Meat may contain natural, spoilage, and pathogenic microorganisms based on the origin and characteristics of its dietary matrix. Several decontamination substances are used during or after meat processing, which include chlorine, organic acids, inorganic phosphates, benzoates, propionates, bacteriocins, or oxidizers. Unfortunately, traditional decontamination methods are often problematic because of their adverse impact on the quality of the raw carcass or processed meat. The extended shelf-life of foods is a response to the pandemic trend, whereby consumers are more likely to choose durable products that can be stored for a longer period between visits to food stores. This includes changing purchasing habits from “just in time” products “for now” to “just in case” products, a trend that will not fade away with the end of the pandemic.
- clean label foods
- ozone
- cold plasma
1. Introduction
2. Nonthermal Decontamination Technologies
2.1. Ozonation
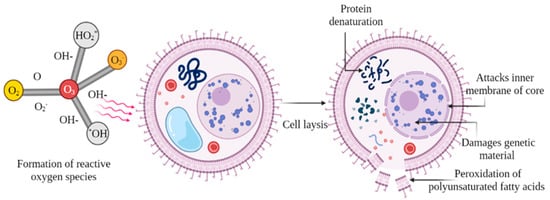
In recent years, ozone (a naturally occurring water-soluble triatomic gas that can act as a strong oxidizing agent) has been of great interest to the processing industry. Bacterial inactivation through cell wall disruption, or lysis by ozone, is faster than other disinfectants that require time to invade the cell membrane [29]. It is, therefore, a very effective germicide against viruses, bacteria, and spores. The two mechanisms of inactivation include: (i) sulfhydryl group and amino acids of enzymes, proteins, and peptides oxidized to smaller peptides and (ii) polyunsaturated fatty acids oxidized to acid peroxides, resulting in cell death [30]. The effect of ozone treatment operating conditions on several microorganisms’ reduction is presented in Table 1.| Sample | Specification | Microbes | Highlights | Reference | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chicken legs | 2–10 mg/L for 1 h combined with vacuum packaging (polyamide/polyethylene bags) stored at 4 °C for 16 days. | TVC, | Pseudomonas | spp., LAB, Yeast-molds, & | Enterobacteriaceae | 6-day shelf-life extension compared to vacuum packaging alone (4-day extension). Positively affected odor, texture, and taste retained an acceptable score for 14–16 days. | [29] | |||||||||
| Chicken meat (freeze-dried) | 0.6 ppm at 4 °C (90% RH) for 10 min. | TAMB, LAB, | E. coli | . & | Salmonella | spp. | 1.1 log CFU/g was observed in TAMB and LAB. | E. coli | . and | Salmonella | spp. was not detected. Combination with MAP (20% CO | 2 | , 80% N | 2 | ) improved the texture and sensory proprieties. | [30] |
| Chicken meat (freeze-dried) | 0.4–0.7 ppm at 4 °C (90% RH) for 10–120 min. | LAB & TAMB | Reduced 4.77 and 6.8 log CFU/g, respectively. The combined use of ozone and lyophilization would be useful for extending shelf-life to 8 months. | [22] | ||||||||||||
| Chicken breast meat | 10 × 10 | −6 | kg O | 3 | /m | 3 | /h for 3 days. | Coliform, aerobic, and anaerobic bacteria | Aerobic: 2.96 log CFU/g (untreated = 5.35 log CFU/g) Anaerobic: 2.18 log CFU/g (untreated = 4.63 log CFU/g) Coliform: 1.74 log CFU/g (untreated = 3.35 log CFU/g) |
[31] | ||||||
| Duck breast meat | Aerobic: 2.52 log CFU/g (untreated = 4.11 log CFU/g) Anaerobic: 3.46 log CFU/g (untreated = 3.95 log CFU/g) Coliform: 1.39 (untreated = 3.28) |
|||||||||||||||
| Turkey breast meat | 1 × 10 | −2 | kg/m | 3 | at 22 °C (21.6% RH) for 8 h. | TAMB, | Enterobacteriaceae | & yeast-mold | Reduced 2.9, 2.3 and 1.9 log CFU/g, respectively. | [32] | ||||||
| Beef (sliced) | 218–286 mg/m | 3 | , 5–20 pulses for 2–40 min with intervals of 30 min. | Heterotrophic microflora & | L. monocytogenes | Decreased 1.5 log CFU/g heterotrophic counts. Decreased inoculated | L. monocytogenes | counts by more than 1 log CFU/g. Exposure times of more than 10 min negatively affected red color and rancidity. | [33] |
2.2. High Hydrostatic Pressure (HHP)
