Polycyclic aromatic hydrocarbons (PAHs) comprise a group of chemical compounds consisting of two or more fused benzene rings. PAHs exhibit hydrophobicity and low water solubility, while some of their members are toxic substances resistant to degradation. Due to their low levels in environmental matrices, a preconcentration step is usually required for their determination. Nowadays, there is a wide variety of sample preparation techniques, including micro-extraction techniques (e.g., solid-phase microextraction and liquid phase microextraction) and miniaturized extraction techniques (e.g., dispersive solid-phase extraction, magnetic solid-phase extraction, stir bar sorptive extraction, fabric phase sorptive extraction etc.).
- PAHs
- sample preparation
- environmental samples
- extraction
- MSPE
- SPME
- FPSE
- SBSE
- DSPE
- PT-SPE.
- PT-SPE
1. Introduction
2. Extraction of PAHs from Environmental Matrices
A plethora of novel sample preparation techniques have been employed for the extraction of PAHs. Figure 2 summarizes the recent advances in sorptive extraction techniques that have been employed for the determination of PAHs from environmental samples.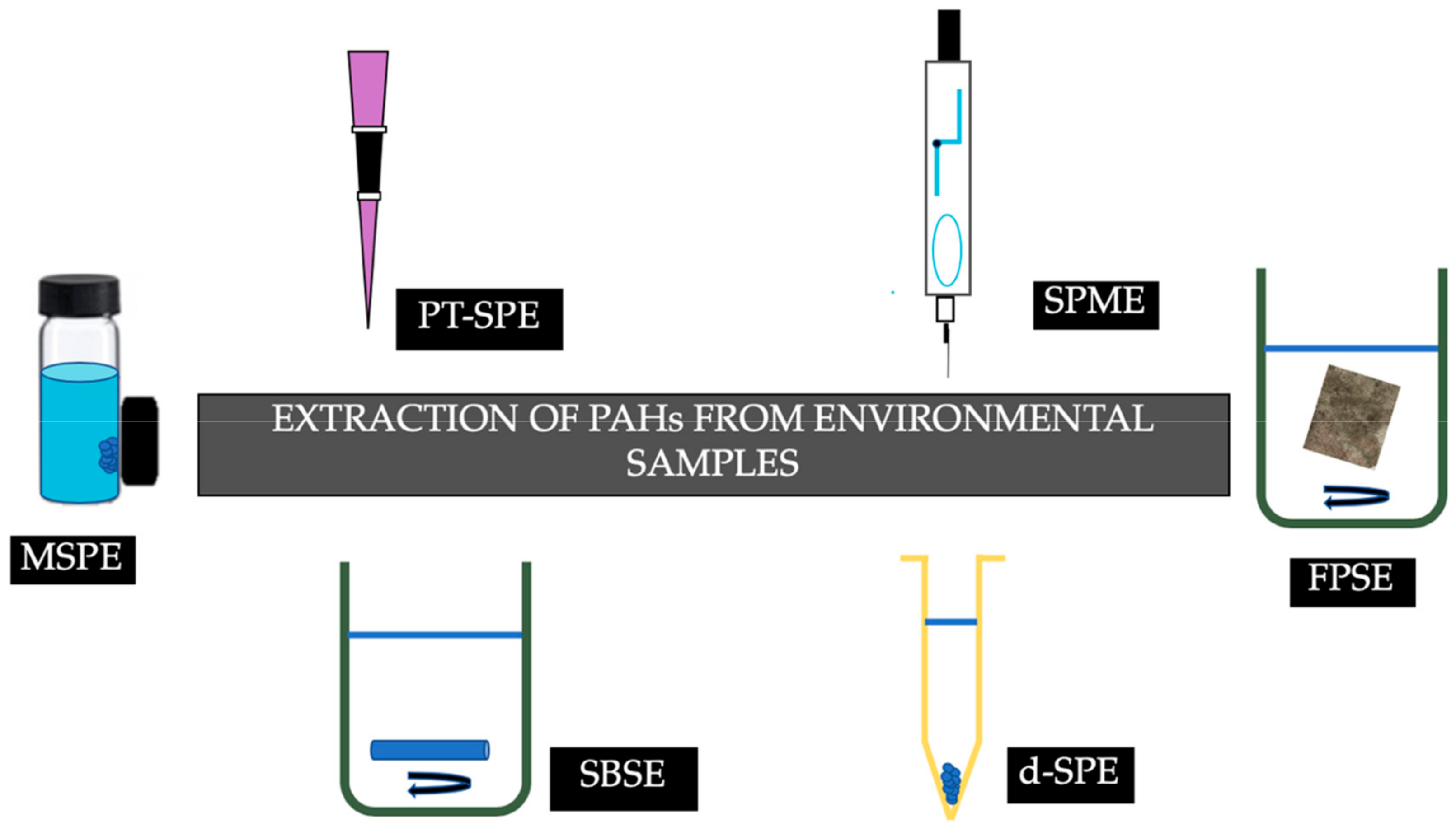
2.1. Dispersive Solid-Phase Extraction of PAHs from Environmental Matrices.
2.1. Dispersive Solid-Phase Extraction of PAHs from Environmental Matrices
Dispersive solid-phase extraction (d-SPE), is a form of SPE in which the desired sorbent is added directly into the sample aqueous solution followed by dispersion. This technique is taking advantage of the contact between the adsorbent and the target analytes. Once the extraction process is completed, the sorbent with the adsorbed analytes is separated from the sample by a mechanical process, such as centrifugation or filtration. Compared to the conventional SPE process, the main benefit of d-SPE is the reduction of sample preparation time, as well as its simplicity, adaptability and easy handling. A wide variety of sorbents have been utilized for the d-SPE of PAHs from environmental samples [30,31][22][23]. This technique gained popularity when Anastassiades et al. [32][24] introduced the QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe) approach for the determination of pesticide residue in food of plant origin. The initial method consists of acetonitrile extraction and addition of a mixture of salts, followed by a dispersive clean-up step with a primary–secondary amine (PSA) as extraction sorbent. QuEChERS was quickly applied for the determination of other analytes in a variety of sample matrices. Cvetkovic et al. [33][25] developed a QuEChERS extraction procedure of PAHs in soil prior to their determination by GC-MS. The researchers evaluated different solvent systems (acetonitrile/water and hexane/water) and sorbents (PSA, C18, Florisil, diatomaceous earth and clinoptilolite). Among the tested parameters, the best results were obtained with acetonitrile/water, as the extraction solvent and diatomaceous earth as the d-SPE extraction adsorbent. Until today, a wide variety of novel extraction sorbents have been evaluated for the d-SPE of PAHs from environmental matrices. Among them, metal-organic frameworks and zeolitic imidazole frameworks are currently the most popular d-SPE adsorbents. MOFs became widely known in 1995, when Yaghi and Li [34][26] reported the hydrothermal synthesis of a MOF material with large rectangular channels. Metal-organic frameworks are a class of hybrid organic-inorganic supramolecular materials, which are based on the coordination of metal ions or clusters with bi- or multidentate organic linkers. What makes MOFs materials so attractive is their unique properties, such as high surface areas (up to 14,600 m2·g−1) [35][27], pore size tunability, structure flexibility, luminosity, thermal stability, charge transfer ability from the ligand to the metal or from the metal to the ligands, etc [21,36,37,38,39][21][28][29][30][31]. As a result, MOFs have gained attention in a plethora of applications, such as gas storage and separation [40][32], catalysis [41][33], sensors [42][34], detoxification [43][35] and drug delivery [44][36]. In analytical chemistry, MOFs have been evaluated as stationary phases for GC [45,46][37][38] and HPLC [47,48][39][40] analysis. However, today, the most popular field of applications of MOFs in analytical chemistry is sample preparation [21,36][21][28]. Other d-SPE sorbents that have been applied for the extraction of PAHs from environmental samples include graphene/sepiolite [53][41] and N-acetyl-l-cysteine modified CdS quantum dots [54][42].2.2. Magnetic Solid-Phase Extraction of PAHs from Environmental Matrices.
