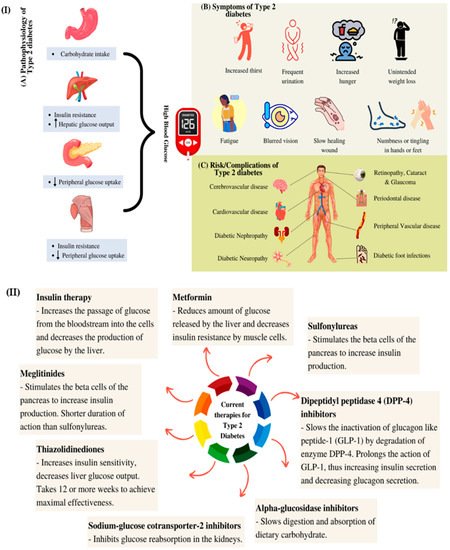
Diabetes mellitus is a prevalent metabolic syndrome that is associated with high blood glucose levels. The number of diabetic patients is increasing every year and the total number of cases is expected to reach more than 600 million worldwide by 2045. Modern antidiabetic drugs alleviate hyperglycaemia and complications that are caused by high blood glucose levels. Due to the side effects of these drugs, plant extracts and bioactive compounds with antidiabetic properties have been gaining attention as alternative treatments for diabetes. Natural products are biocompatible, cheaper and expected to cause fewer side effects than the current antidiabetic drugs. Various nanocarrier systems are discussed, such as liposomes, niosomes, polymeric nanoparticles, nanoemulsions, solid lipid nanoparticles and metallic nanoparticles. These systems have been applied to overcome the limitations of the current drugs and simultaneously improve the efficacy of plant-based antidiabetic drugs. The main challenges in the formulation of plant-based nanocarriers are the loading capacity of the plant extracts and the stability of the carriers. Lipid nanocarriers and the amphipathic properties of phospholipids and liposomes that encapsulate hydrophilic, hydrophobic and amphiphilic drugs is also described. A special emphasis is placed on metallic nanoparticles, with their advantages and associated complications being reported to highlight their effectiveness for treating hyperglycaemia.
Diabetes mellitus is a prevalent metabolic syndrome that is associated with high blood glucose levels. The number of diabetic patients is increasing every year and the total number of cases is expected to reach more than 600 million worldwide by 2045. Modern antidiabetic drugs alleviate hyperglycaemia and complications that are caused by high blood glucose levels. However, due to the side effects of these drugs, plant extracts and bioactive compounds with antidiabetic properties have been gaining attention as alternative treatments for diabetes. Natural products are biocompatible, cheaper and expected to cause fewer side effects than the current antidiabetic drugs. In this review, various nanocarrier systems are discussed, such as liposomes, niosomes, polymeric nanoparticles, nanoemulsions, solid lipid nanoparticles and metallic nanoparticles. These systems have been applied to overcome the limitations of the current drugs and simultaneously improve the efficacy of plant-based antidiabetic drugs. The main challenges in the formulation of plant-based nanocarriers are the loading capacity of the plant extracts and the stability of the carriers. A brief review of lipid nanocarriers and the amphipathic properties of phospholipids and liposomes that encapsulate hydrophilic, hydrophobic and amphiphilic drugs is also described. A special emphasis is placed on metallic nanoparticles, with their advantages and associated complications being reported to highlight their effectiveness for treating hyperglycaemia. The present review could be an interesting paper for researchers who are working in the field of using plant extract-loaded nanoparticles as antidiabetic therapies
- antidiabetic
- plant extract
- nanocarriers
1. Introduction
2. Natural Active Agents in Nanocarriers
Since ancient times, many plant-based extracts and isolated bioactive compounds have been used across the world as therapeutic agents for the prevention and treatment of diseases and ailments [13]. Any plant-based products that are used to preserve or recover health are classified as herbal medicines [14]. About 200 years ago, herbal medicines dominated most medicinal practices. The use of herbal medicines started to decline in the 1960s, especially in the Western world, due to the introduction of allopathic medicines [15]. However, herbal medicines have been regaining public interest and have been slowly becoming more popular for various reasons, including the claims regarding the effectiveness of herbal medicines, changes in consumer preferences for natural medicines, the high costs and adverse effects of modern medicines and improvements in herbal medicines with the development of science and technology [16]. Research into identifying the chemical compounds from medicinal plants and their common uses may lead to new innovative drugs with fewer adverse effects than existing drugs [17]. It has been reported that medicinal plants, such as bitter melon (Momordica charantia), ivy gourd (Coccinia grandis) and ginseng, can be used to treat diabetes and more than 400 plant species with hypoglycaemic activity have been identified [18][19][18,19]; a few of these plants are listed in Table 1. The development of new antidiabetic drugs from plants has been attracting more attention as plants contain bioactive constituents that could have positive effects on the treatment of diabetes mellitus.| Plant Part | Scientific Name | Common Name | Antidiabetic and Other Biological Activities | Ref. |