HHP is a major trend in the food industry nowadays in terms of clean label technology. It is the most modern method of increasing the shelf stability of food products [41][42][41,42]. HHP is a response to the challenges faced by the industry and provides a competitive advantage, which is undoubtedly worth implementing sooner rather than later. According to Lee et al. [43], global revenues from the high-pressure food protection (i.e., HHP) market amounted to USD 1055 million in 2019 and will reach USD 2123 million in 2025, with a compound annual growth rate of 12.34% from 2021–2025. HHP can achieve food safety, inactivate pathogens, such as Salmonella, Listeria, and E. coli, and prevent recontamination, seeing as the packed product is virtually impossible to recontaminate. HHP reduces microorganisms or eliminates them and/or reduces chemical preservatives. Table 2 summarizes the range of parameters used in HHP to decontaminate meat and meat products. In general, HHP (a single step at 86,000 psi for 3 min) as a clean label (no preservatives) technology was able to effectively double the shelf-life of meat products, with the control product lasting for about 30 days compared to 60 days for the HHP product, concerning pathogen control.| Meat Type | Treatment Conditions | Storage Conditions | Findings | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chicken fillets | 500 MPa for 10 min. | 4 and 12 °C | HHP resulted in the reduction of the pathogen population below the detection limit of the enumeration method (0.48 log CFU/g), irrespective of the inoculum. HHP extended the shelf life of chicken fillets by 6 and 2 days, at 4 and 12 °C, respectively. | [44] | ||||||||
| Frozen chicken breast | 500 MPa for 1 min and 400 MPa for 5 min. | _ | HHP showed inactivation of | Salmonella | at 400 MPa for 5 min and 500 MPa for 1 min. | [45] | ||||||
| Ground chicken meat | 350 MPa for 10 min + 0.75% carvacrol. | HHP with 0.60% carvacrol treatment resulted in a >5-log pathogen reduction. | [46] | |||||||||
| Ground beef | 400 MPa for 15 min at 25, 35, and 45 °C. | 4 and −20 °C for up to 5 days | At 25 °C, 5 log reduction in | E. coli | O157:H7 was observed further low-temperature storage serves as the hurdle in its survival and recovery after treatment. HHP showed no effect on the chromatic profile of grounded beef. | [47] | ||||||
| Vacuum-packed ground beef | 200 and 400 MPa for 5 min at 25 °C. | _ | L. sakei | is good pressure-resistant lactic acid bacteria used in combination with HHP at 400 MPa and is efficient in controlling pathogenic | E. coli | strains. | [48] | |||||
| Uncooked ground beef patties | 300, 400, and 500 MPa for 5 min. | 4 °C for 10 days | HHP combine with | Lactobacillus acidophilus | showed less total aerobic count (3.35 log CFU/g) than untreated (6.74 log CFU/g) beef patties with 0.80 log CFU/mL yeast and mold count. The combined treatment showed a delayed decrease in pH value, inhibited lipid oxidation with better color retention and the highest sensory score. | [49] | ||||||
| Beef patty | 400 and 600 MPa for 5 min. | Refrigerated storage for 18 h | An amount of 2 and 4 log CF/mL reductions after 400 and 600 MPa in Shiga toxin-producing | E. coli | O157:H7, respectively. Variations in fat concentration of 10 and 20% did not affect. In contrast, 1% NaCl evident more reduction than 2%, indicating bar protective effect of salt. | [50] | ||||||
| Vacuum-pack ripened mutton patties | 200 and 400 MPa for 10 min. | 4 °C for 28 days | Significant reduction in total plate count after HHP at both levels, with a significant increase in lightness (L*). Redness (a*), yellowness (b*); hardness, gumminess, and chewiness of patties reduced significantly. | [51] | ||||||||
| Beef steak | 450 MPa, 600 MPa 1, 3, 6, 10, 15 min. | _ | HHP have the potential to allow the production of a convenient and safe product by achieving 5 log definition of pasteurization of beef steak inoculated with | E. coli | 0157:H7. | [52] | ||||||
| Beef slurry | 600 MPa for 20 min at 75 °C. | _ | Best inactivation of spores of | Clostridium perfringens | in beef slurry was a 2.2 log reduction. | [53] | ||||||
| Beef slurry | 600 MPa for 20 min at 75 °C. | _ | After HHP, a greater reduction (2.2 log) in | C. perfringens | spores was observed as compared to thermal treatment (no reduction) after 20 min. | [54] | ||||||
