Polycyclic aromatic hydrocarbons (PAHs) comprise a group of chemical compounds consisting of two or more fused benzene rings. PAHs exhibit hydrophobicity and low water solubility, while some of their members are toxic substances resistant to degradation. Due to their low levels in environmental matrices, a preconcentration step is usually required for their determination. Nowadays, there is a wide variety of sample preparation techniques, including micro-extraction techniques (e.g., solid-phase microextraction and liquid phase microextraction) and miniaturized extraction techniques (e.g., dispersive solid-phase extraction, magnetic solid-phase extraction, stir bar sorptive extraction, fabric phase sorptive extraction etc.).
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a large group of chemical compounds composed of two or more fused benzene rings
[1]. PAHs are hydrophobic compounds with low water solubility, and their solubility in water and volatility decrease with an increase in their molecular weight
[2]. PAHs consisting of up to four rings are known as light PAHs, while PAHs that are made of more than four rings are known as heavy PAHs. Heavy PAHs are more stable and more toxic than the light PAHs. These chemical compounds are widespread environmental contaminants that are considered byproducts of the incomplete combustion of organic materials, such as coal, gas, garbage, meat, oil, tobacco and wood, during natural or anthropogenic processes
[1][3][1,3]. PAHs are toxic substances, which are resistant to degradation and exposure to them may increase the risk of cancer
[4]. As a result, PAHs are considered by US Environmental Protection Agency (EPA) and the European Environmental Agency to be priority pollutants
[1]. Therefore, the determination of PAHs in environmental samples is of high importance. Since PAHs exist in traces in environmental matrices, a preconcentration technique is normally required.
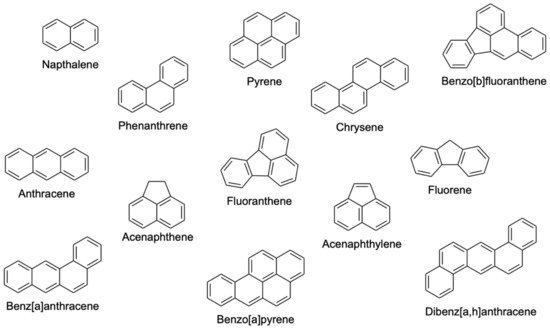
Figure 1 shows the chemical structures of common PAHs.
Figure 1.
Chemical structures of common polycyclic aromatic hydrocarbons (PAHs)
.
Currently, the most widely used methods for analyzing these pollutants in environmental matrices are gas chromatography (GC), high performance liquid chromatography (HPLC) and ultra-high pressure liquid chromatography (UHPLC)
[3][5][3,5]. Various detection systems, including ultraviolet detectors (UV)
[6], diode array detectors (DAD)
[3], fluorescence detectors (FLD)
[1], mass detectors (MS) coupled with HPLC and UHPLC and flame ionization detectors (FID)
[7], MS detectors
[5] and tandem MS detectors (MS/MS)
[8] coupled with GC have been used. Due to the enhanced sensitivity in the determination of PAHs that results in lower LODs, mass detectors and tandem MS detectors are generally preferred.
Solid-phase extraction (SPE) and liquid–liquid extraction (LLE) are two major sample preparation techniques that have been widely used for the extraction and preconcentration of a wide variety of analytes from environmental samples. However, both conventional techniques tend to have many fundamental drawbacks, since they include complicated, time-consuming steps, while they require large amounts of sample and organic solvents. Moreover, in both techniques there are difficulties in automation
[9][10][9,10].
In order to overcome these drawbacks, different microextraction techniques have been proposed as an efficient alternative to classical extraction techniques, since the introduction of solid-phase microextraction (SPME) by the research group of Pawliszyn
[11]. Liquid-phase microextraction (LPME) was introduced a few years later by Liu and Dasgupta, by using organic droplets suspended from the tip of a microsyringe
[12]. Those microextraction techniques are widely used today, and they offer certain benefits compared to the conventional sample preparation techniques. Microextraction techniques require a significantly lower sample amount, number of extraction steps, sample preparation time and organic solvent consumption, and they comply with Green Analytical Chemistry principles
[10][13][10,13].
Typical examples of miniaturized sample preparation techniques include dispersive solid phase extraction (d-SPE)
[14], magnetic solid-phase extraction (MSPE)
[15], pipette tip solid-phase extraction (PT-SPE)
[16], fabric phase sorptive extraction (FPSE)
[17], stir bar sorptive extraction (SBSE)
[18] etc. In recent years, a wide variety of novel sorbents, including molecular imprinted polymers (MIPs), graphene, graphene oxide (GO), carbon nanotubes (CNTs), metal organic frameworks (MOFs), covalent organic frameworks (COFs) and zeolitic imidazole frameworks (ZIFs) have been successfully coupled with miniaturized extraction techniques and microextraction techniques
[14][19][20][21][14,19,20,21].
2. Extraction of PAHs from Environmental Matrices
A plethora of novel sample preparation techniques have been employed for the extraction of PAHs.