2.2. Magnetic Solid-Phase Extraction of PAHs from Environmental Matrices
Magnetic solid-phase extraction (MSPE) is a form of d-SPE in which a magnetic nanomaterial is added into an aqueous sample solution to adsorb the target analytes. After the adsorption of the analyte, an external magnetic field is applied to collect the sorbent and the supernatant solution is discarded. Subsequently, elution of the adsorbed analytes is achieved with the addition of an appropriate solvent, and magnetic separation is performed once again to collect the eluent, which is further analyzed by a suitable analytical technique. Compared to the conventional SPE procedure, in MSPE there is no need for sorbent packing into cartridges, thus avoiding limitations of column blocking and high pressure. Meanwhile, sample and organic solvent consumption is significantly decreased compared to the classic SPE and LLE formats. Finally, the sorbent separation with a magnet is a simple and rapid process, compared to the time-consuming centrifugation and filtration steps that are required in conventional d-SPE [55,56,57][43][44][45]. Magnetic nanoparticles (MNPs) are characterized by the general formula MFe2O4 (M = Fe, Co, Cu, Mn, etc.), and they can be produced by a variety of methods, such as co-precipitation, solvothermal, hydrothermal etc. The most common magnetic nanoparticles that have been used in order to fabricate magnetic sorbents for MSPE are Fe3O4 nanoparticles. Iron oxides have been widely used in MSPE due to their super paramagnetism, their low toxicity, their high magnetic saturation, their simple preparation process and their low price [7]. The application of other magnetic nanoparticles, such as MnFe2O4, have been also reported [58][46]. However, the utilization of MNPs in sample preparation has some drawbacks, since their selectivity is low. Moreover, MNPs exhibit low stability in strong acidic aqueous media and low dispersion ability in many sample matrices. Therefore, surface modification of magnetic nanoparticles is usually required to enhance their stability and selectivity by the introduction of special functional groups [7]. As seen in Table 1, a wide variety of chemical compounds including carbon-based materials, polymeric materials, MOFs and other molecules have been employed for this purpose.|
Sorbent |
Matrix |
Analytical Technique |
Sorbent Mass (mg) |
Time (min) |
LODs (ng L−1) |
Extraction Recovery (%) |
Reusability |
Ref. |
|---|---|---|---|---|---|---|---|---|
|
HKUST-1 |
Water |
UHPLC-FLD |
5 Fe3O4/20 HKUST-1 |
10 |
0.8–12 |
39–59 |
NA |
|
|
MIL-101(Cr) |
Water |
HPLC-PDA |
1 Fe3O4@SiO2/0.6 MIL-101 |
20 |
2.8–27.2 |
NA |
NA |
|
|
Fe@MIL-101(Cr) |
Water |
HPLC-DAD |
50 |
50 |
44–64 |
>80 |
At least 10 times |
|
|
MIL-100(Fe) |
Water |
HPLC-FLD |
10 |
10 |
32–2110 |
>80 |
NA |
|
|
MIL-100(Fe) |
Water |
GC-FID |
12.5 |
15 |
4.6–8.9 |
73–96 |
Up to 10 times |
|
|
Fe3O4@ polydopamine/ZIF-7 |
Water, particulate matter |
GC-MS |
3 Fe3O4@PDA 15 ZIF-7 |
40 |
0.71–5.79 |
>82 |
At least 10 times |
|
|
TpPa-1 COF |
Water |
HPLC-FLD |
5 |
21 |
0.24–1.01 |
73–110 |
NA |
|
|
COF-LZU1@PEI@Fe3O4 |
Water, soil |
HPLC-FLD |
5 |
33 |
0.2–20 |
NA |
At least 6 times |
|
|
G/CNF |
Water |
GC-FID |
20 |
10 |
4–30 |
63.0–84.5 |
Up to 6 times |
|
|
Fe3O4/C |
Water |
HPLC-FLD |
50 |
30 |
2.4. Stir Bar Sorptive Extraction (SBSE) and Stir Rod Sorptive Extraction (SRSE) of PAHs from Environmental Matrices.
2.4. Stir Bar Sorptive Extraction (SBSE) and Stir Rod Sorptive Extraction (SRSE) of PAHs from Environmental Matrices
2.5. Liquid-Phase Microextraction of PAHs from Environmental Matrices.
2.5. Liquid-Phase Microextraction of PAHs from Environmental Matrices
|
Matrix |
Analytical Technique |
Extraction Solvent |
Disperser Solvent |
Phase Separation |
LODs(ng·L−1) |
EF |
Extraction Recovery (%) |
Ref. |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Surface water |
GC-MS |
Tetrachloroethylene |
Acetone |
Centrifugation |
7–30 |
603–1113 |
- |
||||||||||
|
Rainwater |
GC-MS |
n-Hexane |
Acetone |
Addition of demulsification solvent |
3.7–39.1 |
NA |
- |
||||||||||
|
River water |
GC-FID |
Toluene |
Methanol |
Air flotation |
14–41 × 103 |
NA |
- |
||||||||||
|
Sea water |
GC-MS |
Tetrachloroethylene |
Diethyl Ether |
Centrifugation |
1–10 |
722–8133 |
59.2–90.5 |
||||||||||
0.2–0.6 | |||||||||||||||||
76–110 | |||||||||||||||||
At least 10 times | [ | ] | [56] |
||||||||||||||
|
Hydrophilic Fe3O4/C |
|||||||||||||||||
|
Sediment |
HPLC-FLD |
Dichloromethane |
Acetonitrile |
Centrifugation |
2.3–6.8 ng g−1 |
NA |
>94.0 |
||||||||||
|
Tap, sea and spring water |
GC-FID |
Toluene |
- |
Centrifugation |
20–50 |
1776–2714 |
99–103 |
||||||||||
|
Tap, well, surface water etc. |
GC-MS |
Chloroform |
- |
Centrifugation |
1–36 |
NA |
- |
||||||||||
|
Tap, spring, surface water etc. |
GC-MS |
Iso-octane |
- |
Addition of NaCl |
0.001–0.009 |
Up to 100000 |
- |
||||||||||
|
Tap, rain and wastewater |
HPLC-FLD |
Cyclohexane |
- |
Centrifugation |
0.6–62.5 |
90–247 |
95–100 |
||||||||||
|
Well, river, lake water etc. |
HPLC-FLD |
TBAB/2-decanoic acid DES |
- |
Centrifugation/Solidification |
0.7–6.6 |
163–198 |
>80.0 |
Water | |||||||||
|
Tap, bottle, fountain water etc. |
HPLC-FLD GC-MS |
[C8 MiM][PF6 ] 10 |
30 |
Acetone |
Centrifugation 15–335 |
0.03–2 NA |
301–346 NA |
[5] |
|||||||||
- | [ | ] | [ | 135] |
CNF |
||||||||||||
|
Tap, well, surface water etc. |
Water |
GC-FID |
10 |
HPLC-UV 12 |
[BBIM][Tf2N] |
Acetone |
Centrifugation8–30 |
2 NA |
2768–5409 At least 10 times |
- [7] |
|||||||
[ | ] | [ | ] |
G/Fe3O4@PT |
|||||||||||||
|
Tap, rain and surface water | Water |
HPLC-FLD GC-FID |
Trichloroethylene 20 |
Acetonitrile 10 |
- 9–20 |
20–600 83–107 |
At least 17 times |
||||||||||
|
GO |
Water |
HPLC-UV |
40 |
16 |
90–190 |
76.8–103.2 |
NA |
[6] |
|||||||||
|
GO-Fe3O4@PS |
Water |
GC-FID |
15 |
10 |
3–10 |
69.5–88.7 |
NA |
||||||||||
|
Poly(Py-co-Ani)@GO-Fe3O4 |
Water |
GC-FID |
35 |
3–10 |
50.4‒78.3 |
At least 20 times |
|||||||||||
|
CNTs |
Water |
