With the deterioration of the ecological environment and the depletion of fossil energy, fuel cells, representing a new generation of clean energy, have received widespread attention. This review summarized recent progress in noble metal-based core–shell catalysts for oxygen reduction reactions (ORRs) in proton exchange membrane fuel cells (PEMFCs). The novel testing methods, performance evaluation parameters and research methods of ORR were briefly introduced. The effects of the preparation method, temperature, kinds of doping elements and the number of shell layers on the ORR performances of noble metal-based core–shell catalysts were highlighted. The difficulties of mass production and the high cost of noble metal-based core–shell nanostructured ORR catalysts were also summarized. Thus, in order to promote the commercialization of noble metal-based core–shell catalysts, research directions and prospects on the further development of high performance ORR catalysts with simple synthesis and low cost are presented.
With the deterioration of the ecological environment and the depletion of fossil energy, fuel cells, representing a new generation of clean energy, have received widespread attention. Noble metal-based core–shell catalysts for oxygen reduction reactions (ORRs) in proton exchange membrane fuel cells (PEMFCs). The novel testing methods, performance evaluation parameters and research methods of ORR were briefly introduced. The effects of the preparation method, temperature, kinds of doping elements and the number of shell layers on the ORR performances of noble metal-based core–shell catalysts were highlighted. The difficulties of mass production and the high cost of noble metal-based core–shell nanostructured ORR catalysts were also summarized. Thus, in order to promote the commercialization of noble metal-based core–shell catalysts, research directions and prospects on the further development of high performance ORR catalysts with simple synthesis and low cost are presented.
- core–shell nanostructure
- oxygen reduction reaction
- electrocatalyst
- noble metal-based
1. Introduction
2. ORR Testing Technology
The ORR process involves multiple intermediates and multi-step reactions. Many factors, such as electrode potential, type of catalyst, crystal plane structure, reaction temperature and so on, could affect the corresponding electrochemical performances [34,35,36][34][35][36]. The lack of experimental verification methods for the reaction process results in accurate ORR mechanisms not being obtained [37]. However, in recent years, researchers have begun to describe the ORR mechanism by means of characterization [38,39,40][38][39][40] and theoretical calculation [41], which is a benefit in the design of catalysts that meet the requirements. After the desired structural catalysts are obtained, the ORR activity and durability of the catalysts can be analyzed by measuring the electrochemically active surface area (ECSA), Tafel slope, the onset potential (Eonset) and the half-wave potential (E1/2) [42,43,44,45,46][42][43][44][45][46]. Two testing techniques are described in detail below.2.1. Testing Technology of Thin-Film Rotating Disk Electrode (TF-RDE)