|---|---|---|---|---|
| Bark | Pterocarpus marsupium | Indian kino tree | Antidiabetic and hypoglycaemic | [20] |
| Fruit | Momordica charantia | Bitter melon | Antidiabetic and hypoglycaemic | [21] |
| Leaf | Gymnema sylvestre | Gurmar | Hypoglycaemic and hypolipidemic | [22] |
| Catharanthus roseus | Nayantara | Hypoglycaemic | [23] | |
| Azadirachta indica | Neem | Hypoglycaemic | [23] | |
| Ocimum sanctum | Holy basil | Antidiabetic and hypoglycaemic | [20] | |
| Aloe vera | Aloe vera | Antidiabetic, antihypercholesterolemic and antioxidative | [24] | |
| Rhizome | Curcuma longa | Turmeric | Antidiabetic, antioxidant and anticholinesterase | [25] |
| Seed | Allium sativum | Garlic | Hypoglycaemic | [23] |
| Trigonella foenum-graecum | Fenugreek | Antidiabetic and hypoglycaemic | [20] | |
| Stem | Tinospora cordifolia | Giloy | Hypoglycaemic | [26] |
3. Types of Nanocarriers for Plant-Based Antidiabetic Extracts/Active Agents
Nanocarriers have sizes of between 1 and 100 nm and have been used as transporters to deliver active agents to target sites [34]. Nanocarriers are becoming more popular than conventional drug delivery systems due to their effectiveness, stability, improved drug bioavailability, target specificity and ability for the sustained release of the drug [35]. Furthermore, nanocarriers can carry various drugs with various biological properties. Many types of nanocarriers are available that can encapsulate natural compounds, as shown in Figure 3. However, this review only focuses on certain types of nanocarriers that have been studied for use with plant-based antidiabetic agents.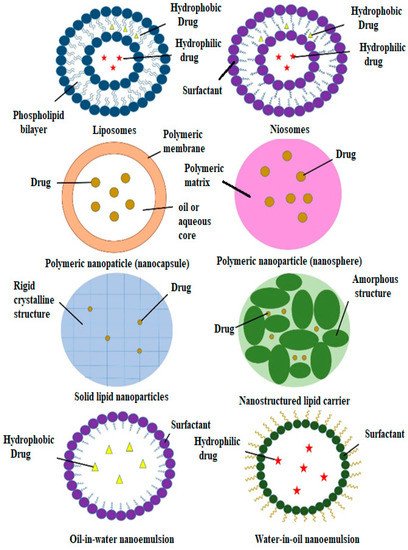
3.1. Liposomes
In 1965, phospholipid molecules were found to self-assemble by forming closed bilayer vesicles in water and were later termed liposomes [36][37][36,37]. Soon after that, liposomes were extensively studied for their potential as drug carriers through various administrative routes, such as parenteral, oral, pulmonary, nasal and transdermal routes [38]. Liposomes have an aqueous compartment that is enclosed by one or more cell-like lipid bilayers, which makes them suitable for cellular investigations. They also have essential cellular functions, such as motility and shape changes and the ability to impersonate the biophysical properties of living cells (Figure 2) [39][40][41][39,40,41]. Phospholipids are amphipathic molecules with water-loving (hydrophilic) and fat-loving (hydrophobic) parts [42]. The hydrophobic parts, which are the tails of the phospholipids, are repulsed by water molecules, which results in the self-assembly of liposomes through hydrophilic interactions, van der Waals interactions and hydrogen bonding interactions [28][43][28,43]. Due to the amphipathic properties of phospholipids, liposomes can encapsulate hydrophilic, hydrophobic and amphiphilic drugs [39][44][39,44]. Hydrophilic drugs are encapsulated in the aqueous part and hydrophobic drugs are encapsulated in the bilayer membrane between the tails of the phospholipids. In contrast, amphiphilic drugs are partitioned at the surface of the bilayers. The different positions of the drugs are due to their affinity to the different parts of the liposomes [44]. As well as the ability to control and sustain the release of drugs at specific sites, the liposomal membrane can also protect the encapsulated drugs from light, moisture and oxygen [45]. Liposomes can improve the physicochemical properties and onset time of the incorporated compounds and decrease their toxicity [46][47][46,47]. Liposomes fulfil the requirements of suitable drug carriers as they are biodegradable, biocompatible and stable in colloidal solutions [37]. When determining the final liposome structures, several crucial factors need to be considered: type and amount of phospholipid; the charge properties of the aqueous solution; hydration time; and the use of mechanical procedures and organic solvents [48]. As oral drug