Single-targeted chimeric antigen receptor (CAR) T cells tremendously improve outcomes for patients with relapsed/refractory hematological malignancies and are considered a breakthrough therapy. However, over half of treated patients experience relapse or refractory disease, with antigen escape being one of the main contributing mechanisms. Dual-targeting CAR T-cell therapy is being developed to minimize the risk of relapse or refractory disease. Preclinical and clinical data on five categories of dual-targeting CAR T-cell therapies and approximately fifty studies were summarized to offer insights and support the development of dual-targeting CAR T-cell therapy for hematological malignancies. The clinical efficacy (durability and survival) is validated and the safety profiles of dual-targeting CAR T-cell therapy are acceptable, although there is still room for improvement in the bispecific CAR structure. It is one of the best approaches to optimize the bispecific CAR structure by boosting T-cell transduction efficiency and leveraging evidence from preclinical activity and clinical efficacy.
- CAR T-cell therapy
- antigen escape
- dual-targeting
- bispecific CAR
- hematological malignancies
1. Introduction
2. Common Dual CAR Strategies
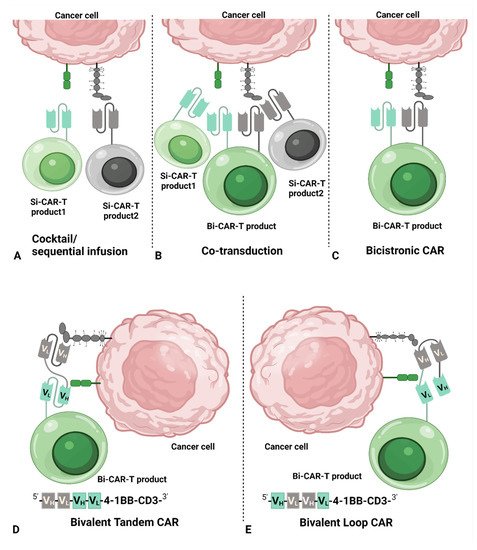
3. Dual-Targeting CAR T-Cell Therapy
The advantage of dual-targeting CAR T-cell therapy over Si-CAR-T-cell therapy is its ability to decrease antigen escaping of tumor cells. Clinical studies of Si-CAR T-cell therapy have already shown >90% complete response (CR) [29][30], leaving little room for improvement in terms of the initial response to dual-targeting CAR T-cell therapy. Therefore, the expectation for dual-targeting CAR T-cell therapy is not only to improve the durability of the response but also to reinduce the response in patients who relapsed or were refractory after treatments with Si-CAR T-cell therapy. Table 1 Tables 3 and Table 2 4 provide data questioning whether dual-targeting CAR T-cell therapy can override Si-CAR T-cell therapy in durability and long-term clinical benefit, e.g., longer duration of response (DOR) and overall survival (OS). It seems that dual-targeting CAR T-cell therapy has demonstrated better DOR and OS than Si-CAR T-cell in a small number of studies. However, there were no head-to-head studies and, therefore, the conclusions should be interpreted with caution due to differences, such as disposition of patients and supportive care between studies. Similar results were found when comparing the data from different studies. In ALL, 6-month RFS and OS were similar between CD19 Si-CAR T-cell product tisagenlecleucel [3] and CD19/CD22 Bi-CAR T-cell therapy [31]. Likewise, the 12-month PFS for NHL patients was close among tisagenlecleucel in diffuse large B-cell lymphoma [24], brexucabtagene autoleucel in mantle-cell lymphoma [4], and CD19/CD20 Tandem Bi-CAR T-cell therapy in B-cell lymphoma [23]; however, the comparison should be viewed with caution among different clinical entities. In particular, in one trial with a head-to-head comparison of CD19 Si-CAR T cells with CD19/CD22 Bi-CAR T cells, the median leukemia-free survival (LFS) in patients without hematopoietic stem cell transplantation (HSCT) after CAR T-cell treatment was 2 months for CD19 Si-CAR T cell treatment, while LFS was 3 months for CD19/CD22 Bi-CAR T cell treatment, demonstrating a better DOR of Bi-CAR T-cell therapy [29].