In principle, the newly synthesized ORR catalyst should be tested and evaluated in the real working environment of the fuel cell, but this testing method is often not feasible in practice. On the one hand, the preparation of membrane electrode assembly (MEA) requires professional skills, complex expansion equipment and a large number of catalysts, so it is difficult to prepare a good MEA in the general laboratory. On the other hand, a rapid screening technique for performance is needed in the early stage of catalyst preparation. However, the complexity of MEA preparation cannot achieve the purpose of rapid screening. TF-RDE is a common laboratory technology based on commercial RDE technology (Figure 1a), which can test the ORR performance of milligram catalysts [47]. TF-RDE is supported by complete hydrodynamic equations and convection–diffusion equations. In the process of continuous improvement, it provides a fine method for membrane preparation technology and electrochemical parameter control [48,49][48][49]. Because of its simple and fast operation, TF-RDE is widely used in the laboratory. Additionally, there is a standard operation flow that can compare the performances of different catalysts to a certain extent [50,51][50][51]. However, it should be noted that there are still differences in the details of the experimental schemes and methods of TF-RDE, which may lead to different measured catalyst activities or different results.2.2. Testing Technology of Gas Diffusion Electrode (GDE)
Although TF-RDE is practical for the initial screening of catalysts in the laboratory, some shortcomings of TF-RDE limit its ability to predict catalysts in complex battery environments. On the one hand, in the procedure of TF-RDE, the reaction gas (H2, O2 or air) is charged into the electrolyte. However, the low solubility of the gas in the electrolyte is very different from the gas concentration in practical application [52]. On the other hand, the catalyst layer used in TF-RDE is usually less than 1 μm, while the thickness is greater than 5 μm in actual fuel cells. In addition, the catalyst coverage area is also very different. So, there is a great difference in the catalyst activity measured by TF-RDE and MEA [53]. Therefore, GDE testing technology has been proposed, which is an intermediate technology between TF-RDE and MEA (Figure 1b–d) [54]. Through the improvement and supplement of GDE testing technology, the gap between laboratory catalyst testing and actual fuel cell catalyst testing can be narrowed under simple and fast conditions [55,56,57][55][56][57].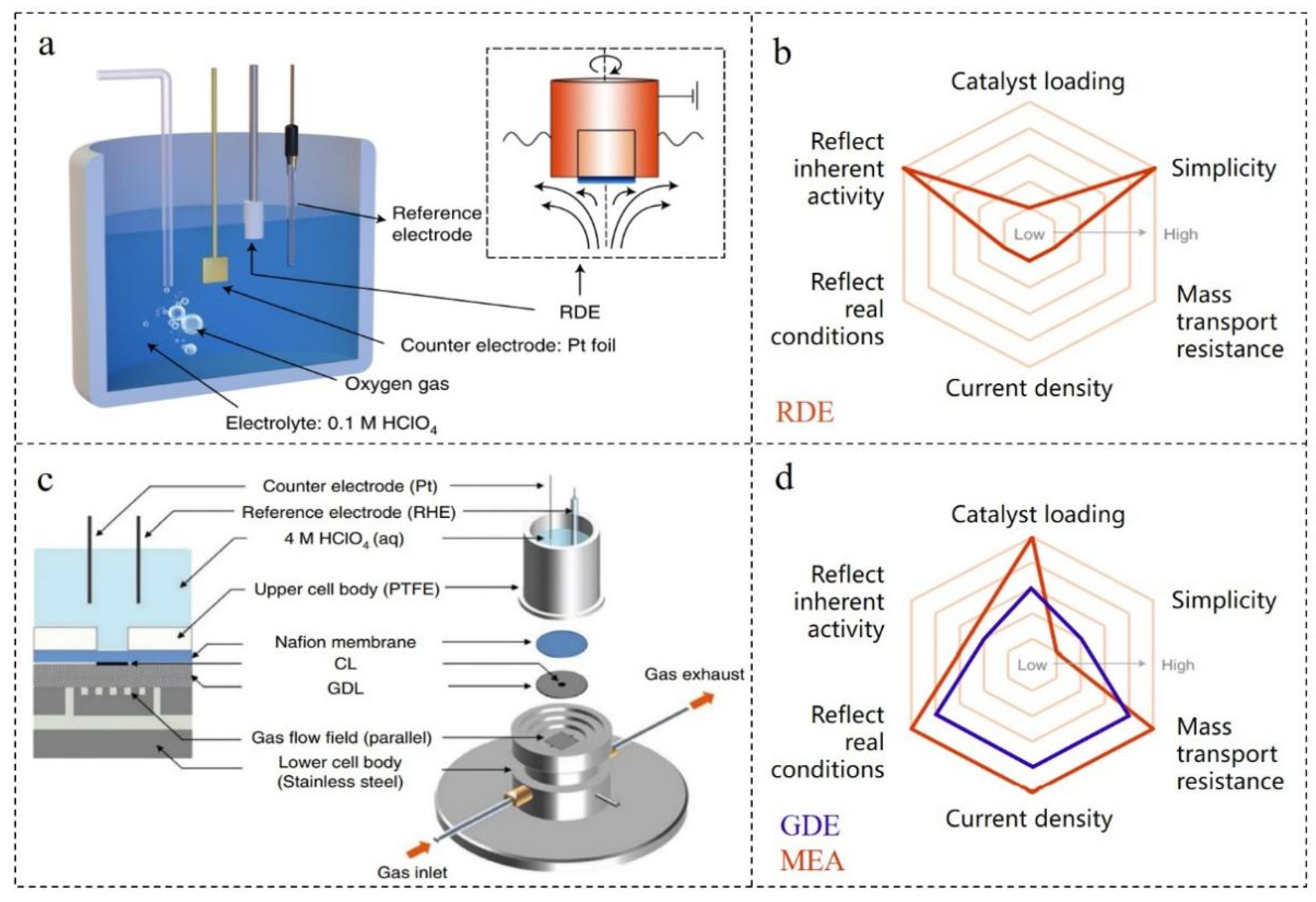
References
- Deng, Q.B.; Jia, H.X.; Yang, B.; Qi, Z.P.; Zhang, Z.Y.; Lee, A.; Hu, N. The electro-chemo-mechanical coupling at the solid-liquid interface and its application into electrocatalysis. Adv. Mech. 2022, 52, 221–252.