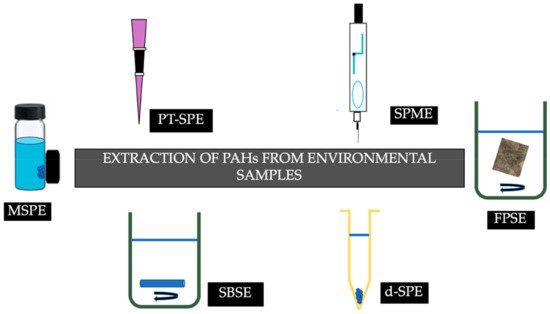
Figure 2 summarizes the recent advances in sorptive extraction techniques that have been employed for the determination of PAHs from environmental samples.
Figure 2.
Recent advances in sorptive extraction techniques for the determination of PAHs from environmental samples.
2.1. Dispersive Solid-Phase Extraction of PAHs from Environmental Matrices
2.1. Dispersive Solid-Phase Extraction of PAHs from Environmental Matrices.
Dispersive solid-phase extraction (d-SPE), is a form of SPE in which the desired sorbent is added directly into the sample aqueous solution followed by dispersion. This technique is taking advantage of the contact between the adsorbent and the target analytes. Once the extraction process is completed, the sorbent with the adsorbed analytes is separated from the sample by a mechanical process, such as centrifugation or filtration. Compared to the conventional SPE process, the main benefit of d-SPE is the reduction of sample preparation time, as well as its simplicity, adaptability and easy handling. A wide variety of sorbents have been utilized for the d-SPE of PAHs from environmental samples
[22][23][30,31].
This technique gained popularity when Anastassiades et al.
[24][32] introduced the QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe) approach for the determination of pesticide residue in food of plant origin. The initial method consists of acetonitrile extraction and addition of a mixture of salts, followed by a dispersive clean-up step with a primary–secondary amine (PSA) as extraction sorbent. QuEChERS was quickly applied for the determination of other analytes in a variety of sample matrices. Cvetkovic et al.
[25][33] developed a QuEChERS extraction procedure of PAHs in soil prior to their determination by GC-MS. The researchers evaluated different solvent systems (acetonitrile/water and hexane/water) and sorbents (PSA, C
18, Florisil, diatomaceous earth and clinoptilolite). Among the tested parameters, the best results were obtained with acetonitrile/water, as the extraction solvent and diatomaceous earth as the d-SPE extraction adsorbent.
Until today, a wide variety of novel extraction sorbents have been evaluated for the d-SPE of PAHs from environmental matrices. Among them, metal-organic frameworks and zeolitic imidazole frameworks are currently the most popular d-SPE adsorbents. MOFs became widely known in 1995, when Yaghi and Li
[26][34] reported the hydrothermal synthesis of a MOF material with large rectangular channels. Metal-organic frameworks are a class of hybrid organic-inorganic supramolecular materials, which are based on the coordination of metal ions or clusters with bi- or multidentate organic linkers. What makes MOFs materials so attractive is their unique properties, such as high surface areas (up to 14,600 m
2·g
−1)
[27][35], pore size tunability, structure flexibility, luminosity, thermal stability, charge transfer ability from the ligand to the metal or from the metal to the ligands, etc
[21][28][29][30][31][21,36,37,38,39]. As a result, MOFs have gained attention in a plethora of applications, such as gas storage and separation
[32][40], catalysis
[33][41], sensors
[34][42], detoxification
[35][43] and drug delivery
[36][44]. In analytical chemistry, MOFs have been evaluated as stationary phases for GC
[37][38][45,46] and HPLC
[39][40][47,48] analysis. However, today, the most popular field of applications of MOFs in analytical chemistry is sample preparation
[21][28][21,36].
Other d-SPE sorbents that have been applied for the extraction of PAHs from environmental samples include graphene/sepiolite
[41][53] and
N-acetyl-
l-cysteine modified CdS quantum dots
[42][54].
2.2. Magnetic Solid-Phase Extraction of PAHs from Environmental Matrices
2.2. Magnetic Solid-Phase Extraction of PAHs from Environmental Matrices.
Magnetic solid-phase extraction (MSPE) is a form of d-SPE in which a magnetic nanomaterial is added into an aqueous sample solution to adsorb the target analytes. After the adsorption of the analyte, an external magnetic field is applied to collect the sorbent and the supernatant solution is discarded. Subsequently, elution of the adsorbed analytes is achieved with the addition of an appropriate solvent, and magnetic separation is performed once again to collect the eluent, which is further analyzed by a suitable analytical technique. Compared to the conventional SPE procedure, in MSPE there is no need for sorbent packing into cartridges, thus avoiding limitations of column blocking and high pressure. Meanwhile, sample and organic solvent consumption is significantly decreased compared to the classic SPE and LLE formats. Finally, the sorbent separation with a magnet is a simple and rapid process, compared to the time-consuming centrifugation and filtration steps that are required in conventional d-SPE
[43][44][45][55,56,57].
