Urea has been widely used in the agricultural industry as a fertilizer. It represents more than 50% of the nitrogen fertilizer market, and its global demand has increased more than 100 times in the last decades. In energy terms, urea has been considered a hydrogen–storage (6.71 wt.%) and ammonia–storage (56.7 wt.%) compound, giving it fuel potential. Urea properties meet the requirements of the US Department of Energy for hydrogen–storage substances, meanly because urea crystalizes, allowing storage and safe transportation. Conventional industrial urea synthesis is energy–intensive (3.2–5.5 GJ ton-1) since it requires high pressures and temperatures, so developing a photocatalyzed synthesis using TiO2 at ambient temperature and pressure is an attractive alternative to conventional synthesis.
- TiO2–based materials
- photocatalysis
- urea
- hydrogen storage
1. Introduction
2. Developments in the Photocatalyzed Production of Urea Using TiO2–Based Materials
Urea production by photocatalysis was first reported in 1998 by S. Kuwabata, H. Yamauchi, and H. Yoneyama [41][9]. This research group presented the simultaneous reduction of CO2 and nitrate ion (NO3−) using titanium dioxide nanocrystals (Q–TiO2) with sizes ranging from 1.0 to 7.5 nm, which were immobilized in a film of polyvinylpyrrolidone gel (Q–TiO2/PVPD). A 500 W high–pressure mercury arc lamp was used as a light source for the experiments, in which wavelengths below 300 nm were removed with a glass filter. S. Kuwabata et al. [41][9] reported a concentration of about 1.9 mM urea in a 5 h photocatalysis using a Q–TiO2/PVPD film in polypropylene carbonate (PC) solutions saturated with CO2 in the presence of LiNO3 and using 2–Propanol as an electron donor species. However, when using Q–TiO2 colloidal and TiO2 P–25 colloidal, under the same conditions, they obtained an approximate concentration of 7.3 × 10−2 mM and 3.3 × 10−2 mM, respectively. The results obtained by the Q–TiO2/PVPD film were promising since a lower concentration of urea was expected as a product because of the belief that the use of the Q–TiO2 photocatalyst particles fixed in a polymeric film reduces the area exposed to the solution as opposed to using suspended photocatalyst particles. S. Kuwabata et al. [41][9] justified their results by studying the photo–oxidation of the urea formed, determining that urea and 2–propanol compete for active sites on the surface of Q–TiO2, degrading and decreasing their concentration in the solution. According to research, using Q–TiO2 in suspension increases the collision frequency and interaction between urea and the catalyst surface. The interface interaction generates a more significant reaction and increases the degradation of the already–formed products. However, the concentration of the products formed by the urea degradation via photo–oxidation does not contribute directly to the secondary reactions leading to by–products such as methanol, ammonia, acetone, and hydrogen. The concentrations observed for the by–products remain proportional to urea formation, as shown in Table 1, whose results were obtained after photoreductions were made for 5 h.| Catalyst | Solvent | Amount of Products (µmol) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Urea | Acetone | Methanol | NH | 4+ | H | 2 | Ti | 3+ | |||