delivery systems, liposomes face some challenges that limit their potential as drug vehicles. Liposomes are vulnerable to the combined effects of the gastric acid, bile salts and pancreatic lipases within the gastrointestinal system [49]. Liposomes also have poor permeability to pass through the intestinal epithelia due to the relatively large size of their particles and the various epithelial barriers. Furthermore, it is difficult to mass-produce liposomes because of inconsistencies between batches [38]. The most important limitation of the use of liposomes as nanocarriers is their inability to retain active agents for prolonged periods compared to polymeric system nanocarriers [50]. The modulation of lipid compositions, surface coating, the addition of absorption enhancers and interior thickening have been carried out to develop better liposomes with special biological effects that could improve their application [38][51][52][53][54][55][38,51,52,53,54,55]. In addition, new apparatus and methods have been developed to overcome the mass production limitations, such as the continuous high-pressure extrusion apparatus and the high-speed dispersion method [56][57][56,57]. All new apparatus and methods need to be suitable for all routes of administration. The interactions between liposomes and cells depend on the composition, surface properties and type of liposomes and those of the interacting cells. Regarding these factors, liposomes are either endocytosed by the cells, adsorbed onto the cell surface or fused with the cell membrane. Some research has been conducted to study the potential of the use of liposomes as nanocarriers of plant-based antidiabetic agents (Table 2). A recent study was carried out by Amjadi et al. (2019) to encapsulate betanin in a nanoliposome. Betanin is a bioactive compound that can be found in many plants, such as red beetroot (Beta vulgaris), amaranth (Amaranthaceae) and pitahayas (Hylocereus undatus) [58]. Betanin exhibits anti-inflammatory, anticarcinogenic and antidiabetic properties [59][60][61][59,60,61]. However, betanin shows less than 1% bioavailability through oral administration due to its insufficient oral absorption and fast degradation from its high reactivity under various conditions, such as temperature, pH and oxygen [62][63][64][62,63,64]. Betanin-incorporated lecithin nanoliposomes are prepared using the thin film hydration method to improve the therapeutic potency of the betanin [65]. These betanin-incorporated nanoliposomes have shown an excellent sustained release property in simulated gastrointestinal fluids (pH 1.2–7.4). This nanoformulation of betanin has exhibited a superior ability to free betanin in the treatment of hyperglycaemia, hyperlipidaemia and oxidative stress in streptozotocin-induced diabetic rats. This formulation has also shown therapeutic potential to reduce hyperglycaemia-induced tissue damage in the kidney, liver and pancreas. In a study that was performed by Bulboacă et al. (2019) [66], the same thin film hydration method was applied for liposome formulation using 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and PEG-2000-DSPE. Curcumin-loaded liposomes have shown better results for lowering plasma glucose concentrations compared to free curcumin. Furthermore, liposomal curcumin has also shown potential as a part of the treatment for reducing the risks of vascular diseases that are caused by diabetes by reducing the level of metalloproteinases. Metalloproteinase expression is always related to cell apoptosis in many pathologies. Hyperglycaemia is believed to induce the production of metalloproteinases, which can increase the risks of vascular complications [67][68][67,68]. Combined herbal extracts can also be used within a single nanoliposomal formulation to improve its therapeutic efficacy. For example, Gauttama and Kalia (2013) combined three extracts (bitter gourd (Momordica charantia) fruit extract, ashwagandha (Withania somnifera) root extract and fenugreek (Trigonella foenum-graecum seed extract) to form a polyherbal antidiabetic that was incorporated into liposomes [69]. These three plants have been scientifically proven to have antidiabetic properties. The vesicle system was developed using phosphatidylcholine and cholesterol. The polyherbal-encapsulated liposomes had improved antidiabetic efficiency compared to the free polyherbal formulation in streptozotocin-induced diabetic rats. The impact of the polyherbal-encapsulated liposomes on lowering blood glucose levels was similar to that of metformin.| Type of Nanocarrier | Formulation (Ratio) | Active Compound | Model | Size Range (nm) | Remark | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Liposomes | Lecithin | Betanin | Streptozotocin-induced diabetic rats | 40.06 ± 6.21 | Increased hypoglycaemic activity; antihyperlipidemic activity; decreased oxidative stress | [65] | ||