| Ref.: First Author | Target | CAR Strategy | Sample Size (CR Patients) | Durability | OS (mon and %) | In Vivo Expansion |
|---|---|---|---|---|---|---|
| Locke [39] | CD19 | One Si-CAR-T product | 7 | 3 ongoing CR at 12+mon | NA | Median Tmax and Mean/Median Cmax NA |
| Locke [40] | CD19 | One Si-CAR-T product | 108 | 11.1 mon (Median DOR), 44% (12-mon PFS) |
59% (12-mon OS) | Median Tmax and Mean/Median Cmax NA |
| Schuster [24][41] | CD19 | One Si-CAR-T product | 93–99 | Median DOR NR (10 mon-NR), 66% (12-mon PFS) |
49% (12-mon OS) | Median Tmax and Mean/Median Cmax NA |
| Jacobson [42] | CD19 | One Si-CAR-T product | 109 | 65.6% (18-mon PFS) | 87.4% (18-mon OS) | Median Tmax: 9 days Median Cmax NA |
| Abramson [1] | CD19 | One Si-CAR-T product | 269 | 6.8 mon (PFS), 51.4% (6-mon PFS), 44.1% (12-mon PFS); Median DOR NR (8.6-NR) |
74.7% (6-mon OS), 57.9% (12-mon OS) | Median Tmax: 12 days Median Cmax: 23,928.2 copies/μg gDNA |
| Wang [4] | CD19 | One Si-CAR-T product | 60 | 61% (12-mon PFS) | 83% (12-mon OS) | Median Tmax: 15 days Median Cmax NA |
| Zhang [43] | CD20 | One Si-CAR-T product | 11 | >6 mon (PFS), 1 CR for 27 mons | NA | Median Tmax: ∼28 days Median Cmax NA |
| Tong [23] | CD19/CD20 | One Tandem Bi-CAR-T product | 27 | 79% (6-mon PFS), 64% (12-mon PFS) |
82% (6-mon OS), 71% (12-mon OS) |
Mean Cmax: 496 CAR-T/μL Median Tmax: NA |
| Shah [25] | CD19/CD20 | One Tandem Bi-CAR-T product | 22 | 12 CR > 6 mon; 6 CR > 12 mon; 8 CR ongoing | NA | Median Tmax and Mean/Median Cmax NA |
| Tholouli [44] | CD19/CD22 | One Bicistronic Bi-CAR-T product | 35 | 4 CR > 10 mon; 4 CR > 5 mon. | NA | Median Tmax and Mean/Median Cmax NA |
| Wang [16] | CD19/CD22 | Cocktail/Sequential infusion of two Si-CAR products | 36 | 9.9 mon (median PFS) 50.0% (12-mon PFS) |
18.0 mon (median OS) 55.3% (12-mon OS) |
Median Tmax and Mean/Median Cmax NA |
| Zhang [45] | CD19/CD22 | One Loop Bi-CAR-T product | 32 | 40.0% (12-mon PFS) 66.7% (12-mon PFS in CR at 3 mon) |
63.3% (12-mon OS) 100% (12-mon OS in CR at 3 mon) |
Median Tmax: 12 days Geometric mean Cmax: 286,294.4 copies/μg DNA |
| Spiegel [37] | CD19/CD22 | One Loop Bi-CAR-T product | 21 | 3.2 mon (median PFS) | 22.5 mon (median OS) | Cmax: 36 CAR-T/μL 1794 copies/50 ng gDNA |
| Ref.: First Author | Target | CAR Strategy | Sample Size (CR Patients) | Durability | OS (mon and %) | In Vivo Expansion |
| Maude [30] | CD19 | One Si-CAR-T product | 30 (27 CR) | NA | 78% (6-mon OS) | Median Cmax: 39.8% Cmax: >5000 copies/μg gDNA (>15,000 copies/μg gDNA in 26 pts) |
| Maude [3] | CD19 | One Si-CAR-T product | 75 (61 CR) | 73% (6-mon RFS), 50% (12-mon RFS) |
19.1 mon (median OS), 90% (6-mon OS), 76% (12-mon OS) |
Median Tmax: 10 days Cmax: NA |
| Grupp [32] | CD19 | One Si-CAR-T product | 79 (65 CR) | 66% (18-mon PFS); Responses were ongoing in 29 pts (max DOR, 29 mon and ongoing) |
70% (18-mon OS) | NA |
| Shah [33] | CD22 | One Si-CAR-T product | 56 (40 CR) | 31.6 mon (EFS), 6 mon (RFS in CR), 11 remain in remission with a median f/u of 9.7 mon |
13.4 mon (median OS) | Tmax: days 14∼21 Median Cmax: 77% CAR+T cells; 480.5 CAR-T/μL |
| Wang [29] | CD19 | One Si-CAR-T product | 35 (31 CR) | ∼2 mon (median LFS in 19 non-HSCT pts) | ∼12 mon (median OS in all pts) | Tmax: day 10.5 Median Cmax: 590.4 CAR-T/μL |