Magnetic nanoparticles (MNPs) are characterized by the general formula MFe
2O
4 (M = Fe, Co, Cu, Mn, etc.), and they can be produced by a variety of methods, such as co-precipitation, solvothermal, hydrothermal etc. The most common magnetic nanoparticles that have been used in order to fabricate magnetic sorbents for MSPE are Fe
3O
4 nanoparticles. Iron oxides have been widely used in MSPE due to their super paramagnetism, their low toxicity, their high magnetic saturation, their simple preparation process and their low price
[7]. The application of other magnetic nanoparticles, such as MnFe
2O
4, have been also reported
[46][58]. However, the utilization of MNPs in sample preparation has some drawbacks, since their selectivity is low. Moreover, MNPs exhibit low stability in strong acidic aqueous media and low dispersion ability in many sample matrices. Therefore, surface modification of magnetic nanoparticles is usually required to enhance their stability and selectivity by the introduction of special functional groups
[7]. As seen in
Table 1, a wide variety of chemical compounds including carbon-based materials, polymeric materials, MOFs and other molecules have been employed for this purpose.
Table 1.
Application of different sorbents for the magnetic solid-phase extraction (MSPE) of PAHs from environmental samples.
|
Sorbent
|
Matrix
|
Analytical Technique
|
Sorbent Mass
(mg)
|
Time (min)
|
LODs
(ng L−1)
|
Extraction Recovery
(%)
|
Reusability
|
Ref.
|
|
HKUST-1
|
Water
|
UHPLC-FLD
|
5 Fe3O4/20 HKUST-1
|
10
|
0.8–12
|
39–59
|
NA
|
[47][59]
|
|
MIL-101(Cr)
|
Water
|
HPLC-PDA
|
1 Fe3O4@SiO2/0.6 MIL-101
|
20
|
2.8–27.2
|
NA
|
NA
|
[48][60]
|
|
Fe@MIL-101(Cr)
|
Water
|
HPLC-DAD
|
50
|
50
|
44–64
|
>80
|
At least 10 times
|
[49][61]
|
|
MIL-100(Fe)
|
Water
|
HPLC-FLD
|
10
|
10
|
32–2110
|
>80
|
NA
|
[50][62]
|
|
MIL-100(Fe)
|
Water
|
GC-FID
|
12.5
|
15
|
4.6–8.9
|
73–96
|
Up to 10 times
|
[51][63]
|
|
Fe3O4@ polydopamine/ZIF-7
|
Water, particulate matter
|
GC-MS
|
3 Fe3O4@PDA
15 ZIF-7
|
40
|
0.71–5.79
|
>82
|
At least 10 times
|
[52][64]
|
|
TpPa-1 COF
|
Water
|
HPLC-FLD
|
5
|
21
|
0.24–1.01
|
73–110
|
NA
|
[53][65]
|
|
COF-LZU1@PEI@Fe3O4
|
Water, soil
|
HPLC-FLD
|
5
|
33
|
0.2–20
|
NA
|
At least 6 times
|
[54][66]
|
|
G/CNF
|
Water
|
GC-FID
|
20
|
10
|
4–30
|
63.0–84.5
|
Up to 6 times
|
[55][67]
|
|
Fe3O4/C
|
Water
|
HPLC-FLD
|
50
|
30
|
|
102][134]. In the latter approach, the researchers developed a novel SPME technology, in which the features of heating the sample, cooling the sorbent and extraction under vacuum condition were combined
[102][134].
PAL (Prep And Load solution) SPME Arrow technique
[103][135] has been also evaluated for the extraction of PAHs from environmental samples. This technique is based on the use of a robust stainless-steel backbone, carrying the connection to the PAL sampler, the coating and an arrow-shaped tip for septum penetration. SPME Arrow combines the benefits of conventional SPME with the larger sorption phase volumes that are used in stir bar sorptive extraction (SBSE). At the same time, the disadvantages of both techniques include the difficulty in automation for SBSE, and the small volume of sorption phase, as well as the low robustness of classical SPME fibers. The results indicated that extraction efficiency significantly benefited from the larger sorption phase volume.
2.4. Stir Bar Sorptive Extraction (SBSE) and Stir Rod Sorptive Extraction (SRSE) of PAHs from Environmental Matrices
2.4. Stir Bar Sorptive Extraction (SBSE) and Stir Rod Sorptive Extraction (SRSE) of PAHs from Environmental Matrices.
Stir bar sorptive extraction was initially introduced by Baltussen et al. in 1999
[104][136]. In SBSE, a coated stir bar is placed into the vial together with the aqueous sample solution. Extraction of the target analytes takes place under rigorous stirring. When equilibrium is reached, the stir bar is removed and elution of the adsorbed analytes takes place either by the addition of an organic solvent or thermally
[18]. SBSE by nature is an equilibrium technique, and for water samples the extraction of the target analytes into the extraction medium is controlled by the partitioning coefficient of the solutes between the coating phase and the aqueous phase
[105][137]. Polydimethylsiloxane (PDMS) is the most used commercially available coating phase for stir bars, however, the synthesis and application of many novel coating materials has been reported.
