The deleterious consequences of snake envenomation are due to the extreme protein complexity of snake venoms. Therefore, the identification of their components is crucial for understanding the clinical manifestations of envenomation pathophysiology and for the development of effective antivenoms. In addition, snake venoms are considered as libraries of bioactive molecules that can be used to develop innovative drugs. Numerous separation and analytical techniques are combined to study snake venom composition including chromatographic and electrophoretic techniques.
- separation techniques
- analytical techniques
- snake venom
- biomolecules
- bioassay-guided fractionation
- snake venomics
1. Introduction
2. Methods Used for Separation of Venom Complex Mixtures
2.1. Chromatographic Techniques
Chromatographic methods are mostly employed for the separation of SVs and are preferred over electrophoretic techniques since they provide better resolution [10]. Liquid chromatography (LC) involves the partition of molecules between two phases: a stationary and a mobile phase. Molecules with different biochemical and physical features will interact differently with the stationary phase and thus will be separated from other components of the mixture [11]. Different types of chromatography are currently used for the separation of SVs, namely (i) size exclusion chromatography, (ii) ion-exchange chromatography, (iii) affinity chromatography and (iv) reversed-phase chromatography. A single chromatographic step is usually insufficient to isolate a molecule or reveal composition of the complex SV mixture. Hence, multiple chromatographic steps can be coupled together, which is known as multidimensional chromatography. Additionally, LC might be coupled with electrophoretic techniques for an improved protein isolation.2.2. Electrophoretic Techniques
