The constant increase in the amount of energy consumed and environmental problems associated with the use of fossil fuels determine the relevance of the search for alternative and renewable energy sources. One of the most promising renewable fuels is hydrogen gas, which can be produced by sunlight-driven photocatalytic water splitting. The decisive role in the efficiency of the process is played by the properties of the photocatalyst. Oxide materials are widely used as photocatalysts due to their appropriate band structure, high-enough photochemical stability and corrosion resistance. However, the bandgap, crystallinity and the surface morphology of oxide materials are subject to improvement. In order to choose an appropriate oxide semiconductor photocatalytic material and strategies of its modification it is necessary to examine the mechanism of the photocatalytic water splitting.
- photocatalytic water splitting
- photoelectrochemical water splitting
- hydrogen production
1. The Main Stages of the Photocatalytic Water Splitting
- –
-
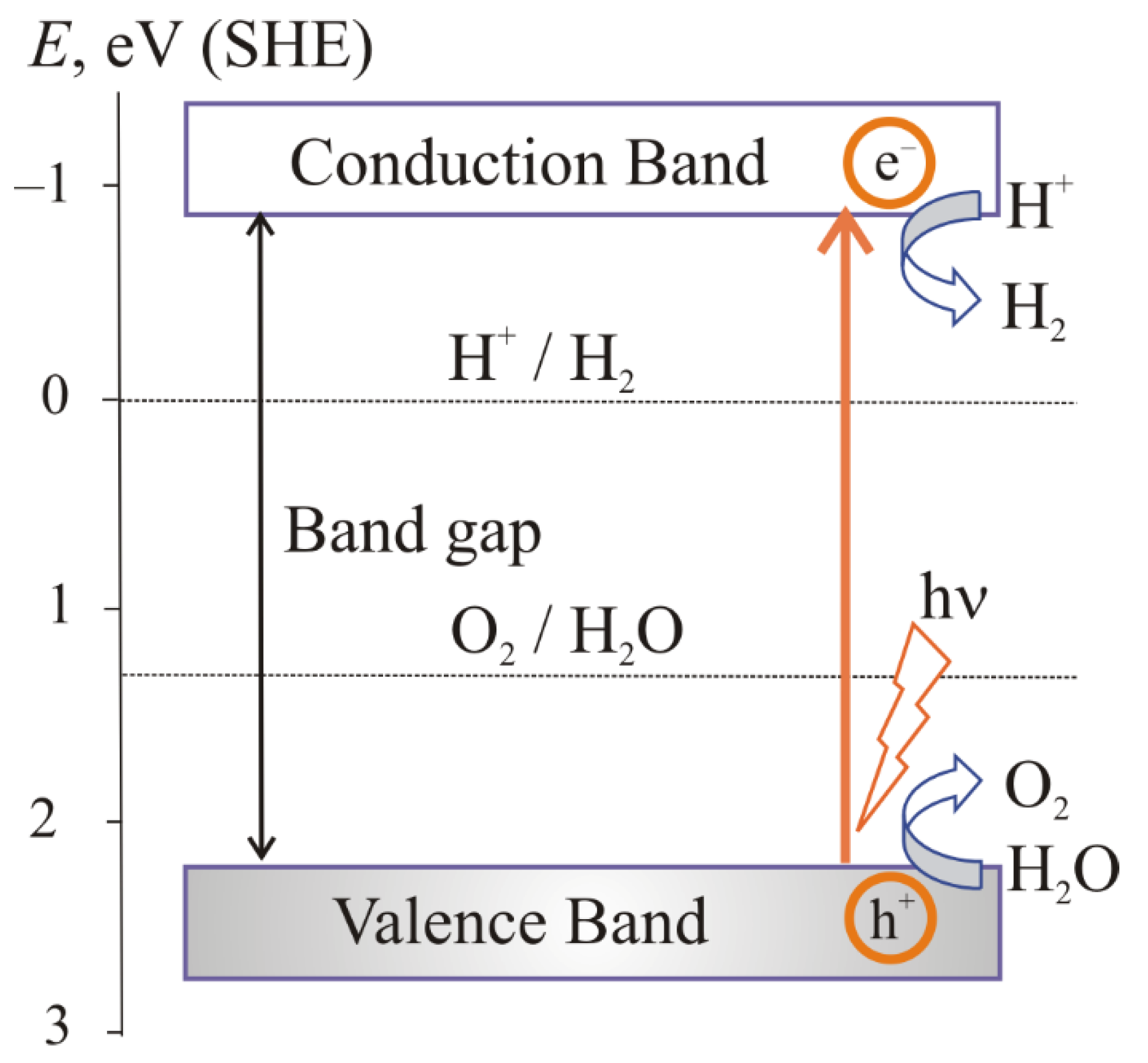
the absorption of light by a semiconductor;
- –
-
excitation of charge carriers;
- –
-
separation and transfer of charge carriers;
- –
-
surface catalytic reactions.