UHPLC-FLD |
5 |
10 |
25–73 |
76.4–106.5 |
Up to 3 times |
||||||||||
86–95 | - | [ | 172] |
mag-MIP |
Water |
HPLC-PDA |
20 |
55 |
1.3–969 |
46–100 |
At least 3 times |
||||||
[ | ] |
mag-MIP |
Water |
GC-MS |
5–20 |
17 |
30–750 |
>76 |
NA |
||||||||
|
RAFT-MIP |
Water |
GC-MS |
10 |
9 |
1–100 |
4.5–97 |
NA |
[8] |
|||||||||
|
PDA |
Water |
HPLC-FLD |
20 |
5 |
0.5–1.9 |
76.4–107 |
NA |
||||||||||
|
PPy |
Water |
GC-MS |
20 |
3 |
0.38–5.01 |
27.4- 115.7 |
NA |
||||||||||
|
PANI/Alginate |
Water |
HPLC-FLD |
400 |
20 |
10 |
86.0–97.8 |
Up to 6 times |
||||||||||
|
PoT |
Water |
GC-FID |
60 |
15 |
0.3–5.5 |
NA |
Up to 15 times |
||||||||||
|
IL-MNPs |
Water |
GC-MS |
30 |
8 |
40–1111 |
75–102 |
Up to 10 times |
||||||||||
|
MNP@CN/IL |
Leachate, sludge |
HPLC-DAD |
30 |
35 |
400–590 |
89.50–110.2 |
NA |
||||||||||
|
MNP-PANI-DICAT |
Water, sludge, soil |
GC-MS |
15 |
40 |
0.8–208.6 |
80.2–111.9 |
Up to 5 times |
||||||||||
|
Fe3O4@IL@MO |
Water |
HPLC-FLD |
18 |
26 |
0.1–2 |
NA |
NA |
||||||||||
|
Fe3O4@SiO2@Nap |
Water |
HPLC-FLD |
40 |
12 |
0.04–0.12 |
>90 |
At least 10 times |
[1] |
|||||||||
|
PC |
Water, milk |
HPLC-FLD |
100 |
10 |
0.2–0.6 |
>90 |
NA |
||||||||||
|
Fe3O4-DVB-SO3- |
Water |
GC-MS |
50 |
10 |
0.6–2.1 |
79.9–115.3 |
NA |
||||||||||
|
MPNP |
Water |
UHPLC-DAD |
200 |
15 |
10.83–18.53 nM |
75.7–106.4 |
At least 5 times |
[3] |
|||||||||
|
Fe3O4/SiO2/TPA |
Water |
HPLC-FLD |
50 |
15 |
0.04–37.5 |
NA |
NA |
||||||||||
|
C18 |
Water |
GC-MS |
50 |
6 |
0.8–36 × 103 |
35–99 |
NA |
||||||||||
|
C10–C18 carboxylates |
Water |
HPLC-FLD |
200 |
18 |
0.1–0.25 |
>90 |
Up to 5 times |
||||||||||
|
n-octadecylphosphonic acid |
Water |
GC-MS |
50 |
1 |
14.1–70.0 × 103 |
61.9–119.1 |
NA |
||||||||||
|
Nylon 6 |
Water |
HPLC-PDA |
40 |
30 |
0.05–0.58 × 103 |
36.2–87.0 |
NA |
||||||||||
|
CTAB |
Water |
UHPLC-FLD |
100 Fe3O4/50 CTAB |
30 |
0.4–10.3 |
59.23–87.95 |
NA |
||||||||||
|
Palm fatty acid |
Leachate, sludge |
HPLC-DAD |
15 |
25 |
10–50 |
>81.1 |
Up to 5 times |
||||||||||
|
TBCD |
Water |
HPLC-FLD |
80 |
15 |
0.03–1.2 |
>80 |
NA |
||||||||||
|
TCT |
Water, urine |
HPLC-FLD |
40 |
13 |
0.09–0.15 |
89–93 |
At least 30 times |
||||||||||
|
C16-HO |
Water |
HPLC-UV |
30 |
24 |
0.14–0.31 |
88–95 |
Up to 4 times |
2.3. Solid-Phase Microextraction of PAHs from Environmental Matrices.
2.3. Solid-Phase Microextraction of PAHs from Environmental Matrices
2.6. Fabric Phase Sorptive Extraction of PAHs from Environmental Matrices.
2.6. Fabric Phase Sorptive Extraction of PAHs from Environmental Matrices
2.7. Other Extraction Techniques for the Determination of PAHs in Environmental Matrices.
2.7. Other Extraction Techniques for the Determination of PAHs in Environmental Matrices
References
- Cai, Y.; Yan, Z.H.; Wang, N.Y.; Cai, Q.Y.; Yao, S.Z. Preparation of naphthyl functionalized magnetic nanoparticles for extraction of polycyclic aromatic hydrocarbons from river waters. RSC Adv. 2015, 5, 56189–56197.
- Pérez, R.A.; Albero, B.; Tadeo, J.L.; Fraile, M.V.; Sánchez-Brunete, C. Determination of PAHs in soil leachates by magnetic solid-phase extraction using nanoparticles and gas chromatography-tandem mass spectrometry. Anal. Methods 2014, 6, 1941–1950.
- Zhang, X.; Xie, S.; Paau, M.C.; Zheng, B.; Yuan, H.; Xiao, D.; Choi, M.M.F. Ultrahigh performance liquid chromatographic analysis and magnetic preconcentration of polycyclic aromatic hydrocarbons by Fe3O4-doped polymeric nanoparticles. J. Chromatogr. A 2012, 1247, 1–9.
- Boffetta, P.; Jourenkova, N.; Gustavsson, P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control 1997, 8, 444–472.
- Bai, L.; Mei, B.; Guo, Q.Z.; Shi, Z.G.; Feng, Y.Q. Magnetic solid-phase extraction of hydrophobic analytes in environmental samples by a surface hydrophilic carbon-ferromagnetic nanocomposite. J. Chromatogr. A 2010, 1217, 7331–7336.
- Han, Q.; Wang, Z.; Xia, J.; Chen, S.; Zhang, X.; Ding, M. Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Talanta 2012, 101, 388–395.
- Sarafraz-Yazdi, A.; Rokhian, T.; Amiri, A.; Ghaemi, F. Carbon nanofibers decorated with magnetic nanoparticles as a new sorbent for the magnetic solid phase extraction of selected polycyclic aromatic hydrocarbons from water samples. New J. Chem. 2015, 39, 5621–5627.
- Azizi, A.; Shahhoseini, F.; Bottaro, C.S. Magnetic molecularly imprinted polymers prepared by reversible addition fragmentation chain transfer polymerization for dispersive solid phase extraction of polycyclic aromatic hydrocarbons in water. J. Chromatogr. A 2020, 1610, 460534.
- Manousi, N.; Raber, G.; Papadoyannis, I. Recent Advances in Microextraction Techniques of Antipsychotics in Biological Fluids Prior to Liquid Chromatography Analysis. Separations 2017, 4, 18.
- Filippou, O.; Bitas, D.; Samanidou, V. Green approaches in sample preparation of bioanalytical samples prior to chromatographic analysis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1043, 44–62.
- Arthur, C.L.; Pawliszyn, J. Solid Phase Microextraction with Thermal Desorption Using Fused Silica Optical Fibers. Anal. Chem. 1990, 62, 2145–2148.
- Liu, H.; Dasgupta, P.K. Analytical chemistry in a drop. solvent extraction in a microdrop. Anal. Chem. 1996, 68, 1817–1821.
- Armenta, S.; Garrigues, S.; de la Guardia, M. The role of green extraction techniques in Green Analytical Chemistry. TrAC - Trends Anal. Chem. 2015, 71, 2–8.
- Manousi, N.; Gomez-Gomez, B.; Madrid, Y.; Deliyanni, E.A.; Zachariadis, G.A. Determination of rare earth elements by inductively coupled plasma-mass spectrometry after dispersive solid phase extraction with novel oxidized graphene oxide and optimization with response surface methodology and central composite design. Microchem. J. 2020, 152.
- Filippou, O.; Deliyanni, E.A.; Samanidou, V.F. Fabrication and evaluation of magnetic activated carbon as adsorbent for ultrasonic assisted magnetic solid phase dispersive extraction of bisphenol A from milk prior to high performance liquid chromatographic analysis with ultraviolet detection. J. Chromatogr. A 2017, 1479, 20–31.