| Wang [29] | CD19/CD22 | One Tandem Bi-CAR-T product | 15 (13 CR) | ∼3 mon (median LFS in 13 non-HSCT pts) | ∼21 mon (median OS in all pts) | Tmax: day 9 Median Cmax: 448.2 CAR-T/μL |
| Wang [16] | CD19/CD22 | Cocktail/Sequential infusion of two Si-CAR-T products | 51 (48 CR) | 52.9% (12-mon PFS) 13.6 mon (median PFS) |
62.8% (12-mon OS) 31 mon (median OS) |
Median Tmax and Mean/Median Cmax NA |
| Pan [15] | CD19/CD22 | Cocktail/Sequential infusion of two Si-CAR-T products | 20 (20 CR) | 79.5% (12-mon LFS) | 92.3% (12-mon OS) | Median Tmax and Mean/Median Cmax NA |
| Schultz [34] | CD19/CD22 | One Bivalent Bi-CAR-T product | 12 (10 CR) | NA | 92% (9.5-mon median f/u) | Median Cmax: 11.13% (Dose Level 1) and 29.1% (Dose Level 2) |
| Dai [35] | CD19/CD22 | One Tandem Bi-CAR-T product | 6 (6 CR) | ≥ 5 mon (RFS in 5 CR, 3 ongo-ing > 8 mon, 1 relapse after 3 mon) | NA | Median Tmax and Mean/Median Cmax NA |
| Yang [36] | CD19/CD22 | One Loop Bi-CAR-T product | 16 (>6/7 CR) | 3 mon (median observed time without relapse) | NA | Median Cmax: 109,000 copies/μg gDNA |
| Tang [31] | CD19/CD22 | One Tandem Bi-CAR-T product | 22 (22 CR) | 76.9% (6-mon RFS), 67.3% (12-mon RFS) |
94.4% (6-mon OS), 57.2% (12-mon OS) | NA |
| Spiegel [37] | CD19/CD22 | One Loop Bi-CAR-T product | 17 (15 CR) | 5.8 mon (PFS) | 11.8 mon (median OS) | Median Cmax: 36 CAR-T/μL 1794 copies/50 ng gDNA Tmax: days 10–14 |
| Cordoba [38] | CD19/CD22 | One Bicistronic Bi-CAR-T product | 15 (13 CR) | 48% (6-mon EFS), 32% (12-mon EFS) |
80% (6-mon OS), 60% (12-mon OS) |
Cmax > 30,000 copies/μg DNA Median Tmax: 12 days |
| T |
| max |
| : days 10–14 |
Strategies involving CD19/CD20 or CD19/CD22 to design Bi-CAR T-cell therapy are based on the hypothesis that targeting CD20 or CD22 would benefit patients with a loss or reduction in CD19 to overcome antigen escape. After careful extraction of data from published clinical trials, details of CD19 expression and the related efficacy were not identified. Only patients with positive CD19 expression were enrolled in several studies, resulting in limited data concerning whether reinduction of CR can be achieved by targeting CD20 or CD22 after the failure of targeting CD19. There were a small number of patients (exact number undisclosed) with CD19-negative/dim expression after treatments with CD19 Si-CAR T-cell therapy who responded to CD22 Si-CAR T-cell therapy [33]. As shown in Table 3, regardless of the small number to date, targeting CD22 or CD20 with Si-CAR T-cell therapy or Bi-CAR T-cell therapy could have helped over twenty patients with CD19 escape achieved CR, among which four patients remained in CR for more than 6 months with 12-month remission in one were patient [14][23][25][46]. In particular, seven patients with prior exposure to CD19 Si-CAR T-cell therapy were enrolled in two studies on CD19/CD20 Bi-CAR T-cell therapy [23][25], amonwith CD19 antig whom five patients managed to achieve CR after Bi-CAR T-cell therapy [23]. There were patientn es with CD19 antigen escape or prior usage of CD19 Si-CAR T-cell therapy who achieved CR after administration of alternative Bi-CAR T cells targeting CD19/CD20 or CD19/CD22. Therefore, clinical data are available to support the targeting of CD10/CD20 or CD19/CD22 in relapsed patients due to resistance to CD19 Si-CAR T-cell therapy.