| Q–TiO | 2 | /PVPD | PC | 5.6 | 61.0 | 1.2 | 2.7 | 0.18 | 0.14 | ||
| Q–TiO | 2 | coloidal | PC | 0.22 | 2.5 | 0.12 | 0 | 0 | 0 | ||
| P–25 TiO | 2 | /PVPD | PC | 2.3 | 30.1 | 0.04 | 1.2 | 0.08 | – | ||
| P–25 Coloidal | PC | 0.1 | 1.1 | 0.05 | 0 | 0.06 | – | ||||
| P–25 TiO | 2 | /PVPD | H | 2 | O | 0 | 0.07 | 0 | 0 | 0.01 | – |
| Solvent | Dielectric Constant, | ε | Amount of Products (mM) | (a) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Urea | NH | 3 | HCO | 2− | CO | |||||
| Ethylene glycol monoethyl ether | 29.6 | 1.00 | 0.20 | 0.80 | 0.50 | |||||
| Acetonitrile | 37.5 | 1.15 | 0.15 | 0.70 | 0.20 | |||||
| Sulfolane | 43.0 | 1.00 | 0.20 | 0.40 | 0.25 | |||||
| PC | 69.0 | 0.85 | 0.25 | 0.10 | 0.05 | |||||
| Water | 78.5 | 2.75 | 0.75 | 0.10 | 0.05 | |||||
| Catalyst | Amount of Products (mMh) | (a) | Urea–Formate Ratio | ||
|---|---|---|---|---|---|
| Urea | Formate | ||||
| TiO | 2 | 0.28 | 0.11 | 2.5 | |
| PFD:TiO | |||||
| 2 | 0.58 | 0.15 | 3.9 | ||
| PFD:TiO | 2 | /Cu | 1.12 | 0.025 | 45 |
| Author | Catalyst | C Source | N Source | Solvent | Illumination Time, h | Urea, mM |
Urea, mM h | −1 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. Kuwabata et al. [41] | S. Kuwabata et al. [9] | Q–TiO | 2 | /PVPD | CO | 2 | , sat. | NH | 2 | OH 0.020 M | (a) | PC | 1 | 5.7 | (a,c) | 5.7 | |||||||
| TiO | 2 | /Cu | 0.40 | 0.050 | 8.0 | ||||||||||||||||||
| B.-J. Liu et al. [42] | B.-J. Liu et al. [10] | Q–TiO | 2 | /SiO | 2 | CO | 2 | , sat. | LiNO | 3 | 0.020 M |
H | 2 | O | 5 | 2.75 | (b) | 0.55 | |||||
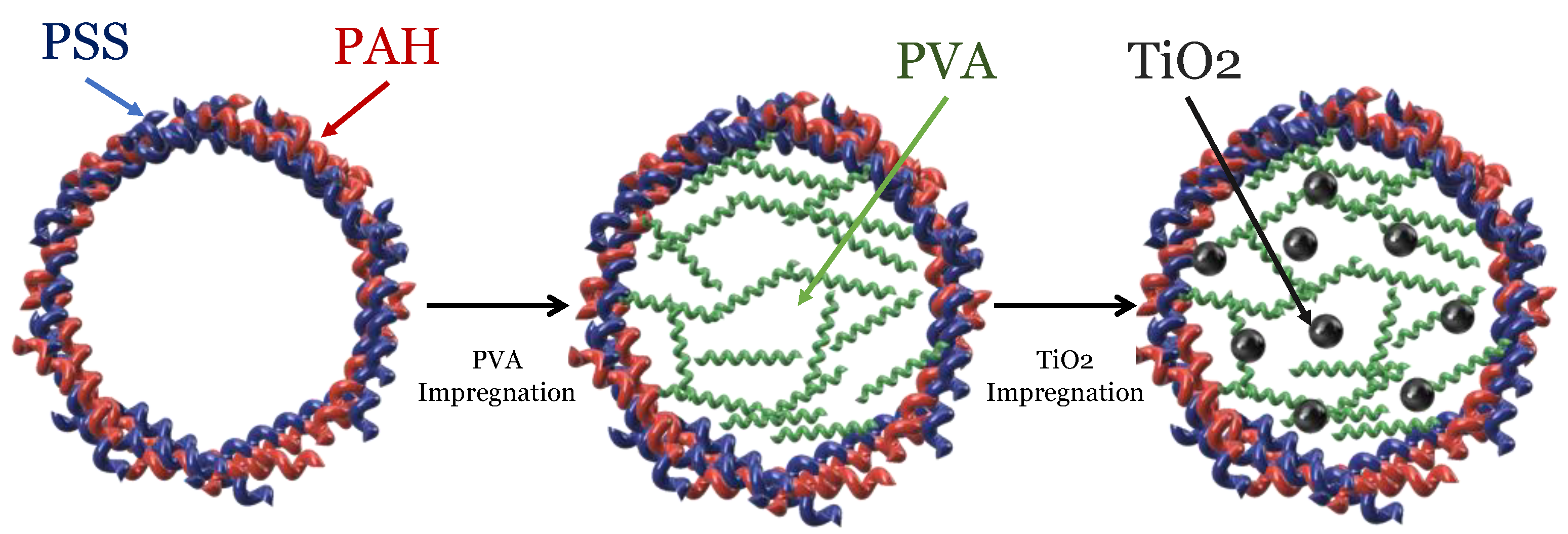
| D.G. Shchukin et al. [44] | D.G. Shchukin et al. [12] | Cu/TiO | 2 | –PVA–PAH/PSS (2.2 μm diameter) |
CO | 2 | , sat. | NaNO | 3 | 0.1 M |
H | 2 | O | 5 | 1.72 | (c) | 0.34 | ||||||
| E.A. Ustinovich et al. [45] | E.A. Ustinovich et al. [13] | Cu/TiO | 2 | :PFD | CO | 2 | , sat. | NaNO | 3 | 1.0 M |
PDF:H | 2 | O | 1 | 1.1 | (c,d) | 1.1 | ||||||
| B. Srinivas et al. [21] | B. Srinivas et al. [15] | Fe | 2 | TiO | 5 | (10wt%)/HZSM–5 | 2–propanol 1 | v | / | v | % | KNO | 3 | 0.016 M |
H | 2 | O | 6 | 0.31 | (e) | 0.052 | ||
| H. Maimaiti et al. [47] | H. Maimaiti et al. [16] | Ti | 3+ | –TiO | 2 | /Fe–CNTs | CO | 2 | (100 mL min | −1 | flow rate) | N | 2 | (100 mL min | −1 | flow rate) | H | 2 | O | 4 | 0.710 | (f) | 0.178 |
References
- Jin, D.; Zhao, S.; Zheng, N.; Beckers, Y.; Wang, J. Urea Metabolism and Regulation by Rumen Bacterial Urease in Ruminants—A Review. Ann. Anim. Sci. 2018, 18, 303–318.