- Chen, Z.; Yu, C.; Xi, J.; Tang, S.; Bao, T.; Zhang, J. A hybrid material prepared by controlled growth of a covalent organic framework on amino-modified MIL-68 for pipette tip solid-phase extraction of sulfonamides prior to their determination by HPLC. Microchim. Acta 2019, 186, 393.
- Karageorgou, E.; Manousi, N.; Samanidou, V.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction for the fast isolation of sulfonamides residues from raw milk followed by high performance liquid chromatography with ultraviolet detection. Food Chem. 2016, 196, 428–436.
- Nazyropoulou, C.; Samanidou, V. Stir bar sorptive extraction applied to the analysis of biological fluids. Bioanalysis 2015, 7, 2241–2250.
- Li, N.; Jiang, H.L.; Wang, X.; Wang, X.; Xu, G.; Zhang, B.; Wang, L.; Zhao, R.S.; Lin, J.M. Recent advances in graphene-based magnetic composites for magnetic solid-phase extraction. TrAC - Trends Anal. Chem. 2018, 102, 60–74.
- Riahi-Zanjani, B.; Balali-Mood, M.; Asoodeh, A.; Es’haghi, Z.; Ghorani-Azam, A. Developing a new sensitive solid-phase microextraction fiber based on carbon nanotubes for preconcentration of morphine. Appl. Nanosci. 2018, 8, 2047–2056.
- Manousi, N.; Zachariadis, G.A.; Deliyanni, E.A.; Samanidou, V.F. Applications of metal-organic frameworks in food sample preparation. Molecules 2018, 23, E2896.
- Islas, G.; Ibarra, I.S.; Hernandez, P.; Miranda, J.M.; Cepeda, A. Dispersive Solid Phase Extraction for the Analysis of Veterinary Drugs Applied to Food Samples: A Review. Int. J. Anal. Chem. 2017, 2017, 8215271.
- Han, L.; Sapozhnikova, Y.; Lehotay, S.J. Streamlined sample cleanup using combined dispersive solid-phase extraction and in-vial filtration for analysis of pesticides and environmental pollutants in shrimp. Anal. Chim. Acta 2014, 827, 40–46.
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F. Fast and Easy Multiresidue Method Employing Acetonitrile. J. AOAC Int. 2003, 86, 412–431.
- Cvetkovic, J.S.; Mitic, V.D.; Stankov Jovanovic, V.P.; Dimitrijevic, M.V.; Petrovic, G.M.; Nikolic-Mandic, S.D.; Stojanovic, G.S. Optimization of the QuEChERS extraction procedure for the determination of polycyclic aromatic hydrocarbons in soil by gas chromatography-mass spectrometry. Anal. Methods 2016, 8, 1711–1720.
- Yaghi, O.M.; Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. J. Am. Chem. Soc. 1995, 117, 10401–10402.
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydin, A.Ö.; Hupp, J.T. Metal-organic framework materials with ultrahigh surface areas: Is the sky the limit? J. Am. Chem. Soc. 2012, 134, 15016–15021.
- Manousi, N.; Giannakoudakis, D.A.; Rosenberg, E.; Zachariadis, G.A. Extraction of metal ions with metal–organic frameworks. Molecules 2019, 24, E4605.
- Vardali, S.C.; Manousi, N.; Barczak, M.; Giannakoudakis, D.A. Novel approaches utilizing metal-organic framework composites for the extraction of organic compounds and metal traces from fish and seafood. Molecules 2020, 25, E513.
- Giliopoulos, D.; Zamboulis, A.; Giannakoudakis, D.; Bikiaris, D.; Triantafyllidis, K. Polymer/metal organic framework (MOF) nanocomposites for biomedical applications. Molecules 2020, 25, 185.
- Giannakoudakis, D.A.; Hu, Y.; Florent, M.; Bandosz, T.J. Smart textiles of MOF/g-C3N4 nanospheres for the rapid detection/detoxification of chemical warfare agents. Nanoscale Horizons 2017, 2, 356–364.
- Li, H.; Li, L.; Lin, R.-B.; Zhou, W.; Zhang, Z.; Xiang, S.; Chen, B. Porous metal-organic frameworks for gas storage and separation: Status and challenges. EnergyChem 2019, 1, 100006.
- Yang, D.; Gates, B.C. Catalysis by Metal Organic Frameworks: Perspective and Suggestions for Future Research. ACS Catal. 2019, 9, 1779–1798.
- Achmann, S.; Hagen, G.; Kita, J.; Malkowsky, I.M.; Kiener, C.; Moos, R. Metal-Organic frameworks for sensing applications in the gas phase. Sensors 2009, 9, 1574–1589.
- Rojas, S.; Baati, T.; Njim, L.; Manchego, L.; Neffati, F.; Abdeljelil, N.; Saguem, S.; Serre, C.; Najjar, M.F.; Zakhama, A.; et al. Metal-Organic Frameworks as Efficient Oral Detoxifying Agents. J. Am. Chem. Soc. 2018, 140, 9581–9586.
- Wang, L.; Zheng, M.; Xie, Z. Nanoscale metal-organic frameworks for drug delivery: A conventional platform with new promise. J. Mater. Chem. B 2018, 6, 707–717.
- Chen, B.; Liang, C.; Yang, J.; Contreras, D.S.; Clancy, Y.L.; Lobkovsky, E.B.; Yaghi, O.M.; Dai, S. A microporous metal-organic framework for gas-chromatographic separation of alkanes. Angew. Chemie - Int. Ed. 2006, 9, 1418–1421.
- Gu, Z.Y.; Jiang, J.Q.; Yan, X.P. Fabrication of isoreticular metal-organic framework coated capillary columns for high-resolution gas chromatographic separation of persistent organic pollutants. Anal. Chem. 2011, 83, 5093–5100.
- Yang, C.X.; Yan, X.P. Metal-organic framework MIL-101(Cr) for high-performance liquid chromatographic separation of substituted aromatics. Anal. Chem. 2011, 83, 7144–7150.
- Yang, C.X.; Chen, Y.J.; Wang, H.F.; Yan, X.P. High-performance separation of fullerenes on metal-organic framework MIL-101(Cr). Chem. - A Eur. J. 2011, 42, 11734–11737.
- Mateos, R.; Vera-López, S.; Saz, M.; Díez-Pascual, A.M.; San Andrés, M.P. Graphene/sepiolite mixtures as dispersive solid-phase extraction sorbents for the anaysis of polycyclic aromatic hydrocarbons in wastewater using surfactant aqueous solutions for desorption. J. Chromatogr. A 2019, 1596, 30–40.
- Yang, X.P.; Luo, N.; Zong, Y.Y.; Jia, Z.H.; Liao, X.J. Quantum dots extraction coupled with high-performance liquid chromatography for the determination of polycyclic aromatic hydrocarbons in water. Appl. Ecol. Environ. Res. 2017, 15, 171–186.
- Hemmati, M.; Rajabi, M.; Asghari, A. Magnetic nanoparticle based solid-phase extraction of heavy metal ions: A review on recent advances. Microchim. Acta 2018, 185, 160.
- Giakisikli, G.; Anthemidis, A.N. Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review. Anal. Chim. Acta 2013, 789, 1–16.
- Manousi, N.; Rosenberg, E.; Deliyanni, E.; Zachariadis, G.A.; Samanidou, V. Magnetic Solid-Phase Extraction of Organic Compounds Based on Graphene Oxide Nanocomposites. Molecules 2020, 25, 1148.
- Hesampour, M.; Ali Taher, M.; Behzadi, M. Synthesis, characterization and application of a (:O-toluidine) nanocomposite for magnetic solid-phase extraction of polycyclic aromatic hydrocarbons. New J. Chem. 2017, 41, 12910–12919.
- Rocío-Bautista, P.; Pino, V.; Ayala, J.H.; Pasán, J.; Ruiz-Pérez, C.; Afonso, A.M. A magnetic-based dispersive micro-solid-phase extraction method using the metal-organic framework HKUST-1 and ultra-high-performance liquid chromatography with fluorescence detection for determining polycyclic aromatic hydrocarbons in waters and fruit tea. J. Chromatogr. A 2016, 1426, 42–50.
