating between parts of the electrolyte. |
| 1909 |
Sorensen described the idea of pH and pH sensors. |
| 1909–1922 |
Nelson and Griffin were the first to discover that enzyme invertase could be immobilized on charcoal aluminium hydroxide [2][3]. | Nelson and Griffin were the first to discover that enzyme invertase could be immobilized on charcoal aluminium hydroxide [15,16]. |
| 1922 |
Hughes observed a pH determination electrode [4]. | Hughes observed a pH determination electrode [17]. |
| 1956 |
Clark first discovered the biosensor electrode that is capable of determining blood oxygen levels [5]. | Clark first discovered the biosensor electrode that is capable of determining blood oxygen levels [18]. |
| 1962 |
Clark also demonstrated the use of an amperometric enzyme electrode for glucose sensing [6]. | Clark also demonstrated the use of an amperometric enzyme electrode for glucose sensing [19]. |
| 1967 |
Hicks et al. [7] enhanced Clark’s work; glucose oxidase was immobilized using an enzyme-based working electrode with an oxygen sensor. | Hicks et al. [20] enhanced Clark’s work; glucose oxidase was immobilized using an enzyme-based working electrode with an oxygen sensor. |
| 1969 |
The first potentiometric enzyme electrode-based urea detection sensor was reported by Montalvo and Guilbault. |
| 1970 |
Bergveld discovered ion-sensitive field-effect transistors (ISFET) [8]. | Bergveld discovered ion-sensitive field-effect transistors (ISFET) [21]. |
| 1973 |
Lubrano and Guilbault demonstrated glucose and lactate enzyme platinum electrode to detect hydrogen peroxide (H | 2 | O | 2) [9]. | ) [22]. |
| 1974 |
Klaus Mosbach group developed a thermistor sensor based on a heat-sensitive enzyme [10]. | Klaus Mosbach group developed a thermistor sensor based on a heat-sensitive enzyme [23]. |
| 1975 |
Opitz and Lubbers developed an optical biosensor for alcohol detection [11]. | Opitz and Lubbers developed an optical biosensor for alcohol detection [24]. |
| 1976 |
Second |
Clemens et al. [12] integrated an electrochemical biosensor for glucose detection into an artificial bedside pancreas. A unique semi-continuous catheter-based blood glucose analyzer was also demonstrated using VIA-based technology. | Clemens et al. [25] integrated an electrochemical biosensor for glucose detection into an artificial bedside pancreas. A unique semi-continuous catheter-based blood glucose analyzer was also demonstrated using VIA-based technology. |
| 1977 |
La Roche introduced the lactate analyzer LA 640, which was utilized to transmit an electron from dehydrogenase to an electrode [13]. | La Roche introduced the lactate analyzer LA 640, which was utilized to transmit an electron from dehydrogenase to an electrode [26]. |
| 1980 |
Peterson was the first to perform in vivo blood gas analysis to create a fiber-optic pH sensor [14]. | Peterson was the first to perform in vivo blood gas analysis to create a fiber-optic pH sensor [27]. |
| 1982 |
Schultz detected glucose by using the fiber-optic biosensor [15]. | Schultz detected glucose by using the fiber-optic biosensor [28]. |
| 1983 |
Third |
Liedberg discovered the reliance-based reactions in real time using the surface plasmon resonance (SPR) method in real time [16]. | Liedberg discovered the reliance-based reactions in real time using the surface plasmon resonance (SPR) method in real time [29]. |
| 1984 |
For glucose detection, the first mediated amperometric biosensor was constructed using ferrocene and glucose oxidase [17]. | For glucose detection, the first mediated amperometric biosensor was constructed using ferrocene and glucose oxidase [30]. |
| 1987 |
University of Cambridge created a pen-sized detector for assessing blood glucose levels. |
| 1990 |
Pharmacia Biacore proposed an SPR-based biosensor [18]. | Pharmacia Biacore proposed an SPR-based biosensor [31]. |
| 1992 |
i-STAT developed a handheld blood biosensor [19]. | i-STAT developed a handheld blood biosensor [32]. |
| 2018 |
Girbi designed a neuron-on-chip biosensor to measure the nerve impulse conduction [20]. | Girbi designed a neuron-on-chip biosensor to measure the nerve impulse conduction [33]. |
| 2021 |
Kulkarni et al. [21] described an Al-foil-based electrode for sensing cysteine. | Kulkarni et al. [34] described an Al-foil-based electrode for sensing cysteine. |
Point-of-care testing provides susceptible and non-invasive devices for detecting various biomarkers such as glucose in biological samples in a continuous, fast, low-cost, and reliable manner. Microfluidics is a form of technology that develops miniaturized biosensors using fluid mechanics. On the other hand, existing commercial devices are large, slow, expensive, and require human intervention. Despite substantial progress in developing microfluidic-based biosensors, which will continue to evolve in tandem with existing or well-established approaches, automating, integrating, and miniaturizing current conventional devices on a single platform is a challenging task. This issue has gained significant attention concerning the production of microfluidic-based small biosensors. Microfluidic devices have the advantages of mobility, operational transparency, controllability, reliability, accuracy, and stability, with a small response volume. Because of the few peripherals required to execute numerous applications, microfluidic-based biosensors fabricated using different techniques enable faster processing and greater efficiency.