2. The Main Conditions for Water Splitting
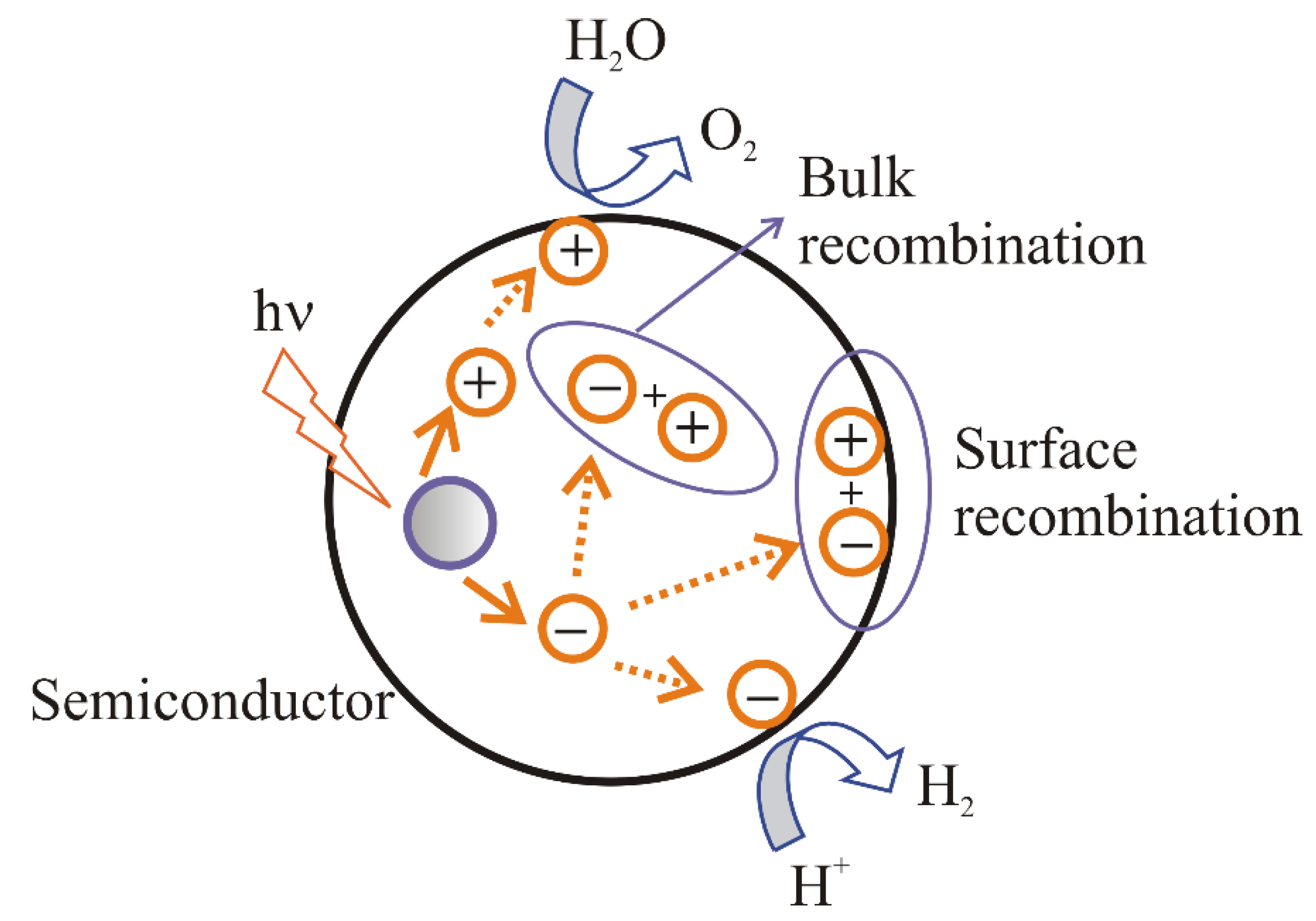
- (i).