- Huo, S.H.; Yan, X.P. Facile magnetization of metal-organic framework MIL-101 for magnetic solid-phase extraction of polycyclic aromatic hydrocarbons in environmental water samples. Analyst 2012, 137, 3445–3451.
- Zhou, Q.; Lei, M.; Wu, Y.; Yuan, Y. Magnetic solid phase extraction of typical polycyclic aromatic hydrocarbons from environmental water samples with metal organic framework MIL-101 (Cr) modified zero valent iron nano-particles. J. Chromatogr. A 2017, 1487, 22–29.
- Du, F.; Qin, Q.; Deng, J.; Ruan, G.; Yang, X.; Li, L.; Li, J. Magnetic metal–organic framework MIL-100(Fe) microspheres for the magnetic solid-phase extraction of trace polycyclic aromatic hydrocarbons from water samples. J. Sep. Sci. 2016, 12, 2356–2364.
- Huo, S.H.; An, H.Y.; Yu, J.; Mao, X.F.; Zhang, Z.; Bai, L.; Huang, Y.F.; Zhou, P.X. Pyrolytic in situ magnetization of metal-organic framework MIL-100 for magnetic solid-phase extraction. J. Chromatogr. A 2017, 1517, 18–25.
- Zhang, S.; Yao, W.; Ying, J.; Zhao, H. Polydopamine-reinforced magnetization of zeolitic imidazolate framework ZIF-7 for magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from the air-water environment. J. Chromatogr. A 2016, 1452, 18–26.
- He, S.; Zeng, T.; Wang, S.; Niu, H.; Cai, Y. Facile synthesis of magnetic covalent organic framework with three-dimensional bouquet-like structure for enhanced extraction of organic targets. ACS Appl. Mater. Interfaces 2017, 9, 2959–2965.
- Wang, R.; Chen, Z. A covalent organic framework-based magnetic sorbent for solid phase extraction of polycyclic aromatic hydrocarbons, and its hyphenation to HPLC for quantitation. Microchim. Acta 2017, 184, 3867–3874.
- Rezvani-Eivari, M.; Amiri, A.; Baghayeri, M.; Ghaemi, F. Magnetized graphene layers synthesized on the carbon nanofibers as novel adsorbent for the extraction of polycyclic aromatic hydrocarbons from environmental water samples. J. Chromatogr. A 2016, 1465, 1–8.
- Zhang, S.; Niu, H.; Hu, Z.; Cai, Y.; Shi, Y. Preparation of carbon coated Fe3O4 nanoparticles and their application for solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. J. Chromatogr. A 2010, 1217, 4757–4764.
- Mehdinia, A.; Khodaee, N.; Jabbari, A. Fabrication of graphene/Fe3O4@polythiophene nanocomposite and its application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Anal. Chim. Acta 2015, 868, 1–9.
- Amiri, A.; Baghayeri, M.; Sedighi, M. Magnetic solid-phase extraction of polycyclic aromatic hydrocarbons using a graphene oxide/Fe3O4@polystyrene nanocomposite. Microchim. Acta 2018, 185, 393.
- Amiri, A.; Baghayeri, M.; Hamidi, E. Poly(pyrrole-: Co -aniline)@graphene oxide/Fe3O4 sorbent for the extraction and preconcentration of polycyclic aromatic hydrocarbons from water samples. New J. Chem. 2018, 42, 16744–16751.
- Corps Ricardo, A.I.; Guzmán Bernardo, F.J.; Zougagh, M.; Rodríguez Martín-Doimeadios, R.C.; Ríos, Á. Magnetic nanoparticles—carbon nanotubes hybrid composites for selective solid-phase extraction of polycyclic aromatic hydrocarbons and determination by ultra-high performance liquid chromatography. Anal. Bioanal. Chem. 2017, 409, 5125–5132.
- Villar-Navarro, M.; Martín-Valero, M.J.; Fernández-Torres, R.M.; Callejón-Mochón, M.; Bello-López, M.Á. Easy, fast and environmental friendly method for the simultaneous extraction of the 16 EPA PAHs using magnetic molecular imprinted polymers (mag-MIPs). J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1044–1045, 63–69.
- Benedetti, B.; Di Carro, M.; Magi, E. Multivariate optimization of an extraction procedure based on magnetic molecular imprinted polymer for the determination of polycyclic aromatic hydrocarbons in sea water. Microchem. J. 2019, 145, 1199–1206.
- Wang, Y.; Wang, S.; Niu, H.; Ma, Y.; Zeng, T.; Cai, Y.; Meng, Z. Preparation of polydopamine coated Fe3O4 nanoparticles and their application for enrichment of polycyclic aromatic hydrocarbons from environmental water samples. J. Chromatogr. A 2013, 1283, 20–26.
- Xu, S.N.; Zhao, Q.; He, H.B.; Yuan, B.F.; Feng, Y.Q.; Yu, Q.W. Rapid determination of polycyclic aromatic hydrocarbons in environmental water based on magnetite nanoparticles/polypyrrole magnetic solid-phase extraction. Anal. Methods 2014, 6, 7046–7053.
- Nurerk, P.; Kanatharana, P.; Bunkoed, O. Polyaniline-coated magnetite nanoparticles incorporated in alginate beads for the extraction and enrichment of polycyclic aromatic hydrocarbons in water samples. Int. J. Environ. Anal. Chem. 2017, 2, 145–158.
- Galán-Cano, F.; Del Carmen Alcudia-León, M.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Ionic liquid coated magnetic nanoparticles for the gas chromatography/mass spectrometric determination of polycyclic aromatic hydrocarbons in waters. J. Chromatogr. A 2013, 1300, 134–140.
- Bakhshaei, S.; Kamboh, M.A.; Nodeh, H.R.; Md Zain, S.; Mahmad Rozi, S.K.; Mohamad, S.; Mohammed Mohialdeen, I.A. Magnetic solid phase extraction of polycyclic aromatic hydrocarbons and chlorophenols based on cyano-ionic liquid functionalized magnetic nanoparticles and their determination by HPLC-DAD. RSC Adv. 2016, 6, 77047–77058.
- Shahriman, M.S.; Ramachandran, M.R.; Zain, N.N.M.; Mohamad, S.; Manan, N.S.A.; Yaman, S.M. Polyaniline-dicationic ionic liquid coated with magnetic nanoparticles composite for magnetic solid phase extraction of polycyclic aromatic hydrocarbons in environmental samples. Talanta 2018, 178, 211–221.
- Liu, X.; Lu, X.; Huang, Y.; Liu, C.; Zhao, S. Fe3O4@ionic orange nanoparticles as a novel nano-adsorbent for magnetic solid-phase extraction of polycyclic aromatic hydrocarbons in environmental water samples. Talanta 2014, 119, 341–347.
- Zhang, S.; Niu, H.; Zhang, Y.; Liu, J.; Shi, Y.; Zhang, X.; Cai, Y. Biocompatible phosphatidylcholine bilayer coated on magnetic nanoparticles and their application in the extraction of several polycyclic aromatic hydrocarbons from environmental water and milk samples. J. Chromatogr. A 2012, 1238, 38–45.
- Xue, S.W.; Tang, M.Q.; Xu, L.; Shi, Z. guo Magnetic nanoparticles with hydrophobicity and hydrophilicity for solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. J. Chromatogr. A 2015, 1411, 9–16.
- Long, Y.; Chen, Y.; Yang, F.; Chen, C.; Pan, D.; Cai, Q.; Yao, S. Triphenylamine-functionalized magnetic microparticles as a new adsorbent coupled with high performance liquid chromatography for the analysis of trace polycyclic aromatic hydrocarbons in aqueous samples. Analyst 2012, 137, 2716–2722.
- Liu, Y.; Li, H.; Lin, J.M. Magnetic solid-phase extraction based on octadecyl functionalization of monodisperse magnetic ferrite microspheres for the determination of polycyclic aromatic hydrocarbons in aqueous samples coupled with gas chromatography-mass spectrometry. Talanta 2009, 77, 1037–1042.