| DPPC, PEG-2000-DSPE and cholesterol (9.5:0.5:1) | Curcumin | Streptozotocin-induced diabetic rats | 140 | Increased hypoglycaemic activity; hepatoprotective effects; decreased oxidative stress | [66] | |||
| Phosphatidylcholine and cholesterol (8:2) | Momordica charantia, Trigonella foenum-graecum | and | Withania somnifera | extracts | Albino Wistar rats | 1176 ± 5.6 | Increased hypoglycaemic activity; antihyperlipidemic activity | [69] |
| Niosomes | Span 60 and cholesterol (1:1) | Lycopene | Alloxan-induced diabetic rats | 202 ± 41 | Increased hypoglycaemic activity; antihyperlipidemic activity | [70] | ||
| Span 60, phospholipid 90G and cholesterol (9:4:1) | Embelin | Streptozotocin-induced diabetic rats | 609–734 | Increased hypoglycaemic activity; antioxidant efficacy | [71] | |||
| Span 40 and cholesterol (1:2) | Gymnema sylvestre | extract | Alloxan-induced diabetic rats | 229.5 ± 30 | Increased hypoglycaemic activity | [72] | ||
| Polymeric Nanoparticles |
Poly-(ε-caprolactone) (PCL) and PLGA-PEG-COOH | Fisetin | In vitro assays | 140–200 | Better α-glucosidase inhibition than acarbose; scavenging capacity | [73] | ||
| Eudragit RS100 | Phoenix dactylifera | seed oil | In vitro assays | 207 | α-amylase and α-glucosidase inhibition | [74] | ||
| Chitosan | Curcumin | In vitro assays | 74 | Increased GLUT-4 levels | [75] | |||
| Chitosan and alginate (3:1) | Naringenin | Streptozotocin-induced diabetic rats | 150–300 | Increased hypoglycaemic activity | [76] | |||
| Chitosan and alginate (1:3) | Quercetin | Streptozotocin-induced diabetic rats | 91.58 | Increased hypoglycaemic activity | [77] | |||
| Chitosan and gum arabic | Glycyrrhizin | Streptozotocin-induced diabetic rats | 165.3 | Increased hypoglycaemic activity | ||||
| Chitosan and tripolyphosphate (4:1) | Ferulic acid | Streptozotocin-induced diabetic rats | 51.2 ± 1.7 | Increased hypoglycaemic activity; increased body weight | [78] | |||
| Chitosan, gum Arabic and Tween 60 | Glycyrrhizin | Streptozotocin- and nicotinamide-induced diabetic rats | 181.4 | Increased hypoglycaemic activity; reduced body weight and lipid levels | [79] | |||
| Polyvinyl alcohol (PVA), Tween 80, gum-rosin polymer and oleic acid | Thymoquinone | Streptozotocin- and nicotinamide-induced diabetic rats | 70.21 | Increased hypoglycaemic activity; reduced body weight and lipid levels | [80] | |||
| Gum rosin, PVA and lecithin | Thymoquinone | Streptozotocin-induced diabetic rats | 36.83 ± 0.32 | Increased hypoglycaemic activity | [81] | |||
| PLGA | Quercetin | Streptozotocin-induced diabetic rats | 179.9 ± 11.2 | Increased hypoglycaemic activity; increased levels of catalase and superoxide dismutase | [82] | |||
| PLGA | Pelargonidin | Streptozotocin-induced diabetic rats | 91.47 ± 2.89 | Increased hypoglycaemic activity; antihyperlipidemic activity | [83] | |||
| PLGA, Pluronic F-127 and chitosan | Silybin | Streptozotocin-induced diabetic rats | 184.6 | Increased hypoglycaemic activity | [84] | |||
| PLGA and PVA | Ethyl acetate | In vitro assays | 365.7 | α-amylase and α-glucosidase inhibition | [85] | |||
| Tween 20 and propylene glycol | Foeniculum vulgare | Mill. essential oil | Streptozotocin-induced diabetic rats | 44–105 | Increased hypoglycaemic activity | [86] | ||
| Nanoemulsions | Tween 20 and polyethylene (PEG) 400 | Ipomoea reptans | extract | - | 15.5 ± 0.8 | - | [87] | |
| Lecithin | Resveratrol | Streptozotocin + nicotinamide-induced diabetic rats | 248 | Increased hypoglycaemic activity; prevention of weight loss | [88] | |||
| Solid Lipid Nanoparticles | Compritol, Tween 80 and Span 20 | Myricitrin | Streptozotocin + nicotinamide-induced diabetic rats | 76.1 | Increased hypoglycaemic activity; antioxidant and anti-apoptotic effects | [89] | ||
| Glycerol tripalmitate and soybean phospholipid | Berberine | Male rats | 76.8 | Increased hypoglycaemic activity; prevention of weight gain | [90] | |||
| Nanostructured Lipid Carriers | Precirol and miglyol (5:2) | Baicalin | Streptozotocin-induced diabetic rats | 92 ± 3.1 | Increased hypoglycaemic activity | [91] |