- Patra, A.K.; Aschenbach, J.R. Ureases in the Gastrointestinal Tracts of Ruminant and Monogastric Animals and Their Implication in Urea-N/Ammonia Metabolism: A Review. J. Adv. Res. 2018, 13, 39–50.
- De Ventura, T.; Zanirato, V. Recent Advances in the Synthesis of Sulfonylureas. Eur. J. Org. Chem. 2021, 2021, 1201–1214.
- Berrada, H.; Font, G.; Moltó, J.C. Determination of Urea Pesticide Residues in Vegetable, Soil, and Water Samples. Crit. Rev. Anal. Chem. 2003, 33, 19–41.
- Amine-Khodja, A.; Boulkamh, A.; Boule, P. Photochemical Behaviour of Phenylurea Herbicides. Photochem.Photobiol.Sci. 2004, 3, 145–156.
- Glibert, P.M.; Harrison, J.; Heil, C.; Seitzinger, S. Escalating Worldwide Use of Urea—A Global Change Contributing to Coastal Eutrophication. Biogeochemistry 2006, 77, 441–463.
- Liu, Y.; Zhao, J.; Lee, J.-M. Conventional and New Materials for Selective Catalytic Reduction (SCR) of NOx. ChemCatChem 2018, 10, 1499–1511.
- Lee, J.; Theis, J.R.; Kyriakidou, E.A. Vehicle Emissions Trapping Materials: Successes, Challenges, and the Path Forward. Appl. Catal. B Environ. 2019, 243, 397–414.
- Kuwabata, S.; Yamauchi, H.; Yoneyama, H. Urea Photosynthesis from Inorganic Carbon and Nitrogen Compounds Using TiO 2 as Photocatalyst. Langmuir 1998, 14, 1899–1904.
- Liu, B.-J.; Torimoto, T.; Yoneyama, H. Photocatalytic Reduction of Carbon Dioxide in the Presence of Nitrate Using TiO2 Nanocrystal Photocatalyst Embedded in SiO2 Matrices. J. Photochem. Photobiol. A Chem. 1998, 115, 227–230.
- Torimoto, T.; Liu, B.-J.; Yoneyama, H. Effect of Solvents on Photocatalytic Reduction of Carbon Dioxide Using Semiconductor Photocatalysts. Stud. Surf. Sci. Catal. 1998, 114, 553–556.
- Shchukin, D.G.; Möhwald, H. Urea Photosynthesis inside Polyelectrolyte Capsules: Effect of Confined Media. Langmuir 2005, 21, 5582–5587.
- Ustinovich, E.A.; Shchukin, D.G.; Sviridov, D.V. Heterogeneous Photocatalysis in Titania-Stabilized Perfluorocarbon-in-Water Emulsions: Urea Photosynthesis and Chloroform Photodegradation. J. Photochem. Photobiol. A Chem. 2005, 175, 249–252.
- Shchukin, D.; Sviridov, D. Photocatalytic Processes in Spatially Confined Micro- and Nanoreactors. J. Photochem. Photobiol. C Photochem. Rev. 2006, 7, 23–39.
- Srinivas, B.; Kumari, V.D.; Sadanandam, G.; Hymavathi, C.; Subrahmanyam, M.; De, B.R. Photocatalytic Synthesis of Urea from in Situ Generated Ammonia and Carbon Dioxide. Photochem. Photobiol. 2012, 88, 233–241.
- Maimaiti, H.; Xu, B.; Sun, J.; Feng, L. Photocatalytic Synthesis of Urea (CO2/N2/H2O) on Coal–Based Carbon Nanotubes with the Fe-Core-Supported Ti3+–TiO2 Composite Catalyst. ACS Sustain. Chem. Eng. 2021, 9, 6991–7002.