- Chhetri, K.; Muthurasu, A.; Dahal, B.; Kim, T.; Mukhiya, T.; Chae, S.H.; Ko, T.H.; Choi, Y.C.; Kim, H.Y. Engineering the abundant heterointerfaces of integrated bimetallic sulfide-coupled 2D MOF-derived mesoporous CoS2 nanoarray hybrids for electrocatalytic water splitting. Mater. Today Nano 2022, 17, 100146.
- Chhetri, K.; Dahal, B.; Mukhiya, T.; Tiwari, A.P.; Muthurasu, A.; Kim, T.; Kim, H.; Kim, H.Y. Integrated hybrid of graphitic carbon-encapsulated CuxO on multilayered mesoporous carbon from copper MOFs and polyaniline for asymmetric supercapacitor and oxygen reduction reactions. Carbon 2021, 179, 89–99.
- Puangsombut, P.; Tantavichet, N. Effect of plating bath composition on chemical composition and oxygen reduction reaction activity of electrodeposited Pt-Co catalysts. Rare Met. 2019, 38, 95–106.
- Zhao, X.; Li, Y.G. Two-electron oxygen reduction reaction by high-loading molybdenum single-atom catalysts. Rare Met. 2020, 39, 455–457.
- Han, Z.F.; Qi, Z.P.; Wei, Q.; Deng, Q.B.; Wang, K. The Mechanical Effect of MnO2 Layers on Electrochemical Actuation Performance of Nanoporous Gold. Nanomaterials 2020, 10, 2056.
- Qiu, W.J.; An, C.H.; Yan, Y.W.; Xu, J.; Zhang, Z.J.; Guo, W.; Wang, Z.; Zheng, Z.J.; Wang, Z.B.; Deng, Q.B.; et al. Suppressed polysulfide shuttling and improved Li+ transport in Li-S batteries enabled by NbN modified PP separator. J. Power Sources 2019, 423, 98–105.
- Wang, A.X.; Deng, Q.B.; Deng, L.J.; Guan, X.Z.; Luo, J.Y. Eliminating Tip Dendrite Growth by Lorentz Force for Stable Lithium Metal Anodes. Adv. Funct. Mater. 2019, 29, 1902630.
- Wang, K.; Deng, Q.B. Constructing Core-Shell Carbon Additives toward Enhanced Hydrogen Storage Performance of Magnesium Hydride. Front. Chem. 2020, 8, 223.
- An, C.H.; Kang, W.; Deng, Q.B.; Hu, N. Pt and Te codoped ultrathin MoS2 nanosheets for enhanced hydrogen evolution reaction with wide pH range. Rare Met. 2022, 41, 378–384.
- Yang, H.; Liu, Y.; Liu, X.; Wang, X.; Tian, H.; Waterhouse, G.I.N.; Kruger, P.E.; Telfer, S.G.; Ma, S. Large-scale synthesis of N-doped carbon capsules supporting atomically dispersed iron for efficient oxygen reduction reaction electrocatalysis. eScience 2022, 2, 227–234.
- Deng, Q.B.; Gopal, V.; Weissmuller, J. Less Noble or More Noble: How Strain Affects the Binding of Oxygen on Gold. Angew. Chem. Int. Ed. 2015, 54, 12981–12985.
- He, J.; Shen, Y.; Yang, M.; Zhang, H.; Deng, Q.; Ding, Y. The effect of surface strain on the CO-poisoned surface of Pt electrode for hydrogen adsorption. J. Catal. 2017, 350, 212–217.