The high versatility, feasibility, and practicality of chromatographic techniques outweighed those of electrophoretic techniques; nevertheless, the latter remain an important means for the separation of a variety of venoms (Table 1). For SVs, electrophoretic techniques are used rigorously for the separation and identification of protein content of whole venom and fractions. Even though electrophoretic methods have several limitations, their use is integral in the process of SV analysis. Two major types of electrophoretic methods are currently used: one-dimensional gel electrophoresis and two-dimensional gel electrophoresis.|
Separation Technique |
Advantages |
Disadvantages |
|---|---|---|
|
Size exclusion chromatography |
|
|
|
Ion-exchange chromatography |
|
|
|
Affinity chromatography |
|
|
|
RP-HPLC |
|
|
|
1D gel electrophoresis |
|
|
|
2-D gel electrophoresis |
|
|
3. Implementation of Separation Methods for SVs
3.1. Bioassay-Guided Fractionation
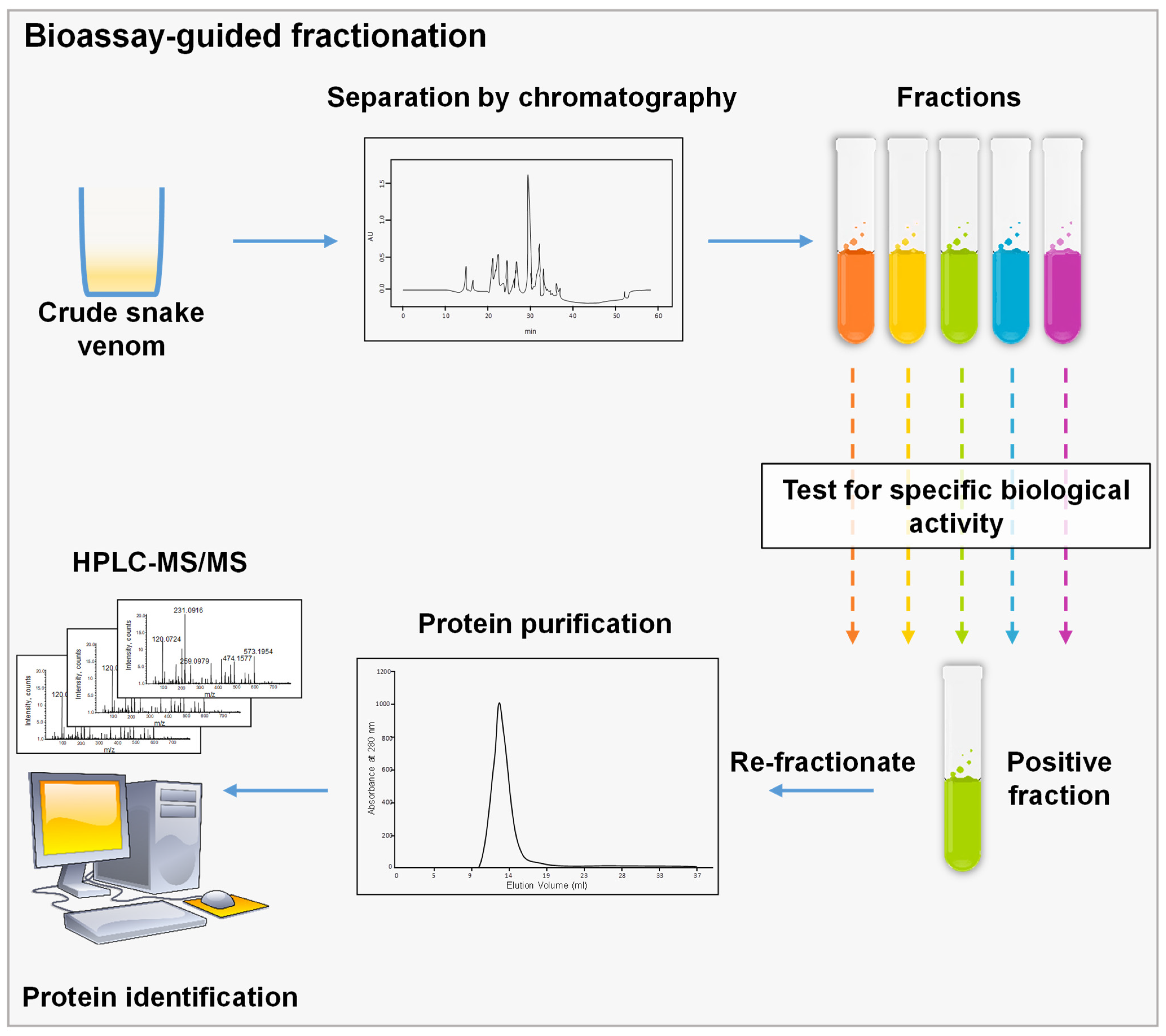
3.2. Whole Proteome Characterization and Identification
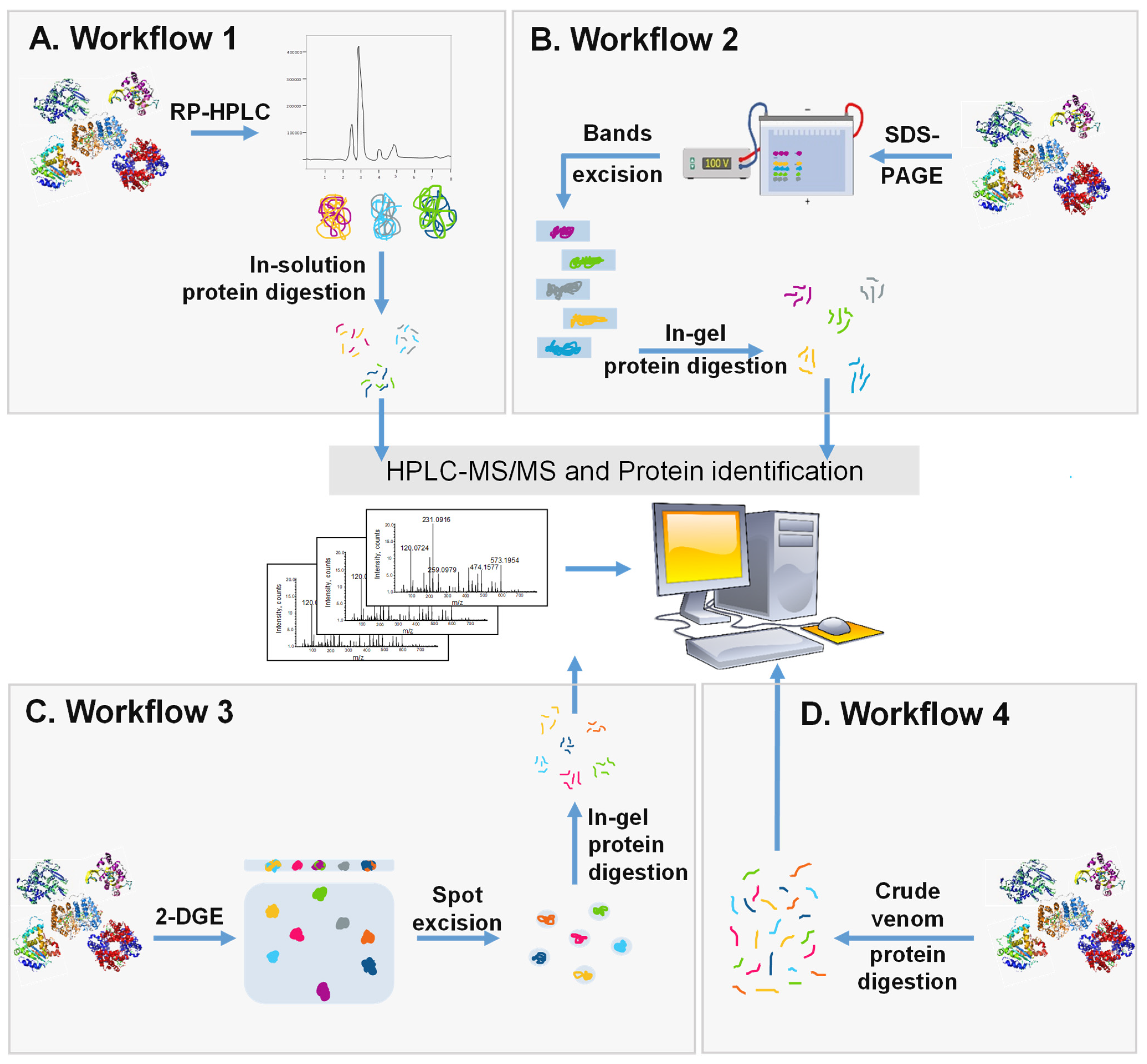
Finally, even with the scientific advancement, there is still no standard technique to be employed for the separation of SVs. Therefore, separation and analytical methods should be carefully chosen based on the objectives of the research and the available resources.
Finally, even with the scientific advancement, there is still no standard technique to be employed for the separation of SVs. Therefore, separation and analytical methods should be carefully chosen based on the objectives of the research and the available resources.
References
- Utkin, Y.N. Animal Venom Studies: Current Benefits and Future Developments. World J. Biol. Chem. 2015, 6, 28–33.
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite Envenoming. Nat. Rev. Dis. Primers 2017, 3, 17079.