- Ballesteros-Gómez, A.; Rubio, S. Hemimicelles of alkyl carboxylates chemisorbed onto magnetic nanoparticles: Study and application to the extraction of carcinogenic polycyclic aromatic hydrocarbons in environmental water samples. Anal. Chem. 2009, 81, 9012–9020.
- Ding, J.; Gao, Q.; Luo, D.; Shi, Z.G.; Feng, Y.Q. n-Octadecylphosphonic acid grafted mesoporous magnetic nanoparticle: Preparation, characterization, and application in magnetic solid-phase extraction. J. Chromatogr. A 2010, 1217, 7351–7358.
- Reyes-Gallardo, E.M.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Magnetic nanoparticles-nylon 6 composite for the dispersive micro solid phase extraction of selected polycyclic aromatic hydrocarbons from water samples. J. Chromatogr. A 2014, 1345, 43–49.
- Wang, H.; Zhao, X.; Meng, W.; Wang, P.; Wu, F.; Tang, Z.; Han, X.; Giesy, J.P. Cetyltrimethylammonium Bromide-Coated Fe3O4 Magnetic Nanoparticles for Analysis of 15 Trace Polycyclic Aromatic Hydrocarbons in Aquatic Environments by Ultraperformance, Liquid Chromatography With Fluorescence Detection. Anal. Chem. 2015, 87, 7667–7675.
- Rozi, S.K.M.; Nodeh, H.R.; Kamboh, M.A.; Manan, N.S.A.; Mohamad, S. Novel palm fatty acid functionalized magnetite nanoparticles for magnetic solid-phase extraction of trace polycyclic aromatic hydrocarbons from environmental samples. J. Oleo Sci. 2017, 66, 771–784.
- Zou, Y.; Chen, Y.; Yan, Z.; Chen, C.; Wang, J.; Yao, S. Magnetic solid-phase extraction based on tetrabenzyl modified Fe3O4 nanoparticles for the analysis of trace polycyclic aromatic hydrocarbons in environmental water samples. Analyst 2013, 138, 5904–5912.
- Zhang, W.; Zhang, Y.; Jiang, Q.; Zhao, W.; Yu, A.; Chang, H.; Lu, X.; Xie, F.; Ye, B.; Zhang, S. Tetraazacalixarencetriazine Coated Fe3O4/SiO2 Magnetic Nanoparticles for Simultaneous Dispersive Solid Phase Extraction and Determination of Trace Multitarget Analytes. Anal. Chem. 2016, 88, 10523–10532.
- Al-rashdi, A.A. Double-functionalized magnetic nanoparticles for preconcentration and determination of polycyclic aromatic hydrocarbons in water samples. Anal. Chem. Res. 2016, 10, 9–17.
- Kataoka, H.; Lord, H.L.; Pawliszyn, J. Applications of solid-phase microextraction in food analysis. J. Chromatogr. A 2000, 880, 35–62.
- Aulakh, J.S.; Malik, A.K.; Kaur, V.; Schmitt-Kopplin, P. A review on solid phase micro extraction - High performance liquid chromatography (SPME-HPLC) analysis of pesticides. Crit. Rev. Anal. Chem. 2005, 35, 71–85.
- Chen, C.; Liang, X.; Wang, J.; Yang, S.; Yan, Z.; Cai, Q.; Yao, S. Development of a highly robust solid phase microextraction fiber based on crosslinked methyl methacrylate-polyhedral oligomeric silsesquioxane hybrid polymeric coating. Anal. Chim. Acta 2013, 792, 45–51.
- Rocío-Bautista, P.; Pacheco-Fernández, I.; Pasán, J.; Pino, V. Are metal-organic frameworks able to provide a new generation of solid-phase microextraction coatings? – A review. Anal. Chim. Acta 2016, 939, 26–41.
- Yu, H.; Ho, T.D.; Anderson, J.L. Ionic liquid and polymeric ionic liquid coatings in solid-phase microextraction. TrAC - Trends Anal. Chem. 2013, 45, 219–232.
- López-Darias, J.; Pino, V.; Anderson, J.L.; Graham, C.M.; Afonso, A.M. Determination of water pollutants by direct-immersion solid-phase microextraction using polymeric ionic liquid coatings. J. Chromatogr. A 2010, 1217, 1236–1243.
- Meng, Y.; Anderson, J.L. Tuning the selectivity of polymeric ionic liquid sorbent coatings for the extraction of polycyclic aromatic hydrocarbons using solid-phase microextraction. J. Chromatogr. A 2010, 1217, 6143–6152.
- Feng, J.; Sun, M.; Li, J.; Liu, X.; Jiang, S. A novel aromatically functional polymeric ionic liquid as sorbent material for solid-phase microextraction. J. Chromatogr. A 2012, 1227, 54–59.
- López-Darias, J.; Pino, V.; Meng, Y.; Anderson, J.L.; Afonso, A.M. Utilization of a benzyl functionalized polymeric ionic liquid for the sensitive determination of polycyclic aromatic hydrocarbons; parabens and alkylphenols in waters using solid-phase microextraction coupled to gas chromatography-flame ionization detection. J. Chromatogr. A 2010, 1217, 7189–7197.
- Zhang, S.; Du, Z.; Li, G. Layer-by-layer fabrication of chemical-bonded graphene coating for solid-phase microextraction. Anal. Chem. 2011, 83, 7531–7541.
- Wang, F.; Zheng, Y.; Qiu, J.; Liu, S.; Tong, Y.; Zhu, F.; Ouyang, G. Graphene-based metal and nitrogen-doped carbon composites as adsorbents for highly sensitive solid phase microextraction of polycyclic aromatic hydrocarbons. Nanoscale 2018, 10, 10073–10078.
- Xu, L.; Feng, J.; Liang, X.; Li, J.; Jiang, S. C18 functionalized graphene oxide as a novel coating for solid-phase microextraction. J. Sep. Sci. 2012, 35, 1531–1537.
- Sun, M.; Feng, J.; Bu, Y.; Duan, H.; Wang, X.; Luo, C. Development of a solid-phase microextraction fiber by the chemical binding of graphene oxide on a silver-coated stainless-steel wire with an ionic liquid as the crosslinking agent. J. Sep. Sci. 2014, 37, 3691–3698.
- Xu, L.; Feng, J.; Li, J.; Liu, X.; Jiang, S. Graphene oxide bonded fused-silica fiber for solid-phase microextraction-gas chromatography of polycyclic aromatic hydrocarbons in water. J. Sep. Sci. 2012, 35, 93–100.
- Behzadi, M.; Noroozian, E.; Mirzaei, M. A novel coating based on carbon nanotubes/poly-ortho-phenylenediamine composite for headspace solid-phase microextraction of polycyclic aromatic hydrocarbons. Talanta 2013, 108, 66–73.
- Maghsoudi, S.; Noroozian, E. HP-SPME of volatile polycyclic aromatic hydrocarbons from water using multiwalled carbon nanotubes coated on a steel fiber through electrophoretic deposition. Chromatographia 2012, 75, 913–921.
- Zhang, X.; Zang, X.H.; Wang, J.T.; Wang, C.; Wu, Q.H.; Wang, Z. Porous carbon derived from aluminum-based metal organic framework as a fiber coating for the solid-phase microextraction of polycyclic aromatic hydrocarbons from water and soil. Microchim. Acta 2015, 182, 2353–2359.
- Mehdinia, A.; Mohammadi, A.A.; Davarani, S.S.H.; Banitaba, M.H. Application of self-assembled monolayers in the preparation of solid-phase microextraction coatings. Chromatographia 2011, 5–6, 421–427.
- Yang, L.; Zhang, J.; Zhao, F.; Zeng, B. Electrodeposition of self-assembled poly(3,4-ethylenedioxythiophene) @gold nanoparticles on stainless steel wires for the headspace solid-phase microextraction and gas chromatographic determination of several polycyclic aromatic hydrocarbons. J. Chromatogr. A 2016, 1471, 80–86.
- Harati, F.; Ghiasvand, A.; Dalvand, K.; Haddad, P.R. Fused-silica capillary internally modified with nanostructured octadecyl silica for dynamic in-tube solid-phase microextraction of polycyclic aromatic hydrocarbons from aqueous media. Microchem. J. 2020, 155, 104672.