- Xu, X.; Liang, T.; Kong, D.; Wang, B.; Zhi, L. Strain engineering of two-dimensional materials for advanced electrocatalysts. Mater. Today Nano 2021, 14, 100111.
- Yang, M.; Zhang, H.; Deng, Q. Understanding the copper underpotential deposition process at strained gold surface. Electrochem. Commun. 2017, 82, 125–128.
- Deng, Q.B.; Weissmuller, J. Electrocapillary Coupling during Electrosorption. Langmuir 2014, 30, 10522–10530.
- Deng, Q.B.; Smetanin, M.; Weissmuller, J. Mechanical modulation of reaction rates in electrocatalysis. J. Catal. 2014, 309, 351–361.
- Xie, Z.J.; Zhang, L.M.; Li, L.; Deng, Q.B.; Jiang, G.X.; Wang, J.Q.; Cao, B.Q.; Wang, Y.J. A ternary FeS2/Fe7S8@nitrogen-sulfur co-doping reduced graphene oxide hybrid towards superior-performance lithium storage. Prog. Nat. Sci. 2021, 31, 207–214.
- Zhang, H.X.; Han, Z.F.; Deng, Q.B. The Effect of an External Magnetic Field on the Electrochemical Capacitance of Nanoporous Nickel for Energy Storage. Nanomaterials 2019, 9, 694.
- Zeng, L.L.; Zhang, Z.J.; Qiu, W.J.; Wei, J.K.; Fang, Z.H.; Deng, Q.B.; Guo, W.; Liu, D.; Xie, Z.Z.; Qu, D.Y.; et al. Multifunctional Polypropylene Separator via Cooperative Modification and Its Application in the Lithium-Sulfur Battery. Langmuir 2020, 36, 11147–11153.
- Li, K.; Xu, J.; Chen, C.; Xie, Z.Z.; Liu, D.; Qu, D.Y.; Tang, H.L.; Wei, Q.; Deng, Q.B.; Li, J.S.; et al. Activating the hydrogen evolution activity of Pt electrode via synergistic interaction with NiS2. J. Colloid Interface Sci. 2021, 582, 591–597.
- Ren, X.F.; Lv, Q.Y.; Liu, L.F.; Liu, B.H.; Wang, Y.R.; Liu, A.M.; Wu, G. Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustain. Energy Fuels 2020, 4, 15–30.
- Song, G.T.; Wang, Y.; Qi, Y.; Li, W.M.; Zhang, L.X. Fabrication of titanium nitride nanoparticles onto carbon nanotubes by atomic layer deposition for utilization as Pt electrocatalyst supports. Rare Met. 2020, 39, 784–791.
- Li, Y.; Wang, H.H.; Priest, C.; Li, S.W.; Xu, P.; Wu, G. Advanced Electrocatalysis for Energy and Environmental Sustainability via Water and Nitrogen Reactions. Adv. Mater. 2021, 33, 2000381.
- He, Y.; Liu, S.; Priest, C.; Shi, Q.; Wu, G. Atomically dispersed metal-nitrogen-carbon catalysts for fuel cells: Advances in catalyst design, electrode performance, and durability improvement. Chem. Soc. Rev. 2020, 49, 3484–3524.
- Nair, A.S.; Pathak, B. Computational Screening for ORR Activity of 3d Transition Metal Based Core-Shell Clusters. J. Phys. Chem. C 2019, 123, 3634–3644.
- Lu, Y.L.; Zhang, H.P.; Wang, Y.F.; Chen, Z. First principles study on the oxygen reduction reaction of core-shell structure. Chem. Phys. 2022, 552, 111356.
- Bharadwaj, N.; Nair, A.S.; Pathak, B. Dimensional-Dependent Effects in Platinum Core-Shell-Based Catalysts for Fuel Cell Applications. ACS Appl. Nano Mater. 2021, 4, 9697–9708.
- Zhou, W.P.; Sasaki, K.; Su, D.; Zhu, Y.; Wang, J.X.; Adzic, R.R. Gram-Scale-Synthesized Pd2Co-Supported Pt Monolayer Electrocatalysts for Oxygen Reduction Reaction. J. Phys. Chem. C 2010, 114, 8950–8957.