- Yap, M.K.; Fung, S.Y.; Tan, K.Y.; Tan, N.H. Proteomic Characterization of Venom of the Medically Important Southeast Asian Naja Sumatrana (Equatorial Spitting Cobra). Acta Trop. 2014, 133, 15–25.
- Mohamed Abd El-Aziz, T.; Soares, A.G.; Stockand, J.D. Snake Venoms in Drug Discovery: Valuable Therapeutic Tools for Life Saving. Toxins 2019, 11, 564.
- Kini, R.M.; Koh, C.Y. Snake Venom Three-Finger Toxins and Their Potential in Drug Development Targeting Cardiovascular Diseases. Biochem. Pharmacol. 2020, 181, 114105.
- Blomback, B.; Blomback, M.; Nilsson, I.M. Coagulation Studies on Reptilase, an Extract of the Venom from Bothrops Jararaca. Thromb. Diath. Haemorrh. 1958, 1, 76–86.
- Frangieh, J.; Rima, M.; Fajloun, Z.; Henrion, D.; Sabatier, J.M.; Legros, C.; Mattei, C. Snake Venom Components: Tools and Cures to Target Cardiovascular Diseases. Molecules 2021, 26, 2223.
- Waheed, H.; Moin, S.F.; Choudhary, M.I. Snake Venom: From Deadly Toxins to Life-Saving Therapeutics. Curr. Med. Chem. 2017, 24, 1874–1891.
- Chan, Y.S.; Cheung, R.C.F.; Xia, L.; Wong, J.H.; Ng, T.B.; Chan, W.Y. Snake Venom Toxins: Toxicity and Medicinal Applications. Appl. Microbiol. Biotechnol. 2016, 100, 6165–6181.
- Tan, C.H.; Tan, K.Y.; Tan, N.H. A Protein Decomplexation Strategy in Snake Venom Proteomics. Methods Mol. Biol. 2019, 1871, 83–92.
- Bird, I.M. High Performance Liquid Chromatography: Principles and Clinical Applications. BMJ 1989, 299, 783–787.
- Kadi-Saci, A.; Laraba-Djebari, F. Purification and Characterization of a Thrombin-like Enzyme Isolated from Vipera Lebetina Venom: Its Interaction with Platelet Receptor. Blood Coagul. Fibrinolysis 2020, 31, 1–10.
- Averin, A.S.; Utkin, Y.N. Cardiovascular Effects of Snake Toxins: Cardiotoxicity and Cardioprotection. Acta Nat. 2021, 13, 4–14.