PDMS coated SBSE bars have been successfully used for the extraction of PAHs resulting in good recoveries and low detection limits
[106][107][108][109][110][138,139,140,141,142]. Apart from the conventional PDMS stir bars, various novel coating materials have been evaluated for the extraction of PAHs from environmental samples. Typical examples are polymeric materials that have been evaluated, as stir bar coatings are polypyrrole and polyaniline copolymer (PPy-PAN)
[111][143] and (octyl methacrylate- ethylene dimethacrylate) copolymer
[112][144]. Poly (ethylene glycol)-grafted multi-walled carbon nanotubes have been also evaluated for the extraction of PAHs from environmental samples
[113][145]. In this case, the extraction efficiency was favored by the superior characteristics of MWCNTs and the ease in operation of the SBSE technique.
Automated stir plate sorptive extraction (SPSE) coupled with HPLC-FLD has been also evaluated for the extraction of PAHs. For this purpose, automatic extraction, desorption and sample loading, was controlled by a programmable flow injection system, and extraction of PAHs took place on the surface of a PDMS/β-cyclodextrin/divinylbenzene (PDMS/β-CD/DVB) coated plates. The researchers investigated three different operation modes, including static, circular flow and continuous flow SPSE. It was found that extraction efficiencies with continuous flow SPSE were slightly better than circular and manual SBSE, probably due to the continuous introduction of new sample solutions
[114][150].
2.5. Liquid-Phase Microextraction of PAHs from Environmental Matrices
2.5. Liquid-Phase Microextraction of PAHs from Environmental Matrices.
Liquid-phase microextraction (LPME) is a miniaturized version of classical LLE, which is characterized by minimum consumption of solvents. LPME can be divided into three main categories, the single-drop microextraction (SDME), the hollow fiber liquid-phase microextraction (HF-LPME) and the dispersive liquid-liquid microextraction (DLLME), with the latter being the most widely used LPME form
[115][151].
HF-LPME is usually based on the use of disposable propylene porous hollow fibers that are filled with a small amount of extracting solvent (acceptor phase). In order to extract the target analytes, the fibers are immersed into the aqueous sample solution (donor phase)
[115][116][151,152].
On the other hand, in single-drop microextraction (SDME), a drop acts as the acceptor phase for the extraction. SDME can be divided into two main categories i.e., the direct-immersion single-drop microextraction (DI-SDME), in which the drop is directly immersed into the sample, and the headspace single-drop microextraction (HS-SDME), in which the drop is suspending over the sample
[117][153].
DLLME is based on the initial fast injection of a suitable mixture of two solvents, an extraction solvent and a dispersive solvent, into an aqueous sample solution with the assistance of a syringe, followed by the formation of a cloudy solution that contains droplets of the extraction solvent dispersed into the sample. After phase separation due to the difference in density of the two phases (e.g., by centrifugation), the extraction phase can be removed and analyzed. In the conventional form of DLLME, the extraction phase is accumulated at the bottom of the extraction container
[115][118][151,154]. DLLME is considered to be simple, cheap and environmentally friendly, while it provides high enrichment. The proper selection of the extraction and dispersive solvents are two critical factors for the optimization of DLLME procedure. Therefore, the dispersive solvent has to be immiscible with the extraction solvent and the aqueous sample, in order to generate a cloudy solution that increases the interaction between the two phases, in order to increase the extraction efficiency
[119][155].
Finally, Fernandez et al.
[120][172] developed a lab on valve DLLME method for the extraction of PAHs, prior to their determination by HPLC-FLD. For this purpose, trichloroethylene was used as the extraction solvent, and acetonitrile was used as the dispersive solvent. The automated instrumentation simplified the extraction process and exhibited satisfactory enhancement factors (86–95).
Table 2 summarizes the applications of DLLME and USAEME in the extraction of PAHs from water samples. Even though DLLME is the predominant form of LPME that has been employed for the extraction of PAHs, the use of SDME
[121][122][173,174] and HF-LPME
[123][124][175,176] has been also evaluated.
Table 2. Applications of dispersive liquid-liquid microextraction (DLLME) and ultrasound-assisted emulsification microextraction (USAEME) in the extraction of PAHs from water samples.
|
Matrix
|
Analytical Technique
|
Extraction Solvent
|
Disperser Solvent
|
Phase Separation
|
LODs(ng·L−1)
|
EF
|
Extraction Recovery (%)
|
Ref.