-
recombination of electrons and holes on the surface of a semiconductor;
- (ii).
-
recombination of electrons and holes in the bulk of a semiconductor;
- (iii).
-
transport of the electrons to the surface and participation in the reduction reaction;
- (iv).
-
transport of the holes to the surface and participation in the oxidation reaction.
| Year, Ref. | Photo-Catalysts | Bandgap, eV | Light Irradiation Parameters |
Reagents | Sacrificial Reagent |
Hydrogen Production, mmol g−1 h−1 |
|---|---|---|---|---|---|---|
| 2014, [6] | Au/TiO2 | 2.77–3.26 | Set of 3 Solarium Philips HB175 lamps each equipped by 4 15 W Philips CLEO florescent tubes |
1 g L−1 photocatalyst 25 vol.% methanol in water pH ~5 |
Methanol | 0.303–1.543 |
| 2015, [7] | Au/TiO2 | 3.03–3.33 | Spectroline model SB-100P/F lamp 100 W 365 nm |
10 vol.% sacrificial reagents | Glycerol | 1.9–27.9 |
| Ethylene glycol | 1.4–20.9 | |||||
| Methanol | 0.9–13.5 | |||||
| Ethanol | 0.4–9.8 | |||||
| 2017, [8] | Zn0.5 Cd0.5S g-C3N4 TiO2 |
- | 300 W Xe lamp, wavelength ≥ 420 nm |
Aqueous solution 0.2 g L−1 photocatalyst powder + 20 vol.% sacrificial reagents |
Triethanol amine | 1.197 |
| Formic acid | 0.845 | |||||
| Methanol | 0.599 | |||||
| Methyl amine | 0.279 | |||||
| Ethylene glycol | 0.116 | |||||
| Ethanol | 0.111 | |||||
| Ethylamine | 0.101 | |||||
| Ethylene diamine | 0.084 | |||||
| 2020, [9] | Cu/In2O3 /TiO2 NPs Cu/In2O3 NRs /TiO2 NWs |
2.69 2.90 |
35 W HID lamp, light intensity 20 mW cm−2, wavelength 450 nm |
0.01 g of photocatalyst was dispersed in 130 mL aqueous solution + 10 vol.% sacrificial reagent | Glycerol | 6.09 |
| Ethylene glycol | 4.85 | |||||
| Methanol | 4.39 | |||||
| Ethanol | 2.84 | |||||
| 2020, [10] | TiO2 NPs TiO2 MPs |
3.20 3.10 |
35 W HID Xenon lamp, 20 mW cm−2, wavelength ~420 nm | 0.1 g of photocatalyst catalyst was dispersed in 100 mL water containing sacrificial reagent | Glycerol | 9.073 |
| Methanol | 4.574 | |||||
| Phenol | 0.146 | |||||
| 0.2 M Na2S/Na2SO3 | 0.508 | |||||
| 0.1 M Na2S/Na2SO3 | 0.124 |