| Beef slurry | 600 MPa at 70 °C for 20 min. | _ | A 4.9 log reduction in | Bacillus cereus | spores after treatment at 70 °C but same temperature thermal processing led to 0.5 log reduction in spore. Increasing HHP temperature from 38 to 70 °C increases the spore inactivation for up to 3 logs. | [55] | ||||||
| Marinated beef ( | Longissimus lumborum | ) | 300, 400, and 600 MPa for 5 min. | Refrigerated storage for 14 days | HHP was proven to provide safe meat along with a sodium reduction in it. Meat marinated with salt and citric acid has no sufficient inactivation of | L. innocua | and | Enterococcus faecium | , while when combine with HHP, a 6 log cycle reduction was observed. | [56] | ||
| Beef burgers | 300 MPa for 10 min at 9.9 °C and 600 MPa 10 min, 10.2 °C. | _ | Mesophilic and psychotropic count remain at the detection limit after HHP at 600 MPa, with no effect on lipid oxidation for at least 6 days. | [57] | ||||||||
| Raw meatballs (beef, veal, beef + veal + pork) | 400 and 600 MPa for 0 and 18 min. | 4 and −12 °C for 18 h | No difference in the extent of inactivation in different species of meat used for meatballs preparation in refrigerated storage (0.9 to 2.9 log CFU/g) as compared to frozen samples (1.0 to 3.0 log CFU/g). A total of 600 MPa requires 1–3 min and 400 MPa requires 9 min for a ≥2.0 log CFU/g reduction. | [58] | ||||||||
| Emulsified beef sausages | 100–400 MPa for 15 min at 10 °C. | _ | HHP proved to be an effective technique to produce microbial safe beef sausages (reduce total viable count equivalent to the sausages having higher salt concentration) with lower salt concentration. | [59] | ||||||||
| Dry fermented sausages | 600 MPa for 3 min. | 4 °C for 4 weeks | Inactivation of | E. coli | O157:H7 in dried fermented sausages was observed to be affected by a | w | . At a | w | ≤ 0.90, or moisture protein ratio in the range of 1.9–2.3, led to 6.4 log reduction. Further drying reduced to 2.2 log reduction. Recovery of | E. coli | O157:H7 was observed for 1 week of storage but in 2-, 3-, and 4-week storage, no further recovery was observed. | [60] |
| Pork cooked sausages | 600 MPa for 3 min. | 4 and 10 °C for 35 days | Cooking of sausages leads to a >6 log reduction in inoculated | L. monocytogenes | . During storage at 4 °C, no significant growth was observed after HHP. But at 10 °C storage, growth remains below the detection limit up to 21 days after the 4.5 log CFU/mL increase in population was observed. No lactic acid bacterial growth was observed till the end of storage. | [61] | ||||||
| Italian salami | 600 MPa for 300 s. | _ | HHP related microbial inactivation depicts an inverse relation with a | w | . All 20 salami samples showed a 5 log reduction in | Salmonella | after treatment. | [62] | ||||
| Italian salami | 600 MPa for 300 s. | _ | An amount of 0.34–4.32 log CFU/g reduction during processing in | L. innocua | was observed which was reduced to 0.48–3.4 log CFU/g after HHP. The efficacy of HHP was associated with a | w | and higher pH after acidification, drying and seasoning phase. | [63] | ||||
| Nitrite-free emulsion-type sausage | 0.1, 500 MPa for 12 min + 0, 1, 2% vinegar | 4 °C for two weeks followed by at 20 °C for three weeks | HHP (500 MPa; four cycles and each for 3 min) + vinegar (1%) reduced vegetative cells and spores of | C. perfringens | by 4.8 and 2.8 log CFU/g, respectively. | [64]. | ||||||
| Traditional Portuguese ready-to-eat meat sausage ( | Chouriço de carne | ) | 300 MPa for 5 min at 10 °C + lactic acid bacteria (Pediococcus acidilactici, HA-6111-2) and its bacteriocin (bacHA-6111-2). | Refregrated storage for 60 days. | The hurdle technology (bacteriocin and pressurization) showed a 0.5 log CFU/g decrease in | L. innocua | cells compared to non-treated cells. | [65] | ||||
| Dry-cured ham | 450 MPa for 10 min and 600 MPa for 5 min. | 4 °C for 30 days | The efficacy of HHP against | L. monocytogenes | was reduced by low a | w | values. The changes in HHP-surviving bacteria gene transcription patterns were strain-dependent. | [66] | ||||
| Cooked ham | 400 MPa for 10 min at 17 °C + alginate films containing enterocins. | 1 or 6 °C for 2 months | Both antimicrobial packaging and pressurization delayed the growth of | L. monocytogenes | levels below the detection limit (day 90) during 6 °C storage. | [67] |