- Price, S.W.T.; Speed, J.D.; Kannan, P.; Russell, A.E. Exploring the First Steps in Core-Shell Electrocatalyst Preparation: In Situ Characterization of the Underpotential Deposition of Cu on Supported Au Nanoparticles. J. Am. Chem. Soc. 2011, 133, 19448–19458.
- Wang, X.; Orikasa, Y.; Inaba, M.; Uchimototo, Y. Reviving Galvanic Cells to Synthesize Core-Shell Nanoparticles with a Quasi-Monolayer Pt Shell for Electrocatalytic Oxygen Reduction. ACS Catal. 2020, 10, 430–434.
- Gamler, J.T.L.; Leonardi, A.; Sang, X.H.; Koczkur, K.M.; Unocic, R.R.; Engel, M.; Skrabalak, S.E. Effect of lattice mismatch and shell thickness on strain in nanocrystals. Nanoscale Adv. 2020, 2, 1105–1114.
- Sasaki, K.; Kuttiyiel, K.A.; Adzic, R.R. Designing high performance Pt monolayer core-shell electrocatalysts for fuel cells. Curr. Opin. Electrochem. 2020, 21, 368–375.
- Keith, J.A.; Jerkiewicz, G.; Jacob, T. Theoretical Investigations of the Oxygen Reduction Reaction on Pt(111). ChemPhysChem 2010, 11, 2779–2794.
- Raj, C.R.; Samanta, A.; Noh, S.H.; Mondal, S.; Okajima, T.; Ohsaka, T. Emerging new generation electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2016, 4, 11156–11178.
- Hansen, H.A.; Viswanathan, V.; Norskov, J.K. Unifying Kinetic and Thermodynamic Analysis of 2 e− and 4 e− Reduction of Oxygen on Metal Surfaces. J. Phys. Chem. C 2014, 118, 6706–6718.
- Jiao, Y.; Zheng, Y.; Jaroniec, M.T.; Qiao, S.Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086.
- Raciti, D.; Kubal, J.; Ma, C.; Barclay, M.; Gonzalez, M.; Chi, M.F.; Greeley, J.; More, K.L.; Wang, C. Pt3Re alloy nanoparticles as electrocatalysts for the oxygen reduction reaction. Nano Energy 2016, 20, 202–211.
- Malacrida, P.; Casalongue, H.G.S.; Masini, F.; Kaya, S.; Hernandez-Fernandez, P.; Deiana, D.; Ogasawara, H.; Stephens, I.E.L.; Nilsson, A.; Chorkendorff, I. Direct observation of the dealloying process of a platinum-yttrium nanoparticle fuel cell cathode and its oxygenated species during the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2015, 17, 28121–28128.
- Kim, J.; Renault, C.; Nioradze, N.; Arroyo-Curras, N.; Leonard, K.C.; Bard, A.J. Electrocatalytic Activity of Individual Pt Nanoparticles Studied by Nanoscale Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2016, 138, 8560–8568.
- Yue, J.; Du, Z.; Shao, M.H. Mechanisms of Enhanced Electrocatalytic Activity for Oxygen Reduction Reaction on High-Index Platinum n(111)-(111) Surfaces. J. Phys. Chem. Lett. 2015, 6, 3346–3351.
- Bhalothia, D.; Krishnia, L.; Yang, S.S.; Yan, C.; Hsiung, W.H.; Wang, K.W.; Chen, T.Y. Recent Advancements and Future Prospects of Noble Metal-Based Heterogeneous Nanocatalysts for Oxygen Reduction and Hydrogen Evolution Reactions. Appl. Sci. 2020, 10, 7708.
- Voiry, D.; Chhowalla, M.; Gogotsi, Y.; Kotov, N.A.; Li, Y.; Penner, R.M.; Schaak, R.E.; Weiss, P.S. Best Practices for Reporting Electrocatalytic Performance of Nanomaterials. ACS Nano 2018, 12, 9635–9638.
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801.
- Lin, R.; Cai, X.; Zeng, H.; Yu, Z.P. Stability of High-Performance Pt-Based Catalysts for Oxygen Reduction Reactions. Adv. Mater. 2018, 30, 1705332.