|
|
Surface water
|
GC-MS
|
Tetrachloroethylene
|
Acetone
|
Centrifugation
|
7–30
|
603–1113
|
-
|
[125][156]
|
|
Rainwater
|
GC-MS
|
n-Hexane
|
Acetone
|
Addition of demulsification solvent
|
3.7–39.1
|
NA
|
-
|
[126][157]
|
|
River water
|
GC-FID
|
Toluene
|
Methanol
|
Air flotation
|
14–41 × 103
|
NA
|
-
|
[127][158]
|
|
Sea water
|
GC-MS
|
Tetrachloroethylene
|
Diethyl Ether
|
Centrifugation
|
1–10
|
722–8133
|
59.2–90.5
|
[128][159]
|
0.2–0.6 |
|
|
|
|
76–110 |
|
|
| At least 10 times |
|
| [ | 56 | ] | [68]
|
|
Hydrophilic Fe3O4/C
|
|
Sediment
|
HPLC-FLD
|
Dichloromethane
|
Acetonitrile
|
Centrifugation
|
2.3–6.8 ng g−1
|
NA
|
>94.0
|
[129][160]
|
|
Tap, sea and spring water
|
GC-FID
|
Toluene
|
-
|
Centrifugation
|
20–50
|
1776–2714
|
99–103
|
[130][161]
|
|
Tap, well, surface water etc.
|
GC-MS
|
Chloroform
|
-
|
Centrifugation
|
1–36
|
NA
|
-
|
[131][162]
|
|
Tap, spring, surface water etc.
|
GC-MS
|
Iso-octane
|
-
|
Addition of NaCl
|
0.001–0.009
|
Up to 100000
|
-
|
[132][163]
|
|
Tap, rain and wastewater
|
HPLC-FLD
|
Cyclohexane
|
-
|
Centrifugation
|
0.6–62.5
|
90–247
|
95–100
|
[133][164]
|
|
Well, river, lake water etc.
|
HPLC-FLD
|
TBAB/2-decanoic acid DES
|
-
|
Centrifugation/Solidification
|
0.7–6.6
|
163–198
|
>80.0
|
[134][167]
|
Water |
|
Tap, bottle, fountain water etc.
|
|
|
HPLC-FLD
GC-MS
|
[C8 MiM][PF6 ]
10
|
30 |
Acetone
|
|
Centrifugation
15–335
|
0.03–2
NA
|
301–346
NA
|
[5]
|
|
| - |
|
| [ | 135 | ] | [ | 169]
|
CNF
|
|
Tap, well, surface water etc. |
Water
|
GC-FID
|
10
|
|
HPLC-UV
12
|
[BBIM][Tf2N]
|
Acetone
|
Centrifugation8–30
|
|
2
NA
|
2768–5409
At least 10 times
|
|
-
[7]
|
|
| [ | 136 | ] | [ | 170 | ] |
|
G/Fe3O4@PT
|
|
|
Tap, rain and surface water
| Water
|
HPLC-FLD
GC-FID
|
Trichloroethylene
20
|
Acetonitrile
10
|
|
-
9–20
|
20–600
83–107
|
At least 17 times
|
[57][69]
|
|
GO
|
Water
|
HPLC-UV
|
40
|
16
|
90–190
|
76.8–103.2
|
NA
|
[6]
|
|
GO-Fe3O4@PS
|
Water
|
GC-FID
|
15
|
10
|
3–10
|
69.5–88.7
|
NA
|
[58][70]
|
|
Poly(Py-co-Ani)@GO-Fe3O4
|
Water
|
GC-FID
|
35
|
|
3–10
|
50.4‒78.3
|
At least 20 times
|
[59][71]
|
|
CNTs
|
Water
|
UHPLC-FLD
|
5
|
10
|
25–73
|
76.4–106.5
|
Up to 3 times
|
[60][72]
|
|
| 86–95 |
|
| - |
|
| [ | 120] |
mag-MIP
|
Water
|
HPLC-PDA
|
20
|
55
|
1.3–969
|
46–100
|
At least 3 times
|
[61][73]
|
[ | 172 | ] |
|
mag-MIP
|
Water
|
GC-MS
|
5–20
|
17
|
30–750
|
>76
|
NA
|
[62][74]
|
|
RAFT-MIP
|
Water
|
GC-MS
|
10
|
9
|
1–100
|
4.5–97
|
NA
|
[8]
|
|
PDA
|
Water
|
HPLC-FLD
|
20
|
5
|
0.5–1.9
|
76.4–107
|
NA
|
[63][75]
|
|
PPy
|
Water
|
GC-MS
|
20
|
3
|
0.38–5.01
|
27.4- 115.7
|
NA
|
[64][76]
|
|
PANI/Alginate
|
Water
|
HPLC-FLD
|
400
|
20
|
10
|
86.0–97.8
|
Up to 6 times
|
[65][77]
|
|
PoT
|
Water
|
GC-FID
|
60
|
15
|
0.3–5.5
|
NA
|
Up to 15 times
|
[46][58]
|
|
IL-MNPs
|
Water
|
GC-MS
|
30
|
8
|
40–1111
|
75–102
|
Up to 10 times
|
[66][78]
|
|
MNP@CN/IL
|
Leachate, sludge
|
HPLC-DAD
|
30
|
35
|
400–590
|
89.50–110.2
|
NA
|
[67][79]
|
|
MNP-PANI-DICAT
|
Water, sludge, soil
|
GC-MS
|
15
|
40
|
0.8–208.6
|
80.2–111.9
|
Up to 5 times
|
[68][80]
|
|
Fe3O4@IL@MO
|
Water
|
HPLC-FLD
|
18
|
26
|
0.1–2
|
NA
|
NA
|
[69][81]
|
|
Fe3O4@SiO2@Nap
|
Water
|
HPLC-FLD
|
40
|
12
|
0.04–0.12
|
>90
|
At least 10 times
|
[1]
|
|
PC
|
Water, milk
|
HPLC-FLD
|
100
|
10
|
0.2–0.6
|
>90
|
NA
|
[70][82]
|
|
Fe3O4-DVB-SO3-
|
Water
|
GC-MS
|
50
|
10
|
0.6–2.1
|
79.9–115.3
|
NA
|
[71][83]
|
|
MPNP
|
Water
|
UHPLC-DAD
|
200
|
15
|
10.83–18.53 nM
|
75.7–106.4
|
At least 5 times
|
[3]
|
|
Fe3O4/SiO2/TPA
|
Water
|
HPLC-FLD
|
50
|
15
|
0.04–37.5
|
NA
|
NA
|
[72][84]
|
|
C18
|
Water
|
GC-MS
|
50
|
6
|
0.8–36 × 103