3. The Mechanism of Photoelectrochemical Water Splitting
- –
-
a photoanode (n-type semiconductor electrode) and a metal (platinum) cathode (Figure 3a);
-
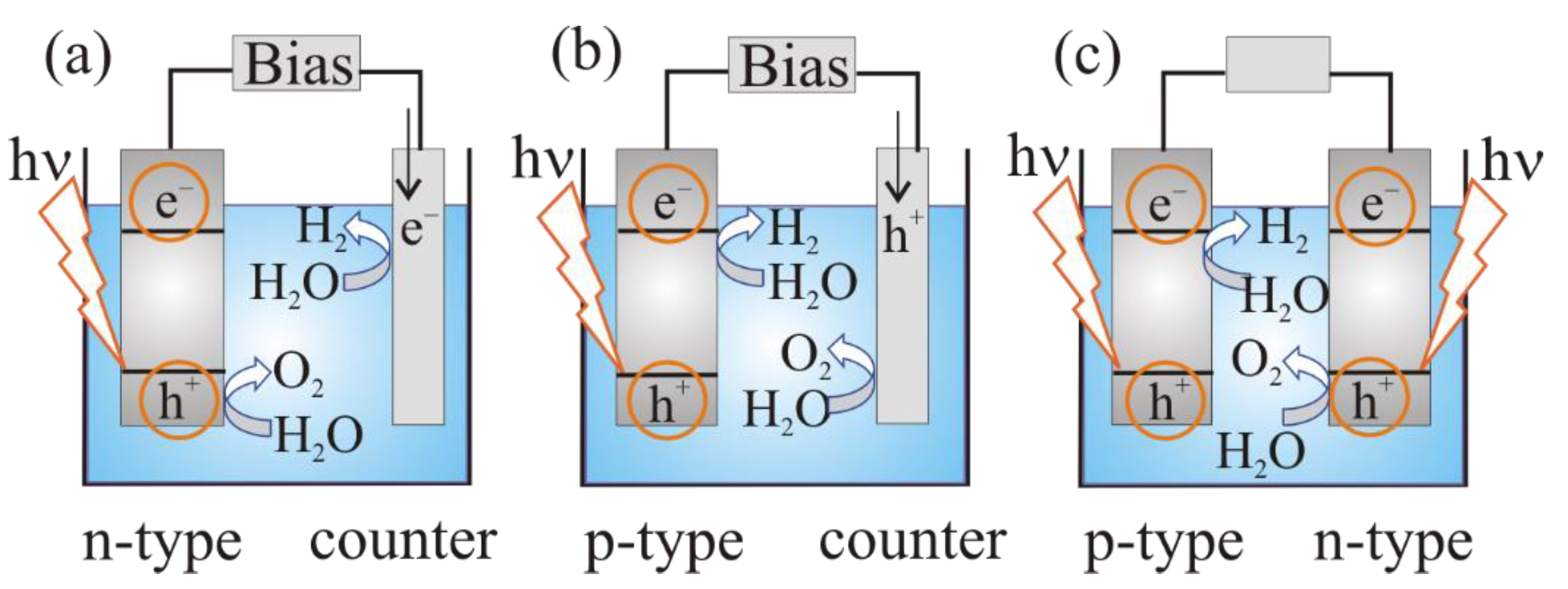 Figure 3. Photoelectrochemical cell for water splitting: (a) n-type semiconducting (SC) photoanode and Pt cathode; (b) p-type semiconducting (SC) photocathode and Pt anode; (c) semiconducting photoanode and photocathode (tandem system) [19].
Figure 3. Photoelectrochemical cell for water splitting: (a) n-type semiconducting (SC) photoanode and Pt cathode; (b) p-type semiconducting (SC) photocathode and Pt anode; (c) semiconducting photoanode and photocathode (tandem system) [19]. - –
-
a photocathode (p-type semiconductor) and a metal (platinum) anode (Figure 3b):
- –
-
a photoanode (n-type semiconductor electrode) and a photocathode (p-type semiconductor (Figure 3c).
-
References
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrog. Energy 2019, 44, 540–577.
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. Part B 2017, 391, 72–123.
- Chen, J.; Yang, D.; Song, D.; Jiang, J.; Ma, A.; Hu, M.Z.; Ni, C. Recent progress in enhancing solar-to-hydrogen efficiency. J. Power Source 2015, 280, 649–666.
- Rongé, J.; Bosserez, T.; Huguenin, L.; Dumortier, M.; Haussener, S.; Martens, J.A. Solar hydrogen reaching maturity. Oil Gas Sci. Technol. Rev. D’ifp Energ. Nouv. 2015, 70, 863–876.
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570.
- Ortega Méndez, J.A.; López, C.R.; Pulido Melián, E.; González Díaz, O.; Doña Rodríguez, J.M.; Fernández Hevia, D.; Macías, M. Production of hydrogen by water photo-splitting over commercial and synthesised Au/TiO2 catalysts. Appl. Catal. B Environ. 2014, 147, 439–452.
- Chen, W.-T.; Chan, A.; Al-Azri, Z.H.N.; Dosado, A.G.; Nadeem, M.A.; Sun-Waterhouse, D.; Idriss, H.; Waterhouse, G.I.N. Effect of TiO2 polymorph and alcohol sacrificial agent on the activity of Au/TiO2 photocatalysts for H2 production in alcohole-water mixtures. J. Catal. 2015, 329, 499–513.