- Karabuva, S.; Lukšić, B.; Brizić, I.; Latinović, Z.; Leonardi, A.; Križaj, I. Ammodytin L Is the Main Cardiotoxic Component of the Vipera ammodytes ammodytes Venom. Toxicon 2017, 139, 94–100.
- Sahyoun, C.; Krezel, W.; Mattei, C.; Sabatier, J.-M.; Legros, C.; Fajloun, Z.; Rima, M. Neuro- and Cardiovascular Activities of Montivipera bornmuelleri Snake Venom. Biology 2022, 11, 888.
- Zanotty, Y.; Álvarez, M.; Perdomo, L.; Sánchez, E.E.; Giron, M.E.; Jimenez, J.C.; Suntravat, M.; Guerrero, B.; Ibarra, C.; Montero, Y. Mutacytin-1, a New C-Type Lectin-like Protein from the Venezuelan Cuaima (Lachesis muta muta Linnaeus, 1766)(Serpentes: Viperidae) Snake Venom Inducing Cardiotoxicity in Developing Zebrafish (Danio rerio) Embryos. Zebrafish 2019, 16, 379–387.
- Menezes, T.N.; Naumann, G.B.; Peixoto, P.; Rouver, W.N.; Gomes, H.L.; Campos, F.V.; Borges, M.H.; Dos Santos, R.L.; Bissoli, N.S.; Sanchez, E.F.; et al. Bothrops leucurus Venom Induces Acute Hypotension in Rats by Means of Its Phospholipase A2 (blD-PLA2). Toxicon 2020, 185, 5–14.
- Tan, L.C.; Kuruppu, S.; Smith, A.I.; Reeve, S.; Hodgson, W.C. Isolation and Pharmacological Characterisation of Hostoxin-1, a Postsynaptic Neurotoxin from the Venom of the Stephen’s Banded Snake (Hoplocephalus stephensi). Neuropharmacology 2006, 51, 782–788.
- Lumsden, N.G.; Banerjee, Y.; Kini, R.M.; Kuruppu, S.; Hodgson, W.C. Isolation and Characterization of Rufoxin, a Novel Protein Exhibiting Neurotoxicity from Venom of the Psammophiine, Rhamphiophis oxyrhynchus (Rufous Beaked Snake). Neuropharmacology 2007, 52, 1065–1070.
- Marcon, F.; Purtell, L.; Santos, J.; Hains, P.G.; Escoubas, P.; Graudins, A.; Nicholson, G.M. Characterization of Monomeric and Multimeric Snake Neurotoxins and Other Bioactive Proteins from the Venom of the Lethal Australian Common Copperhead (Austrelaps superbus). Biochem. Pharmacol. 2013, 85, 1555–1573.
- Venkatesh, M.; Prasad, N.; Sing, T.; Gowda, V. Purification, Characterization, and Chemical Modification of Neurotoxic Peptide from Daboia Russelii Snake Venom of India. J. Biochem. Mol. Toxicol. 2013, 27, 295–304.
- Lomonte, B.; Calvete, J.J. Strategies in “snake Venomics” Aiming at an Integrative View of Compositional, Functional, and Immunological Characteristics of Venoms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 26.
- Abd El-Aziz, T.M.; Soares, A.G.; Stockand, J.D. Advances in Venomics: Modern Separation Techniques and Mass Spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1160, 122352.
- Slagboom, J.; Kaal, C.; Arrahman, A.; Vonk, F.J.; Somsen, G.W.; Calvete, J.J.; Wüster, W.; Kool, J. Analytical Strategies in Venomics. Microchem. J. 2022, 175, 107187.
- Tasoulis, T.; Pukala, T.L.; Isbister, G.K. Investigating Toxin Diversity and Abundance in Snake Venom Proteomes. Front. Pharmacol. 2021, 12, 768015.
- Tan, K.Y.; Liew, J.L.; Tan, N.H.; Quah, E.S.H.; Ismail, A.K.; Tan, C.H. Unlocking the Secrets of Banded Coral Snake (Calliophis intestinalis, Malaysia): A Venom with Proteome Novelty, Low Toxicity and Distinct Antigenicity. J. Proteom. 2019, 192, 246–257.
- Oh, A.M.F.; Tan, C.H.; Tan, K.Y.; Quraishi, N.H.; Tan, N.H. Venom Proteome of Bungarus sindanus (Sind Krait) from Pakistan and in Vivo Cross-Neutralization of Toxicity Using an Indian Polyvalent Antivenom. J. Proteom. 2019, 193, 243–254.