|
35–99
|
NA
|
[73][85]
|
|
C10–C18 carboxylates
|
Water
|
HPLC-FLD
|
200
|
18
|
0.1–0.25
|
>90
|
Up to 5 times
|
[74][86]
|
|
n-octadecylphosphonic acid
|
Water
|
GC-MS
|
50
|
1
|
14.1–70.0 × 103
|
61.9–119.1
|
NA
|
[75][87]
|
|
Nylon 6
|
Water
|
HPLC-PDA
|
40
|
30
|
0.05–0.58 × 103
|
36.2–87.0
|
NA
|
[76][88]
|
|
CTAB
|
Water
|
UHPLC-FLD
|
100 Fe3O4/50 CTAB
|
30
|
0.4–10.3
|
59.23–87.95
|
NA
|
[77][89]
|
|
Palm fatty acid
|
Leachate, sludge
|
HPLC-DAD
|
15
|
25
|
10–50
|
>81.1
|
Up to 5 times
|
[78][90]
|
|
TBCD
|
Water
|
HPLC-FLD
|
80
|
15
|
0.03–1.2
|
>80
|
NA
|
[79][91]
|
|
TCT
|
Water, urine
|
HPLC-FLD
|
40
|
13
|
0.09–0.15
|
89–93
|
At least 30 times
|
[80][92]
|
|
C16-HO
|
Water
|
HPLC-UV
|
30
|
24
|
0.14–0.31
|
88–95
|
Up to 4 times
|
[81][93]
|
2.3. Solid-Phase Microextraction of PAHs from Environmental Matrices
2.3. Solid-Phase Microextraction of PAHs from Environmental Matrices.
Solid-phase microextraction is a sample preparation microextraction technique in which the analytes are directly extracted and preconcentrated at the outer coating of a fused-silica fiber
[82][104]. There are two approaches of SPME that can be used to extract analytes, the headspace (HS-SPME), where the fiber is exposed to the gas phase above the sample, and direct immersion (DI-SPME), where the fiber is directly immersed into the sample solution
[83][105]. After the extraction, desorption takes place either thermally in the injection port of a gas chromatograph or by the addition of an organic solvent
[82][83][104,105].
Until now, there are many different commercial SPME coated fibers, such as polydimethylsiloxane (PDMS), polydimethylsiloxane/divinylbenzene (PDMS/DVB), polyacrylate (PA), carboxen/polydimethylsiloxane(CAR/PDMS) and divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS)
[83][105]. However, most of them more or less have some disadvantages, such as low selectivity, ease of fiber breakage, a short lifetime and swelling in organic solvents. In order to overcome them, various new coatings have been prepared and evaluated
[84][106].
Ionic liquids and polymeric ionic liquids (PILs) have been successfully employed as SPME coatings, due to their simplicity of synthesis and their high tuneability, that enable the preparation of highly selective fibers
[85][107]. PILs are polymers prepared from IL monomers. Compared with conventional ILs, PILs exhibit a number of advantages when used as coatings in SPME. Generally, PILs often have solid nature and good thermal and mechanical strength, while extraction selectivity is similar with ILs. As a result, they have proved to be more stable coatings
[85][86][107,108]. Various PILs including poly(1-vinyl-3-hexadecylimidazolium) bis[(trifluoromethyl)sulfonyl]imide
[87][109], poly(1-4-vinylbenzyl)-3-hexadecylimidazolium bis[(trifluoromethyl)sulfonyl]imide
[88][110], poly (1-vinyl-3-octylimidazolium) 2-naphthalene-sulfonate
[89][111] and poly(1-(4-vinylbenzyl)-3-hexadecylimidazolium bis[(trifluoromethyl)sulfonyl]imide
[90][112] have been successfully used as coatings used for the SPME of PAHs. In most cases, the PIL was initially prepared and diluted in a volatile solvent (i.e., acetone or chloroform), and a bare fiber (usually made from stainless steel) was immersed in the solution, followed by slow removal and air drying to remove any excess of solvent that may contribute to high background signals in gas chromatography
[87][88][90][109,110,112]. However, in situ polymerization of the IL and creation of the SPME coating on the surface of a stainless steel wire has also been reported
[89][111].