| Ref.: First Author | Target | Characteristics of CD19 and CD22 Expression | Outcome |
|---|---|---|---|
| Fry [46] | CD22 | 10 ALL pts with CD19neg or CD19dim | CR: 6/10 *, 4 in CR for ≥ 6 mon; 1 in CR for 12 mon; 1 in CR for 9 mon ongoing |
| Tong [23] | CD19/CD20 | 4 NHL pts with CD19neg | CR: 2/4; PR: 1/4; PD:1/4 |
| Shah [25] | CD19/CD20 | 4 NHL pts with < 40% CD19 | CR: 3/4; PR: 1/4 |
| Gardner [14] | CD19/CD22 | 13 ALL pts with diverse expression of CD19 and CD22 | CR: approximately 9–11/13 |
Overcoming BCMA-negative or BCMA-low escape has been proposed as capable of reversing the resistance of malignant plasma cells to BCMA Si-CAR T-cell therapy [47]. However, after examining more than 200 patients treated with BCMA Si-CAR T-cell therapy in several trials [8][48][49][50][51][52][53][54], BCMA-negative cells were detected in only two patients who relapsed. A BCMA-negative plasma cell population was present in one patient [50], while BCMA-negative and BCMA-positive plasma cells were present in the other patient [55]. By comparison, 10 patients relapsed with BCMA-positive expression or BCMA expression returning to the baseline level [53][56]. Therefore, evidence of relapse resulting from loss or down-regulation of BCMA expression derived from current clinical data is scarce, making the evidence of BCMA-negative or BCMA-low escape not as robust as that for CD19.
Despite limited evidence on the failure of response due to BCMA escape among trials with Si-CAR T-cell therapy targeting BCMA, the combination of BCMA CAR and a second CAR is still being explored in MM [11][12]. Given that the DOR of present BCMA Si-CAR T-cell therapy in MM is far from satisfactory, Bi-CAR T-cell therapy targeting other antigens together with BCMA might warrant further investigation. Although clinical efficacy, such as response and survival, has been reported to be irrelevant to BCMA expression [48][57], the data on the detailed expression pattern over time in responders who relapsed are limited. Meanwhile, it is unclear whether patients with reduced BCMA expression have been enrolled in current trials of Bi-CAR T-cell therapy. It may be worthwhile to design trials that include the tracking of BCMA expression and the related response in the individual patient during the clinical course, especially among patients treated with Bi-CAR T cells after relapse of BCMA Si-CAR T-cell therapy, to identify patients with reduced BCMA expression compared to baseline who could benefit from BCMA Bi-CAR T-cell therapy.
Bi-CAR T-cell therapy is less likely to cause severe CRS and NT than Si-CAR T-cell therapy. There seems to be a difference in the safety profile with respect to the occurrence of CRS and NT between Bi-CAR T-cell therapy and Si-CAR T-cell therapy by simply looking at the numbers; however, considering the sample size, different clinical sites, and possible inadequate management of CRS and NT during early development of CAR T-cell therapy, it seems more investigations are needed to confirm this conclusion.
No matter how effective dual CAR strategies have been in preclinical models, it may only be considered a true success when it benefits patients in the clinic. Emerging clinical data in 2021 permit a fair comparison of different dual-targeting CAR T-cell therapies possible in patients (Table 4Table 1).
Table 41. Comparison of optimization process, transduction efficiencies, DOR, and OS among different dual-targeting CAR T-cell therapies (n > 10; ALL and NHL).