- Chen, P.C.; Huang, M.N.; Chang, J.F.; Liu, C.C.; Chen, C.K.; Hsieh, C.H. Snake Venom Proteome and Immuno-Profiling of the Hundred-Pace Viper, Deinagkistrodon Acutus, in Taiwan. Acta Trop. 2019, 189, 137–144.
- Lee, L.P.; Tan, K.Y.; Tan, C.H. Snake Venom Proteomics and Antivenomics of Two Sundaic Lance-Headed Pit Vipers: Trimeresurus wiroti (Malaysia) and Trimeresurus puniceus (Indonesia). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100875.
- Tan, C.H.; Tan, K.Y.; Ng, T.S.; Sim, S.M.; Tan, N.H. Venom Proteome of Spine-Bellied Sea Snake (Hydrophis curtus) from Penang, Malaysia: Toxicity Correlation, Immunoprofiling and Cross-Neutralization by Sea Snake Antivenom. Toxins 2018, 11, 3.
- Tan, K.Y.; Wong, K.Y.; Tan, N.H.; Tan, C.H. Quantitative Proteomics of Naja annulifera (Sub-Saharan Snouted Cobra) Venom and Neutralization Activities of Two Antivenoms in Africa. Int. J. Biol. Macromol. 2020, 158, 605–616.
- Hia, Y.L.; Tan, K.Y.; Tan, C.H. Comparative Venom Proteomics of Banded Krait (Bungarus fasciatus) from Five Geographical Locales: Correlation of Venom Lethality, Immunoreactivity and Antivenom Neutralization. Acta Trop. 2020, 207, 105460.
- Kumkate, S.; Chanhome, L.; Thiangtrongjit, T.; Noiphrom, J.; Laoungboa, P.; Khow, O.; Vasaruchapong, T.; Sitprija, S.; Chaiyabutr, N.; Reamtong, O. Venomics and Cellular Toxicity of Thai Pit Vipers (Trimeresurus macrops and T. hageni). Toxins 2020, 12, 54.
- Yang, Z.M.; Yang, Y.E.; Chen, Y.; Cao, J.; Zhang, C.; Liu, L.L.; Wang, Z.Z.; Wang, X.M.; Wang, Y.M.; Tsai, I.H. Transcriptome and Proteome of the Highly Neurotoxic Venom of Gloydius intermedius. Toxicon 2015, 107, 175–186.
- Bocian, A.; Urbanik, M.; Hus, K.; Łyskowski, A.; Petrilla, V.; Andrejčáková, Z.; Petrillová, M.; Legáth, J. Proteomic Analyses of Agkistrodon Contortrix Contortrix Venom Using 2D Electrophoresis and MS Techniques. Toxins 2016, 8, 372.
- Hus, K.K.; Buczkowicz, J.; Petrilla, V.; Petrillová, M.; Łyskowski, A.; Legáth, J.; Bocian, A. First Look at the Venom of Naja ashei. Molecules 2018, 23, 609.
- Leonardi, A.; Sajevic, T.; Pungerčar, J.; Križaj, I. Comprehensive Study of the Proteome and Transcriptome of the Venom of the Most Venomous European Viper: Discovery of a New Subclass of Ancestral Snake Venom Metalloproteinase Precursor-Derived Proteins. J. Proteome Res. 2019, 18, 2287–2309.
- Choksawangkarn, W.; Sriswasdi, S.; Kalpongnukul, N.; Wongkongkathep, P.; Saethang, T.; Chanhome, L.; Laoungbua, P.; Khow, O.; Sumontha, M.; Chaiyabutr, N.; et al. Combined Proteomic Strategies for In-Depth Venomic Analysis of the Beaked Sea Snake (Hydrophis schistosus) from Songkhla Lake, Thailand. J. Proteom. 2022, 259, 104559.
- Katali, O.; Shipingana, L.; Nyarangó, P.; Pääkkönen, M.; Haindongo, E.; Rennie, T.; James, P.; Eriksson, J.; Hunter, C.J. Protein Identification of Venoms of the African Spitting Cobras. Toxins 2020, 12, 520.