Graphene
[91][92][113,114], graphene oxide
[93][94][95][115,116,117], MWCNTs
[96][97][118,119] and other carbon based materials
[98][120] have also been successfully employed as SPME sorbent coatings, either neat or combined with other materials in order to generate more efficient composites. These materials exhibit high chemical, thermal and mechanical stability, as well as great affinity towards PAHs. Additionally, due to their unique structures and sufficient surface areas, rapid extraction and desorption of the target analytes can be achieved
[85][107]. Various techniques for the preparation of the coated fibers including the chemical bonding
[91][93][95][113,115,117], electrophoretic deposition
[97][119] and sol-gel approaches
[98][120] have been also evaluated.
Examples of other materials that have been employed to fabricate SPME coatings for the extraction of PAHs include polyaminithiophenol (PATP) with Au coating
[99][131], poly(3,4-ethylenedioxythiophene)@gold nanoparticles
[100][132], crosslinked methyl methacrylate–polyhedral oligomeric silsesquioxane hybrid polymeric coating
[84][106], nanostructured octadecyl silica
[101][133] and polythiophene/carboxylic acid modified multi-walled carbon nanotube composite
[
2.6. Fabric Phase Sorptive Extraction of PAHs from Environmental Matrices
2.6. Fabric Phase Sorptive Extraction of PAHs from Environmental Matrices.
Fabric phase sorptive extraction (FPSE) is a novel sample preparation technique proposed by Kabir and Furton in 2014. FPSE utilizes a natural or synthetic fabric substrate, which is chemically coated in the form of ultra-thin coating with sol-gel organic-inorganic hybrid sorbent as the extraction medium. For the FPSE procedure, the sol-gel sorbent coated FPSE media is immersed into a mixture of appropriate solvents, to remove any undesirable impurities from the material, and rinsed with deionized water to remove residual organic solvents. Subsequently, the FPSE media is submerged into the sample solution placed in a glass vial. A magnet is added into the sample solution, and the sample is magnetically stirred for certain time span for the adsorption of the target analytes. Finally, the FPSE media is removed, and elution of the analytes takes place into another vial containing appropriate elution solvent. Analysis of the eluent can take place after centrifugation and or/filtration
[137][138][139][140][177,178,179,180].
FPSE provides high primary contact surface area, thus, rapid and efficient analyte extraction can be easily achieved. Moreover, FPSE is also characterized by low organic solvent consumption, ease in operation, reusability and good selectivity towards the target analytes, which is based directly on the different nature of the fabric substrates and the sol-gel coating. Until today, the use of various fabric substrates, including cellulose, fiber glass and polyester, as well as several sol-gel coatings, including polyethylene glycol, polytetrahydrofuran and polydimethyldiphenylsiloxane have been evaluated as adsorbents for a wide variety of analytes
[17][138][141][142][17,178,181,182].
A trace-level determination of selected PAHs in environmental water samples using FPSE prior to their determination by HPLC-FLD has been reported. For this purpose, a non-polar sol-gel C
18 coated FPSE media was prepared and conditioned in a mixture of methanol and acetonitrile for 5 min, and then rinsed with deionized water. The extraction of PAHs took place in a glass vial containing 10 mL of the aqueous sample solution, in which the sol-gel C
18 coated FPSE media was directly immersed. After 30 min under constant stirring at 1000 rpm, the FPSE media was removed and PAHs were eluted with acetonitrile under ultrasonic radiation for 5 min. The developed FPSE-HPLC-FLD protocol was proved to simple, efficient, fast, sensitive, green, economical and reliable for trace level determination of environmentally important PAHs
[143][183].
Recently, fabric-phase sorptive extraction was coupled with ion mobility spectrometry (IMS) for on-site rapid detection of PAHs in aquatic environment
[144][184]. Ion mobility spectrometry is a rapid and sensitive gas-phase analytical technique, which can be employed for the in the field testing of various chemical compounds, due to its fast analysis and compact size
[145][185]. For the fabrication of the FPSE media, PDMS was coated on the glass fiber cloth through a sol-gel reaction. Glass wool was chosen based on the inlet temperature of IMS, since the thermal desorption of the PAHs was performed after inserting the FPSE media the inlet port of the IMS instrument directly after analyte extraction. Under optimum conditions, low LODs and satisfactory recoveries were obtaining, thus enabling the on-site monitoring water quality.