- Wang, M.; Shen, S.; Li, L.; Tang, Z.; Yang, J. Effects of sacrificial reagents on photocatalytic hydrogen evolution over different photocatalysts. J. Mater. Sci. 2017, 52, 5155–5167.
- Tahir, B.; Tahir, M. Morphological effect of 1D/1D In2O3/TiO2 NRs/NWs heterojunction photo-embedded with Cu-NPs for enhanced photocatalytic H2 evolution under visible light. Appl. Surf. Sci. 2020, 506, 145034.
- Tahir, B.; Er, P.W.; Tahir, M.; Nawawi, M.G.M.; Siraj, M.; Alias, H.; Fatehmulla, A. Tailoring metal/support interaction in 0D TiO2 NPs/MPs embedded 2D MAX composite with boosted interfacial charge carrier separation for stimulating photocatalytic H2 production. J. Environ. Chem. Eng. 2020, 8, 104529.
- Acar, C.; Dincer, I.; Zamfirescu, C. A review on selected heterogeneous photocatalysts for hydrogen production. Int. J. Energy Res. 2014, 38, 1903–1920.
- Tasleem, S.; Tahir, M.; Zakaria, Z.Y. Fabricating structured 2D Ti3AlC2 MAX dispersed TiO2 heterostructure with Ni2P as a cocatalyst for efficient photocatalytic H2 production. J. Alloys Compd. 2020, 842, 155752.
- Vaiano, V.; Iervolino, G. Visible light driven photocatalytic hydrogen evolution using different sacrificial reagents. Chem. Eng. Trans. 2019, 74, 547–552.
- Yang, R.; Song, K.; He, J.; Fan, Y.; Zhu, R. Photocatalytic hydrogen production by RGO/ZnIn2S4 under visible light with simultaneous organic amine degradation. ACS Omega 2019, 4, 11135–11140.
- Li, Q.; Li, X.; Yu, J. Surface and interface modification strategies of CdS-based photocatalysts. In Interface Science and Technology, 1st ed.; Yu, J., Jaroniec, M., Jiang, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 313–348.
- Zhao, W.; Ma, W.H.; Chen, C.C.; Zhao, J.C.; Shuai, Z.G. Efficient degradation of toxic organic pollutants with Ni2O3/TiO2−xBx under visible irradiation. J. Am. Chem. Soc. 2004, 126, e4783.
- Fang, Y.; Hou, Y.; Fu, X.; Wang, X. Semiconducting Polymers for Oxygen Evolution Reaction under Light Illumination. Chem. Rev. 2022, 122, 4204–4256.
- Nozik, A.J.; Memming, R. Physical chemistry of semiconductor—Liquid interfaces. J. Phys. Chem. 1996, 100, 13061–13078.
- He, H.; Liao, A.; Guo, W.; Luo, W.; Zhou, Y.; Zou, Z. State-of-the-art progress in the use of ternary metal oxides as photoelectrode materials for water splitting and organic synthesis. Nano Today 2019, 28, 100763.
- Meng, X.-D.; Wang, D.-Z.; Liu, J.-H.; Zhang, S.-Y. Preparation and characterization of sodium titanate nanowires from brookite nanocrystallites. Mater. Res. Bull. 2004, 39, 2163–2170.
- Corredor, J.; Rivero, M.J.; Rangel, C.M.; Gloaguen, F.; Ortiz, I. Comprehensive review and future perspectives on the photocatalytic hydrogen production. J. Chem. Technol. Biotechnol. 2019, 94, 3049–3063.
- Grewe, T.; Meggouh, M.; Tüysüz, H. Nanocatalysts for solar water splitting and a perspective on hydrogen economy. Chem. Asian J. 2016, 11, 22–42.
- Chen, Z.; Jaramillo, T.F.; Deutsch, T.G.; Kleiman-Shwarsctein, A.; Forman, A.J.; Gaillard, N.; Garland, R.; Takanabe, K.; Heske, C.; Sunkara, M.; et al. Accelerating materials development for photoelectrochemical hydrogen production: Standards for methods, definitions, and reporting protocols. J. Mater. Res. 2010, 25, 3–16.
- Sivula, K. Solar-to-chemical energy conversion with photoelectrochemical tandem cells. Chimia 2013, 67, 155–161.
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473.