- Zhang, J.W.; Yuan, Y.L.; Gao, L.; Zeng, G.M.; Li, M.F.; Huang, H.W. Stabilizing Pt-Based Electrocatalysts for Oxygen Reduction Reaction: Fundamental Understanding and Design Strategies. Adv. Mater. 2021, 33, 2006494.
- Shinozaki, K.; Zack, J.W.; Richards, R.M.; Pivovar, B.S.; Kocha, S.S. Oxygen Reduction Reaction Measurements on Platinum Electrocatalysts Utilizing Rotating Disk Electrode Technique I. Impact of Impurities, Measurement Protocols and Applied Corrections. J. Electrochem. Soc. 2015, 162, F1144–F1158.
- Kocha, S.S.; Shinozaki, K.; Zack, J.W.; Myers, D.J.; Kariuki, N.N.; Nowicki, T.; Stamenkovic, V.; Kang, Y.J.; Li, D.G.; Papageorgopoulos, D. Best Practices and Testing Protocols for Benchmarking ORR Activities of Fuel Cell Electrocatalysts Using Rotating Disk Electrode. Electrocatalysis 2017, 8, 366–374.
- Shinozaki, K.; Zack, J.W.; Pylypenko, S.; Pivovar, B.S.; Kocha, S.S. Oxygen Reduction Reaction Measurements on Platinum Electrocatalysts Utilizing Rotating Disk Electrode Technique II. Influence of Ink Formulation, Catalyst Layer Uniformity and Thickness. J. Electrochem. Soc. 2015, 162, F1384–F1396.
- Wei, C.; Rao, R.R.; Peng, J.Y.; Huang, B.T.; Stephens, I.E.L.; Risch, M.; Xu, Z.C.J.; Shao-Horn, Y. Recommended Practices and Benchmark Activity for Hydrogen and Oxygen Electrocatalysis in Water Splitting and Fuel Cells. Adv. Mater. 2019, 31, 1806296.
- Ehelebe, K.; Seeberger, D.; Paul, M.T.Y.; Thiele, S.; Mayrhofer, K.J.J.; Cherevko, S. Evaluating Electrocatalysts at Relevant Currents in a Half-Cell: The Impact of Pt Loading on Oxygen Reduction Reaction. J. Electrochem. Soc. 2019, 166, F1259–F1268.
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51.
- Pinaud, B.A.; Bonakdarpour, A.; Daniel, L.; Sharman, J.; Wilkinson, D.P. Key Considerations for High Current Fuel Cell Catalyst Testing in an Electrochemical Half-Cell. J. Electrochem. Soc. 2017, 164, F321–F327.
- Fan, J.T.; Chen, M.; Zhao, Z.L.; Zhang, Z.; Ye, S.Y.; Xu, S.Y.; Wang, H.J.; Li, H. Bridging the gap between highly active oxygen reduction reaction catalysts and effective catalyst layers for proton exchange membrane fuel cells. Nat. Energy 2021, 6, 475–486.
- Sievers, G.W.; Jensen, A.W.; Brüser, V.; Arenz, M.; Escudero-Escribano, M. Sputtered Platinum Thin-films for Oxygen Reduction in Gas Diffusion Electrodes: A Model System for Studies under Realistic Reaction Conditions. Surfaces 2019, 2, 336–348.
- Hu, Y.; Jiang, Y.L.; Jensen, J.O.; Cleemann, L.N.; Li, Q.F. Catalyst evaluation for oxygen reduction reaction in concentrated phosphoric acid at elevated temperatures. J. Power Sources 2018, 375, 77–81.
- Wiberg, G.K.H.; Fleige, M.; Arenz, M. Gas diffusion electrode setup for catalyst testing in concentrated phosphoric acid at elevated temperatures. Rev. Sci. Instrum. 2015, 86, 024102.
- Du, J.; Quinson, J.; Zana, A.; Arenz, M. Elucidating Pt-Based Nanocomposite Catalysts for the Oxygen Reduction Reaction in Rotating Disk Electrode and Gas Diffusion Electrode Measurements. ACS Catal. 2021, 11, 7584–7594.