- Ghiasvand, A.; Yazdankhah, F.; Paull, B. Heating-, Cooling- and Vacuum-Assisted Solid-Phase Microextraction (HCV-SPME) for Efficient Sampling of Environmental Pollutants in Complex Matrices. Chromatographia 2020, 83, 531–540.
- Kremser, A.; Jochmann, M.A.; Schmidt, T.C. PAL SPME Arrow - Evaluation of a novel solid-phase microextraction device for freely dissolved PAHs in water. Anal. Bioanal. Chem. 2016, 408, 943–952.
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: Theory and principles. J. Microcolumn Sep. 1999, 10, 737–747.
- David, F.; Sandra, P. Stir bar sorptive extraction for trace analysis. J. Chromatogr. A 2007, 1152, 54–69.
- García-Falcón, M.S.; Cancho-Grande, B.; Simal-Gándara, J. Stirring bar sorptive extraction in the determination of PAHs in drinking waters. Water Res. 2004, 38, 1679–1684.
- Popp, P.; Bauer, C.; Wennrich, L. Application of stir bar sorptive extraction in combination with column liquid chromatography for the determination of polycyclic aromatic hydrocarbons in water samples. Anal. Chim. Acta 2001, 436, 1–9.
- Popp, P.; Bauer, C.; Hauser, B.; Keil, P.; Wennrich, L. Extraction of polycyclic aromatic hydrocarbons and organochloride compounds from water: A comparison between solid-phase microextraction and stir bar sorptive extraction. J. Sep. Sci. 2003, 9–10, 961–967.
- Bourdat-Deschamps, M.; Daudin, J.J.; Barriuso, E. An experimental design approach to optimise the determination of polycyclic aromatic hydrocarbons from rainfall water using stir bar sorptive extraction and high performance liquid chromatography-fluorescence detection. J. Chromatogr. A 2007, 1167, 143–153.
- Mao, X.; Hu, B.; He, M.; Fan, W. Stir bar sorptive extraction approaches with a home-made portable electric stirrer for the analysis of polycyclic aromatic hydrocarbon compounds in environmental water. J. Chromatogr. A 2012, 1260, 16–24.
- Mollahosseini, A.; Rokue, M.; Mojtahedi, M.M.; Toghroli, M.; Kamankesh, M.; Motaharian, A. Mechanical stir bar sorptive extraction followed by gas chromatography as a new method for determining polycyclic aromatic hydrocarbons in water samples. Microchem. J. 2016, 126, 431–437.
- Huang, X.; Yuan, D. Preparation of stir bars for sorptive extraction based on monolithic material. J. Chromatogr. A 2007, 1154, 152–157.
- Ekbatani Amlashi, N.; Hadjmohammadi, M.R. Sol–gel coating of poly(ethylene glycol)-grafted multiwalled carbon nanotubes for stir bar sorptive extraction and its application to the analysis of polycyclic aromatic hydrocarbons in water. J. Sep. Sci. 2016, 39, 3445–3456.
- Yu, C.; Hu, B. Automated stir plate (bar) sorptive extraction coupled to high-performance liquid chromatography for the determination of polycyclic aromatic hydrocarbons. J. Sep. Sci. 2010, 33, 2176–2183.
- Rutkowska, M.; Płotka-Wasylka, J.; Sajid, M.; Andruch, V. Liquid–phase microextraction: A review of reviews. Microchem. J. 2019, 149, 103989.
- Bello-López, M.Á.; Ramos-Payán, M.; Ocaña-González, J.A.; Fernández-Torres, R.; Callejón-Mochón, M. Analytical Applications of Hollow Fiber Liquid Phase Microextraction (HF-LPME): A Review. Anal. Lett. 2012, 45, 804–830.
- Kokosa, J.M. Recent trends in using single-drop microextraction and related techniques in green analytical methods. TrAC - Trends Anal. Chem. 2015, 71, 194–204.
- Kocúrová, L.; Balogh, I.S.; Šandrejová, J.; Andruch, V. Recent advances in dispersive liquid-liquid microextraction using organic solvents lighter than water. A review. Microchem. J. 2012, 102, 11–17.
- Rykowska, I.; Ziemblińska, J.; Nowak, I. Modern approaches in dispersive liquid-liquid microextraction (DLLME) based on ionic liquids: A review. J. Mol. Liq. 2018, 259, 319–339.
- Fernández, M.; Clavijo, S.; Forteza, R.; Cerdà, V. Determination of polycyclic aromatic hydrocarbons using lab on valve dispersive liquid-liquid microextraction coupled to high performance chromatography. Talanta 2015, 138, 190–195.
- Pena-Pereira, F.; Costas-Mora, I.; Lavilla, I.; Bendicho, C. Rapid screening of polycyclic aromatic hydrocarbons (PAHs) in waters by directly suspended droplet microextraction-microvolume fluorospectrometry. Talanta 2012, 89, 217–222.
- Santos, L.O.; dos Anjos, J.P.; Ferreira, S.L.C.; de Andrade, J.B. Simultaneous determination of PAHS, nitro-PAHS and quinones in surface and groundwater samples using SDME/GC-MS. Microchem. J. 2017, 133, 431–440.
- Sibiya, P.; Cukrowska, E.; Jönsson, J.Å.; Chimuka, L. Hollow-fibre liquid-phase microextraction for the determination of polycyclic aromatic hydrocarbons in Johannesburg Jukskei River, South Africa. Chromatographia 2013, 76, 427–436.
- Ratola, N.; Alves, A.; Kalogerakis, N.; Psillakis, E. Hollow-fibre liquid-phase microextraction: A simple and fast cleanup step used for PAHs determination in pine needles. Anal. Chim. Acta 2008, 618, 70–78.
- Rezaee, M.; Assadi, Y.; Milani Hosseini, M.R.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid-liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9.
- Guo, L.; Lee, H.K. Low-density solvent-based solvent demulsification dispersive liquid-liquid microextraction for the fast determination of trace levels of sixteen priority polycyclic aromatic hydrocarbons in environmental water samples. J. Chromatogr. A 2011, 1218, 5040–5046.
- Hosseini, M.H.; Rezaee, M.; Akbarian, S.; Mizani, F.; Pourjavid, M.R.; Arabieh, M. Homogeneous liquid-liquid microextraction via flotation assistance for rapid and efficient determination of polycyclic aromatic hydrocarbons in water samples. Anal. Chim. Acta 2013, 762, 54–60.
- Song, X.; Li, J.; Liao, C.; Chen, L. Ultrasound-assisted dispersive liquid-liquid microextraction combined with low solvent consumption for determination of polycyclic aromatic hydrocarbons in seawater by GC-MS. Chromatographia 2011, 74, 89–98.
- Leng, G.; Lui, G.; Chen, Y.; Yin, H.; Dan, D. Vortex-assisted extraction combined with dispersive liquid-liquid microextraction for the determination of polycyclic aromatic hydrocarbons in sediment by high performance liquid chromatography. J. Sep. Sci. 2012, 35, 2796–2804.
- Saleh, A.; Yamini, Y.; Faraji, M.; Rezaee, M.; Ghambarian, M. Ultrasound-assisted emulsification microextraction method based on applying low density organic solvents followed by gas chromatography analysis for the determination of polycyclic aromatic hydrocarbons in water samples. J. Chromatogr. A 2009, 1216, 6673–6679.
- Ozcan, S.; Tor, A.; Aydin, M.E. Determination of polycyclic aromatic hydrocarbons in waters by ultrasound-assisted emulsification-microextraction and gas chromatography-mass spectrometry. Anal. Chim. Acta 2010, 665, 193–199.
- Avino, P.; Notardonato, I.; Perugini, L.; Russo, M.V. New protocol based on high-volume sampling followed by DLLME-GC-IT/MS for determining PAHs at ultra-trace levels in surface water samples. Microchem. J. 2017, 133, 251–257.