- Dias, Ê.; de Oliveira, L.A.; Sales Lauria, P.S.; Bordon, K.C.F.; Rodrigues Domênico, A.M.; da Silva Guerreiro, M.L.; Wiezel, G.A.; Cardoso, I.A.; Rossini, B.C.; Marino, C.L.; et al. Bothrops Leucurus Snake Venom Protein Profile, Isolation and Biological Characterization of Its Major Toxin PLA. Toxicon 2022, 213, 27–42.
- Gopcevic, K.; Karadzic, I.; Izrael-Zivkovic, L.; Medic, A.; Isakovic, A.; Popović, M.; Kekic, D.; Stanojkovic, T.; Hozic, A.; Cindric, M. Study of the Venom Proteome of Vipera ammodytes ammodytes (Linnaeus, 1758): A Qualitative Overview, Biochemical and Biological Profiling. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 37, 100776.
- Nie, X.; He, Q.; Zhou, B.; Huang, D.; Chen, J.; Chen, Q.; Yang, S.; Yu, X. Exploring the Five-Paced Viper (Deinagkistrodon acutus) Venom Proteome by Integrating a Combinatorial Peptide Ligand Library Approach with Shotgun LC-MS/MS. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200196.
- Hus, K.K.; Marczak, Ł.; Petrilla, V.; Petrillová, M.; Legáth, J.; Bocian, A. Different Research Approaches in Unraveling the Venom Proteome of Naja Ashei. Biomolecules 2020, 10, 1282.
- Choudhury, M.; McCleary, R.J.R.; Kesherwani, M.; Kini, R.M.; Velmurugan, D. Comparison of Proteomic Profiles of the Venoms of Two of the “Big Four” Snakes of India, the Indian Cobra (Naja naja) and the Common Krait (Bungarus caeruleus), and Analyses of Their Toxins. Toxicon 2017, 135, 33–42.
- Ghezellou, P.; Garikapati, V.; Kazemi, S.M.; Strupat, K.; Ghassempour, A.; Spengler, B. A Perspective View of Top-down Proteomics in Snake Venom Research. Rapid Commun. Mass Spectrom. 2019, 33 (Suppl. 1), 20–27.
- Calvete, J.J.; Pla, D.; Els, J.; Carranza, S.; Damm, M.; Hempel, B.F.; John, E.B.O.; Petras, D.; Heiss, P.; Nalbantsoy, A.; et al. Combined Molecular and Elemental Mass Spectrometry Approaches for Absolute Quantification of Proteomes: Application to the Venomics Characterization of the Two Species of Desert Black Cobras, Walterinnesia aegyptia and Walterinnesia morgani. J. Proteome Res. 2021, 20, 5064–5078.
- Melani, R.D.; Skinner, O.S.; Fornelli, L.; Domont, G.B.; Compton, P.D.; Kelleher, N.L. Mapping Proteoforms and Protein Complexes From King Cobra Venom Using Both Denaturing and Native Top-down Proteomics. Mol. Cell. Proteom. 2016, 15, 2423–2434.
- Ainsworth, S.; Petras, D.; Engmark, M.; Süssmuth, R.D.; Whiteley, G.; Albulescu, L.O.; Kazandjian, T.D.; Wagstaff, S.C.; Rowley, P.; Wüster, W.; et al. The Medical Threat of Mamba Envenoming in Sub-Saharan Africa Revealed by Genus-Wide Analysis of Venom Composition, Toxicity and Antivenomics Profiling of Available Antivenoms. J. Proteom. 2018, 172, 173–189.
- Petras, D.; Heiss, P.; Harrison, R.A.; Süssmuth, R.D.; Calvete, J.J. Top-down Venomics of the East African Green Mamba, Dendroaspis angusticeps, and the Black Mamba, Dendroaspis polylepis, Highlight the Complexity of Their Toxin Arsenals. J. Proteom. 2016, 146, 148–164.
- Ghezellou, P.; Albuquerque, W.; Garikapati, V.; Casewell, N.R.; Kazemi, S.M.; Ghassempour, A.; Spengler, B. Integrating Top-Down and Bottom-Up Mass Spectrometric Strategies for Proteomic Profiling of Iranian Saw-Scaled Viper, Echis carinatus sochureki, Venom. J. Proteome Res. 2021, 20, 895–908.
- Hempel, B.F.; Damm, M.; Mrinalini; Göçmen, B.; Karış, M.; Nalbantsoy, A.; Kini, R.M.; Süssmuth, R.D. Extended Snake Venomics by Top-Down In-Source Decay: Investigating the Newly Discovered Anatolian Meadow Viper Subspecies. J. Proteome Res. 2020, 19, 1731–1749.