2.7. Other Extraction Techniques for the Determination of PAHs in Environmental Matrices
2.7. Other Extraction Techniques for the Determination of PAHs in Environmental Matrices.
Yang et al.
[146][186] synthesized MOF-5 from terephthalic acid and zinc acetate, and evaluated it as a sorbent for the SPE of PAHs from environmental samples. Therefore, 300 mg of MOF-5 was packed into SPE cartridges. Although MOF-5 is known to be unstable when exposed to water, the researchers reported that the derived material of MOF-5 still demonstrated good extraction characteristics. This was attributed to the π–π conjugate effect between the terephthalic acid molecules of the framework and the PAHs and the π-complexation between PAHs and the central zinc ions. The analytes were further separated and detected in a HPLC-UV system. The method exhibited satisfactory extraction ability and low LODs (0.4–4.0 ng L
−1), however, no sorbent reusability was reported.
Hu et al.
[147][187] evaluated two zeolitic imidazolate frameworks for the SPE disk extraction of PAHs in aid of filter membrane of PAHs from environmental water samples. The studied ZIFs were both composed of the same metal ion (zinc) and organic linker (benzimidazole), thus differing in spatial structures with one in cube (ZIF-7), while the other was in rhombic dodecahedron (ZIF-11). ZIF-11 with markedly better extraction efficiencies due to its unique spatial structure with large cages and its molecular composition that was composed of abundant benzyl groups and metal sites on the surface.
ZIF-8
[148][188] has been also evaluated for the porous membrane-protected micro-solid-phase extraction (μ-SPE) of PAHs. For this purpose, the sorbent was packed in a sealed porous polypropylene membrane envelope. The novel extraction devices exhibited good extraction characteristics and decreased consumption of the organic solvent.
A molecularly imprinted polymer has been also applied for the SPE of 16 PAHs from seawater, prior to their determination by GC-MS
[149][189]. MIPs were prepared by using the 16 PAHs mixture through non-covalent polymerization as a template based on sol-gel surface imprinting. The developed sorbent exhibited good affinity towards the target analytes. Other examples of novel SPE sorbents that have been implemented for the extraction and preconcentration of PAHs from environmental samples include a cyclodextrin-silica microporous composite
[150][151][190,191], aminopropyl imidazole-modified silica gel
[152][192], tetraazacalix[2]arene[2]triazine
[153][193], titanium oxide nanotubes
[154][194] and a titanate nanotube array modified by cetyltrimethylammonium bromide
[155][195].
Krupadam et al.
[156][196] prepared MIP microspheres in a continuous segmented flow microfluidic reactor, and used them as packing material for microtraps for the selective extraction of benzo[a]pyrene from environmental water samples. For this purpose, the pumping of monodisperse droplets of acetonitrile containing methacrylic acid as the functional monomer took place, benzo[a]pyrene was used as a template, and ethylene glycol dimethacrylate as cross-linking monomer into the microchannels of the microfluidic reactor. The obtained microspheres exhibited high extraction efficiency and selectivity towards benzo[a]pyrene. In comparison with commercially available activated carbon, the novel microspheres showed 300% higher adsorption capacity.
A portable system for the in situ extraction of PAHs was proposed by Foan et al.
[157][197]. The researchers designed a microfluidic device for the fast extraction of PAHs using low volume samples. The work was performed on a lab-on-a-chip, made of a silicon/glass microfluidic device functionalized with PDMS. Among the benefits of the novel technique was the low organic solvent consumption and the portability. A comparison of the novel device with SBSE showed approximately 50 times less sample preparation time for the high molecular weight PAHs. However, for the lightest PAHs, the performance of the microchip required improvement.
Flow injection solid-phase extraction (FI-SPE) of PAHs from environmental samples with novel extraction sorbents has been also proposed by the research groups of Wu
[158][198] and Zhou
[159][199]. The first group used a multi-walled carbon nanotubes (MWCNTs)-packed micro-column for the extraction of PAHs, prior to their determination by GC-MS, while the second group prepared a copper(II) isonicotinate coordination polymer packed in a pre-column for the extraction of PAHs, prior to their determination by HPLC-DAD. Both methods exhibited good extraction characteristics. In the case of GC-MS detection, after the FI-SPE process, the eluates were collected, and manual injection was performed, while for the HPLC-DAD analysis elution of the adsorbed analytes was also performed on-line in the backflush mode by the HPLC mobile phase directly into the chromatographic column, thus minimizing the required analysis steps.
In-syringe solid-phase extraction of PAHs has been also proposed for the on-site sampling of water samples. In-syringe SPE is characterized by portability, simplicity in use, low cost and short extraction time. Zhang et al.
[160][200] evaluated the application of MIL-101 as a novel sorbent, due to its good thermal and mechanical stability, as well as its resistance towards organic solvent and waters. The proposed technique exhibited excellent adsorption performance, since the analytes could be completely adsorbed during one adsorption cycle, thus reducing the extraction time. Moreover, it was found that the adsorbed analytes remained stable on the in-syringe device for at least 7 days.