- Cheng, J.; Matsadiq, G.; Liu, L.; Zhou, Y.W.; Chen, G. Development of a novel ultrasound-assisted surfactant-enhanced emulsification microextraction method and its application to the analysis of eleven polycyclic aromatic hydrocarbons at trace levels in water. J. Chromatogr. A 2011, 1218, 2476–2482.
- Yousefi, S.M.; Shemirani, F.; Ghorbanian, S.A. Hydrophobic Deep Eutectic Solvents in Developing Microextraction Methods Based on Solidification of Floating Drop: Application to the Trace HPLC/FLD Determination of PAHs. Chromatographia 2018 81, 1201–1211.
- Pena, M.T.; Casais, M.C.; Mejuto, M.C.; Cela, R. Development of an ionic liquid based dispersive liquid-liquid microextraction method for the analysis of polycyclic aromatic hydrocarbons in water samples. J. Chromatogr. A 2009, 1216, 6356–6364.
- Liu, L.; He, L.; Jiang, X.; Zhao, W.; Xiang, G.; Anderson, J.L. Macrocyclic polyamine-functionalized silica as a solid-phase extraction material coupled with ionic liquid dispersive liquid-liquid extraction for the enrichment of polycyclic aromatic hydrocarbons. J. Sep. Sci. 2014, 37, 1004–1011.
- Aznar, M.; Alfaro, P.; Nerin, C.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction: An innovative sample preparation approach applied to the analysis of specific migration from food packaging. Anal. Chim. Acta 2016, 936, 97–107.
- Zilfidou, E.; Kabir, A.; Furton, K.G.; Samanidou, V. Fabric phase sorptive extraction: Current state of the art and future perspectives. Separations 2018, 5, 40.
- Kabir, A.; Furton, K.G. Sample preparation in food analysis: Practices, problems and future outlook. In Analytical Chemistry: Developments, Applications and Challenges in Food Analysis; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017; ISBN 9781536122824.
- Locatelli, M.; Kabir, A.; Innosa, D.; Lopatriello, T.; Furton, K.G. A fabric phase sorptive extraction-High performance liquid chromatography-Photo diode array detection method for the determination of twelve azole antimicrobial drug residues in human plasma and urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1040, 192–198.
- Samanidou, V.; Galanopoulos, L.D.; Kabir, A.; Furton, K.G. Fast extraction of amphenicols residues from raw milk using novel fabric phase sorptive extraction followed by high-performance liquid chromatography-diode array detection. Anal. Chim. Acta 2015, 855, 41–50.
- Samanidou, V.; Michaelidou, K.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction of selected penicillin antibiotic residues from intact milk followed by high performance liquid chromatography with diode array detection. Food Chem. 2017, 224, 131–138.
- Saini, S.S.; Kabir, A.; Rao, A.L.J.; Malik, A.K.; Furton, K.G. A novel protocol to monitor trace levels of selected polycyclic aromatic hydrocarbons in environmental water using fabric phase sorptive extraction followed by high performance liquid chromatography-fluorescence detection. Separations 2017, 4, 22.
- Sun, T.; Wang, D.; Tang, Y.; Xing, X.; Zhuang, J.; Cheng, J.; Du, Z. Fabric-phase sorptive extraction coupled with ion mobility spectrometry for on-site rapid detection of PAHs in aquatic environment. Talanta 2019, 195, 109–116.
- Armenta, S.; Alcala, M.; Blanco, M. A review of recent, unconventional applications of ion mobility spectrometry (IMS). Anal. Chim. Acta 2011, 703, 114–123.
- Yang, S.; Chen, C.; Yan, Z.; Cai, Q.; Yao, S. Evaluation of metal-organic framework 5 as a new SPE material for the determination of polycyclic aromatic hydrocarbons in environmental waters. J. Sep. Sci. 2013, 36, 1283–1290.
- Hu, H.; Liu, S.; Chen, C.; Wang, J.; Zou, Y.; Lin, L.; Yao, S. Two novel zeolitic imidazolate frameworks (ZIFs) as sorbents for solid-phase extraction (SPE) of polycyclic aromatic hydrocarbons (PAHs) in environmental water samples. Analyst 2014, 139, 5818–5826.
- Ge, D.; Lee, H.K. Water stability of zeolite imidazolate framework 8 and application to porous membrane-protected micro-solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. J. Chromatogr. A 2011, 1218, 8490–8495.
- Song, X.; Li, J.; Xu, S.; Ying, R.; Ma, J.; Liao, C.; Liu, D.; Yu, J.; Chen, L. Determination of 16 polycyclic aromatic hydrocarbons in seawater using molecularly imprinted solid-phase extraction coupled with gas chromatography-mass spectrometry. Talanta 2012, 99, 75–82.
- Mauri-Aucejo, A.; Amorós, P.; Moragues, A.; Guillem, C.; Belenguer-Sapiña, C. Comparison of the solid-phase extraction efficiency of a bounded and an included cyclodextrin-silica microporous composite for polycyclic aromatic hydrocarbons determination in water samples. Talanta 2016, 32, 659–665.
- Soler-Seguí, S.; Belenguer-Sapiña, C.; Amorós, P.; Mauri-Aucejo, A. Evaluation of a cyclodextrin-silica hybrid microporous composite for the solid-phase extraction of polycyclic aromatic hydrocarbons. Anal. Sci. 2016, 32, 659–665.
- Wang, N.; Guo, Y.; Wang, L.; Liang, X.; Liu, S.; Jiang, S. Preparation of an aminopropyl imidazole-modified silica gel as a sorbent for solid-phase extraction of carboxylic acid compounds and polycyclic aromatic hydrocarbons. Analyst 2014, 139, 2531–2537.
- Zhao, W.; Yang, L.; He, L.; Zhang, S. Simultaneous Enrichment of Polycyclic Aromatic Hydrocarbons and Cu2+ in Water Using Tetraazacalixarenetriazine as a Solid-Phase Extraction Selector. J. Agric. Food Chem. 2016, 64, 6233–6239.
- Kefi, B.B.; El Atrache, L.L.; Kochkar, H.; Ghorbel, A. TiO2 nanotubes as solid-phase extraction adsorbent for the determination of polycyclic aromatic hydrocarbons in environmental water samples. J. Environ. Sci. 2011, 23, 860–867.
- Huang, Y.; Zhou, Q.; Xie, G. Development of micro-solid phase extraction with titanate nanotube array modified by cetyltrimethylammonium bromide for sensitive determination of polycyclic aromatic hydrocarbons from environmental water samples. J. Hazard. Mater. 2011, 183, 82–89.
- Krupadam, R.J.; Korde, B.A.; Ashokkumar, M.; Kolev, S.D. Novel molecularly imprinted polymeric microspheres for preconcentration and preservation of polycyclic aromatic hydrocarbons from environmental samples. Anal. Bioanal. Chem. 2014, 406, 5313–5321.
- Foan, L.; Ricoul, F.; Vignoud, S. A novel microfluidic device for fast extraction of polycyclic aromatic hydrocarbons (PAHs) from environmental waters – comparison with stir-bar sorptive extraction (SBSE). Int. J. Environ. Anal. Chem. 2015, 95, 1171–1185.
- Wu, H.; Wang, X.; Liu, B.; Lu, J.; Du, B.; Zhang, L.; Ji, J.; Yue, Q.; Han, B. Flow injection solid-phase extraction using multi-walled carbon nanotubes packed micro-column for the determination of polycyclic aromatic hydrocarbons in water by gas chromatography-mass spectrometry. J. Chromatogr. A 2010, 1217, 2911–2917.
- Zhou, Y.Y.; Yan, X.P.; Kim, K.N.; Wang, S.W.; Liu, M.G. Exploration of coordination polymer as sorbent for flow injection solid-phase extraction on-line coupled with high-performance liquid chromatography for determination of polycyclic aromatic hydrocarbons in environmental materials. J. Chromatogr. A 2006, 1116, 172–178.
- Zhang, X.; Wang, P.; Han, Q.; Li, H.; Wang, T.; Ding, M. Metal–organic framework based in-syringe solid-phase extraction for the on-site sampling of polycyclic aromatic hydrocarbons from environmental water samples. J. Sep. Sci. 2018, 41, 1856–1863.