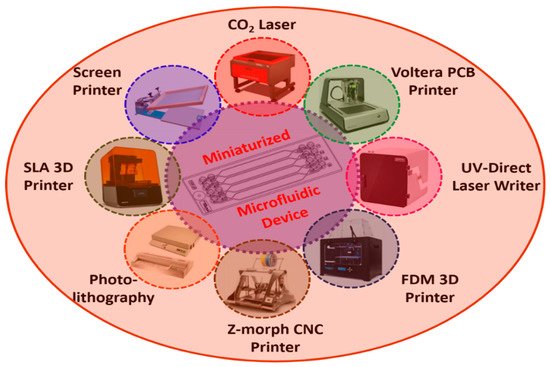
Figure 27 shows the schematic representation of various microfabrication techniques which are widely used in microfluidic-based biosensor development
[22][100].
Figure 27.
Schematic representation of various fabrication techniques used in the development of miniaturized microfluidic biosensors.
Table 25 summarizes the various microfabrication techniques used in the development of miniaturized microfluidic biosensors with different materials/substrates employed in building microfluidic devices, and their advantages and disadvantages.
3. Applications
3.1. Food Processing and Environmental Monitoring
Biosensors are utilized to increase quality of life in a variety of applications. The quality, safety, and upkeep of food items as well as their processing are difficult in food processing industries, and hence the food sector would benefit from cost-effective technologies for food authentication and monitoring
[31][32][33][121,122,123]. Potentiometric alternating biosensing devices are also used to detect the changes in pH caused by ammonia, and have been used to identify
E. coli [34][124]; ingredients were mixed in a sonicator to separate the bacteria from the food
[35][125].
Enzyme-based biosensors are also used in the dairy industry
[36][126]; flow cells have been linked to biosensors based on screen-printed carbon electrodes, and enzymes are encapsulated by the polymer mounted onto the electrodes. Three organophosphate insecticides in milk have thus been quantified using automated flow-based biosensors
[37][127]. Sugar substitutes are one of the most widely used food additives that are related to a number of conditions, including dental cavities, cardiovascular disease, obesity, and type II diabetes. In this case, multichannel biosensors are considered to be effective for merging lipid films via the electrochemical approaches for rapid and sensitive sweetener screening. Here, MATLAB software is used with a spatiotemporal method to evaluate the signals of glucose and sucrose in natural sugars, saccharin, and cyclamate, which represent artificial sweeteners
[38][128].
3.2. Biomedical Domain
In the field of medicine, biosensor applications are fast-growing. Glucose biosensors are frequently used in clinical settings to diagnose diabetes mellitus, requiring precise blood glucose control
[39][129]. A lateral flow assay, also known as a rapid test, is a simple device intended to detect the presence of a target substance in a liquid sample without the need for specialized and costly equipment. Around 85% of the large global market for blood glucose biosensors consists of items used at home. In the medical area, biosensors are frequently employed to diagnose infectious diseases such as urinary tract infections (UTIs), pathogen identification, and antibiotic susceptibility
[40][130]. Human interleukin-II has been detected early by using a novel biosensor based on hafnium (IV) oxide (HfO2). For early cytokine detection, recombinant human IL-10 with a monoclonal antibody is used. The interaction between antibodies and antigens has also been studied with fluorescence patterns and electromechanical impedance spectroscopy, while fluorescence patterns have been employed to produce the bio-recognition of protein. Chen et al.
[41][131] used HfO2 as a bio-field-effect transistor with high sensitivity
[42][132].
3.3. Plant Biology
New revolutionary technologies in nucleic acid molecular imaging and sequencing have enabled advances in plant research
[43][133]. Conventional mass spectroscopy can accurately identify cellular and subcellular localization and metabolite levels using critical data on the dynamics and location of enzymes, although transporters, substrates, and receptors still need advancement. Biosensors can access these data to quantify an active process under physiological circumstances. Strategies to envision actual processes, such as changing one metabolite into another or generating signal events, have also been attempted
[43][133]. Tsien
[44][134] was the first to produce protein prototype sensors for detecting caspase activity and calcium levels in living cells. These sensors were made using fluorescence resonance energy transfer (FRET) from green fluorescent protein (GFP) spectrum variants. The in vivo detection of calcium oscillations with a high temporal resolution was achieved by the use of a chameleon sensor
[45][135].
3.4. Biodefense Sensing
In the case of a biological agent, biosensors can be employed for military purposes. Here, the primary goal is to promptly and correctly identify biowarfare agents (BWAs), including bacteria (vegetative and spores), poisons, and viruses. Several attempts have been made to use molecular techniques to build biosensors that can recognize the chemical markers of BWAs. Furthermore, the human papillomavirus (HPV) is a DNA virus with two strands that have been related to aggressive cervical cancer. HPV is divided into two types: HPV 16 and HPV 18
[46][47][136,137].