|
Ref.: First Author |
Target |
CAR Strategy |
Optimization Process |
Final CAR Transduction Efficiency (Normal Donor vs. Patient) |
Durability |
OS (mon and %) |
|
Schneider , Shah |
CD19/CD20 |
One Tandem Bi-CAR-T product |
2 constructs Change order of CAR19 and CAR20 Final: CD20 scFv distal to 4-1BB |
85%–89% vs. 7.4–28% |
NHL: 12 CR > 6 mon; 6 CR > 12 mon; 8 CR ongoing |
NHL: NA |
|
Tong |
CD19/CD20 |
One Tandem Bi-CAR-T product |
8 constructs Change order of CAR19 and CAR20 Final: CD20 scFv distal to 4-1BB |
35% vs. 10.1%-35.1% |
NHL: 64% (12-mon PFS) |
NHL: 71% (12-mon OS) |
|
Wang |
CD19/CD22 |
One Tandem Bi-CAR-T product |
Undisclosed Final: CD19 scFv distal to 4-1BB |
Undisclosed vs. 60.1 (30–75.1)% |
ALL: ~3 mon (median LFS in 13 non-HSCT pts) |
ALL: ~21 mon (median OS in all pts) |
|
Wang |
CD19/CD22 |
Cocktail/Sequential infusion of two Si-CAR products |
Not required |
52.2% vs. 40.4% ± 18.4% (CAR19); 53.8% vs. 42.8% ± 19.6% (CAR22) |
ALL: 52.9% (12-mon PFS) 13.6 mon (median PFS) NHL: 9.9 mon (median PFS) 50.0% (12-mon PFS) |
ALL: 62.8% (12-mon OS) 31 mon (median OS) NHL: 18.0 mon (median OS) 55.3% (12-mon OS) |
|
Qin , Spiegel |
CD19/CD22 |
One Loop Bi-CAR-T product |
Co-transduction vs. 4 Bivalent/Tan constructs vs. 6 Loop constructs Final: CD22 scFv distal to 4-1BB |
82% vs. 60.1% (34.6–75.2%) |
ALL: 5.8 mon (PFS) ~0% (12-mon PFS) NHL: 3.2 mon (PFS) ~25% (12-mon PFS) |
ALL: 11.8 mon (median OS in all pts) ~25% (12-mon OS) NHL: 22.5 mon (median OS) ~64% (12-mon OS) |
|
Zhang |
CD19/CD22 |
One Loop Bi-CAR-T product |
Undisclosed Final: CD19 scFv distal to 4-1BB |
Undisclosed vs. 20-(~)78% |
NHL: 40.0% (12-mon PFS) 66.7% (12-mon PFS in CR at 3 mon) |
NHL: 63.3% (12-mon OS) 100% (12-mon OS in CR at 3 mon) |
|
Cordoba [32] |
CD19/CD22 |
One Bicistronic Bi-CAR-T product |
Binder humanization |
56.8% vs. 17.7% (8.6–39.3%) |
ALL: 32% (12-mon EFS) |
ALL: 60% (12-mon OS) |
Abbreviations: ALL, acute lymphoblastic leukemia; CR, complete response; DOR, duration of response; EFS, event-free survival; f/u, follow-up; LFS, leukemia-free survival; mon, month(s); NA, not available; non-HSCT, no hematopoietic stem cell transplantation; OS, overall survival; PFS, progression-free survival; pts, patients; Ref., reference; RFS, relapse-free survival.
When comparing clinical outcomes on CD19/CD22 Loop Bi-CAR T-cell therapy with different locations of CD19 scFv and CD22 scFv on CAR in NHL subjects, the PFS in the trial on CAR T cells expressing Bi-CAR with CD19 scFv distal to 4-1BB [45] was longer than the one with CD22 scFv distal to 4-1BB [37], both of which have similar OS. Of note, the transduction efficiency of the CD19/CD22 Loop CAR with CD19 scFv distal to the 4-1BB was lower than the lowest one of the CD19/CD22 Loop CAR with CD22 scFv distal to 4-1BB, indicating that optimization may still be needed. Transduction efficiencies of CD19/CD20 Tandem Bi-CAR T-cell product in patients with NHL dropped to nearly one-third of the those in vitro [23]. However, the PFS and OS rates of patients given CD19/CD20 Tandem Bi-CAR T-cell therapy were higher than those with CD19/CD22 Loop Bi-CAR T-cell therapy, despite higher transduction efficiencies observed in the latter [23][37]. Of course, caution is needed to interpret non-head-to-head studies, and the differences may be due to different targets in NHL. Overall, poor transduction efficiencies may not necessarily worsen clinical outcomes, though improving transduction efficiencies still matters in optimization.
When comparing clinical outcomes on CD19/CD22 Loop Bi-CAR T-cell therapy with different locations of CD19 scFv and CD22 scFv on CAR in NHL subjects , the PFS in the trial on CAR T cells expressing Bi-CAR with CD19 scFv distal to 4-1BB was longer than the one with CD22 scFv distal to 4-1BB [34], both of which have similar OS. Of note, the transduction efficiency of the CD19/CD22 Loop CAR with CD19 scFv distal to the 4-1BB was lower than the lowest one of the CD19/CD22 Loop CAR with CD22 scFv distal to 4-1BB, indicating that optimization may still be needed. Transduction efficiencies of CD19/CD20 Tandem Bi-CAR T-cell product in patients with NHL dropped to nearly one-third of the those in vitro. However, the PFS and OS rates of patients given CD19/CD20 Tandem Bi-CAR T-cell therapy were higher than those with CD19/CD22 Loop Bi-CAR T-cell therapy, despite higher transduction efficiencies observed in the latter. Of course, caution is needed to interpret non-head-to-head studies, and the differences may be due to different targets in NHL. Overall, poor transduction efficiencies may not necessarily worsen clinical outcomes, though improving transduction efficiencies still matters in optimization.
Despite a great deal of effort directed at optimizing the Bi-CAR T-cell product, the outcomes have not been able to outperform the simple strategy of the cocktail/sequential infusion of two Si-CAR T-cell products without relentless optimization. For ALL and NHL, the cocktail/sequential infusion of CD19/CD22 Si-CAR T-cell products achieved the longest median OS [16], not only providing convincing clinical evidence of dual-targeting CAR T-cell therapy to improve survival but also dwarfing other time- and cost-consuming trials from preclinic to clinic. It is time for different research groups to collaborate and share details on optimizing Bi-CAR structure and standardize clinical trials to compare different dual-targeting therapeutic strategies in a quest for the ideal construct to produce Bi-CAR. Meanwhile, the cocktail/sequential infusion of two Si-CAR T-cell products still merits clinical application to save the lives of patients with ALL and NHL if the commercialization of other dual CAR strategies requires additional time.
In terms of experience gained from the cocktail/sequential infusion of two Si-CAR T-cell products, the timing of the second infusion warrants further exploration. During the trial on the cocktail infusion of BCMA/CD19 Si-CAR T-cell products, CD19 Si-CAR T and BCMA Si-CAR T-cell product were infused on the same day [58]. During the cocktail/sequential infusion of CD19/CD22 Si-CAR T-cell products, CD22 Si-CAR T cells were infused one day before CD19 Si-CAR T cells [16]. Comparing the cocktail infusion of BCMA/CD19 Si-CAR T-cell products [58] with BCMA/CD38 Tandem Bi-CAR T-cell product [59], the PFS of the former was much shorter than that of the latter. Again, the variance may be attributed to the difference in targets. However, it may be worthwhile to adjust the timing of the cocktail/sequential infusion of two Si-CAR T-cell products to standardize the comparison and investigate the influence of the timing of the infusion on the expansion of two different Si-CAR T-cell products in preclinical models.
4. Challenges and Perspectives
Advancing technologies have made Bi-CAR T-cell therapy readily available; however, three main limitations remain for Bi-CAR T-cell therapy: (1) Bi-CAR T-cell therapy does not address other proposed resistance mechanisms outside of target antigen loss; (2) evidence on the safety profile and in vivo activity of Bi-CAR T cells are insufficient; and (3) increased difficulty in manufacturing since the size of construct is bigger. The specific challenges within Bi-CAR T cell manufacturing are the complicated optimization process to find the suitable vectors for manufacturing, increased inconsistency in batch manufacture of viral vector, low transduction efficiency in Bi-CAR T cells, and high manufacturing failure rate due to the size of the bivalent and bicistronic vector.
In conclusion, dual-targeting CAR T-cell therapy has offered another hope for patients in the post era after the use of Si-CAR T-cell therapy. The clinical efficacy has been validated in trials on cocktail/sequential infusion of Si-CAR T-cell products and in a few trials of Bi-CAR T-cell therapy. However, an optimal Bi-CAR structure has not been established. The pooled safety profiles of Bi-CAR T-cell appear better than those of Si-CAR T-cell therapy, with a lower incidence of severe CRS and NT. No apparent effect of 1 + 1 > 2 in terms of DOR, OS, and PFS has been demonstrated in trials on Bi-CAR T-cell therapy, indicating that further optimization is needed. The optimization of the Bi-CAR should focus on finding the right targets for different indications, the appropriate spatial structure of two different scFvs, the suitable linker for scFvs, and the proper transduction efficiencies using patients’ T cells to enhance the efficacy and the persistence of Bi-CAR T cells in patients.
The lack of a magic bullet as Bi-CAR structure calls for collaboration of different research groups to develop solutions to benefit the global community. Models integrating clinical data with preclinical data to predict the optimal Bi-CAR may help design an ideal vector for Bi-CAR introduction. Meanwhile, more trials with Bi-CAR T-cell therapy in patients without prior exposure to Si-CAR T-cell therapy are also needed to compare the two types of CAR T-cell therapy.
