The sol–gel method produces glass and ceramic materials at lower temperatures than the corresponding products molded by melting. This method uses alkoxides, water, catalyst, and solvent. Depending on the relationship between alkoxide/water and the kind of catalyst, one can obtain a one-dimensional, two-dimensional, or three-dimensional network where one takes fibers, thin films, or bulk materials. Some years ago, scientists attempted to produce coatings on metals to protect against corrosion.
- corrosion
- CBC
- Sol–Gel
1. Brief Introduction of Sol–Gel Processing
Using a multiple immersion technique in the sol solution followed by a densification process, they deposited on copper, nickel, iron, and aluminum metals protective ZrO2, SiO2, TiO2, and B2O3-3SiO2 coatings. They were using the dip-coating method of metals in the sol solution, where they were first left to dry and next heat-treated slowly (5 °C/min) up to the temperature of 500 °C. The effectiveness of the sol–gel coatings against corrosion in the early studies was examined with weight loss measurements by exposing them to salt solutions. These studies revealed that the protection offered by the coating depends on its thickness. The increased thickness led to cracks during their thermal densification treatment or the alternating temperature during operation [1]. From the above studies, inorganic oxide coatings can protect metal substrates but do not significantly replace chromium-based corrosion barrier coatings (CBCs) [2]. One needs to reduce the densification temperature to 200 °C to avoid the metal’s destruction and develop flexible coatings that will adapt their physical properties (e.g., anticorrosion protection, the thermal expansion coefficient of the coating) to the properties of the metal. To realize that requirement, one needs precursors consisting of two primary components, the one organic and the other inorganic, the one responsible for the flexibility of the CBC, and the other for the anticorrosion properties.
2. Silanes
| Material | Structure |
|---|---|
| Vinyl trimethoxy silane | 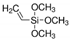 |
| Bis-l,2-(trimethoxysilyl) ethane | 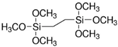 |
| Bis-[trimethoxysilylpropyl]amine |  |
| (3-Aminopropyl)trimethoxysilane | 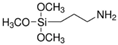 |
| 3-Glycidyloxypropyl- trimethoxysilane | 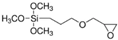 |
| 3-(Trimethoxysilyl)propyl methacrylate | 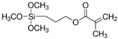 |
| 3-(2-Aminoethylamino)-propyldimethoxymethylsilane | 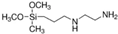 |
| 1,2-Bis(trimethoxysilyl)ethane | 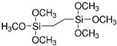 |
3. ORMOSIL
- a.
-
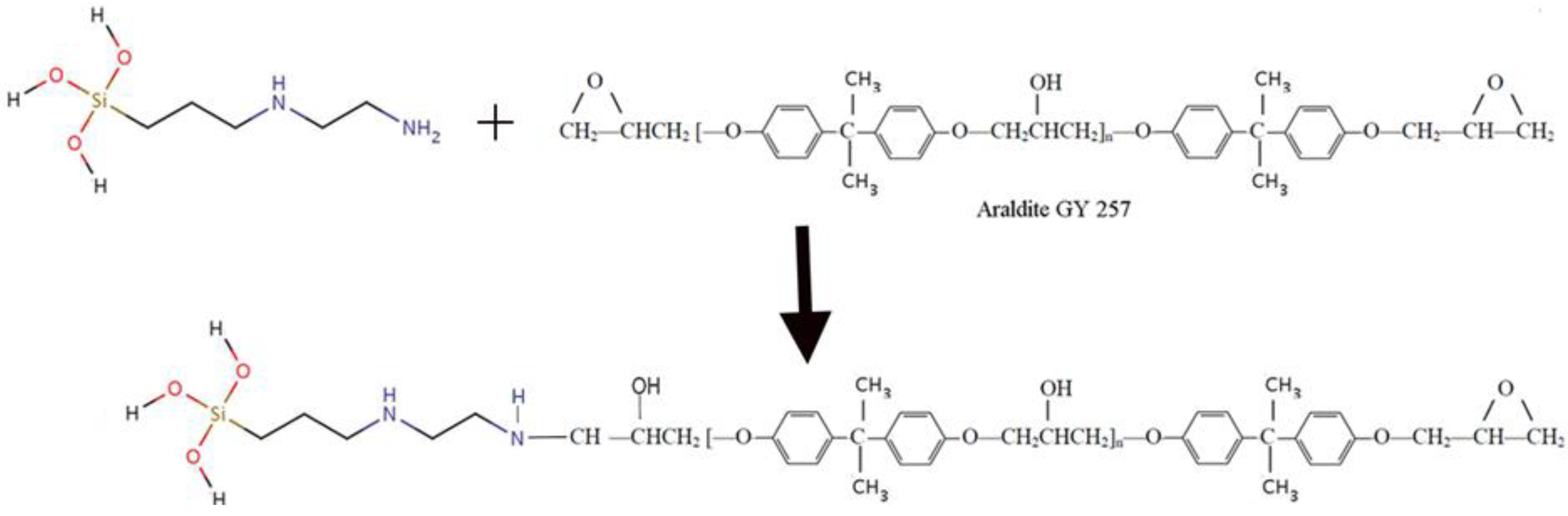
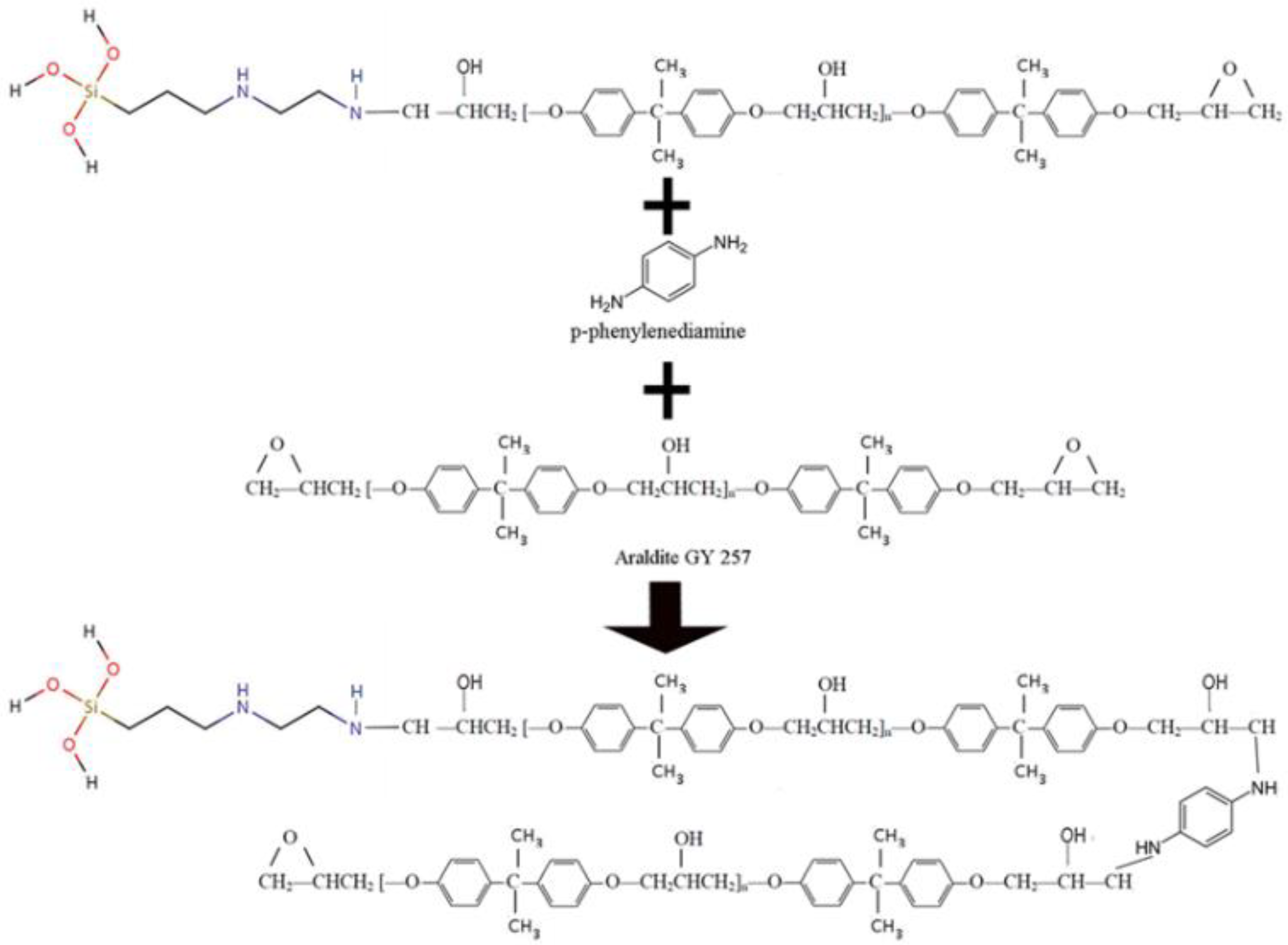
Precursors and Reactions
- b.
-
Organically Modified Silane
- c.
-
Epoxy Resin
- d.
-
Hardener
- e.
-
Solvents
- f.
-
Preparation of ORMOSIL
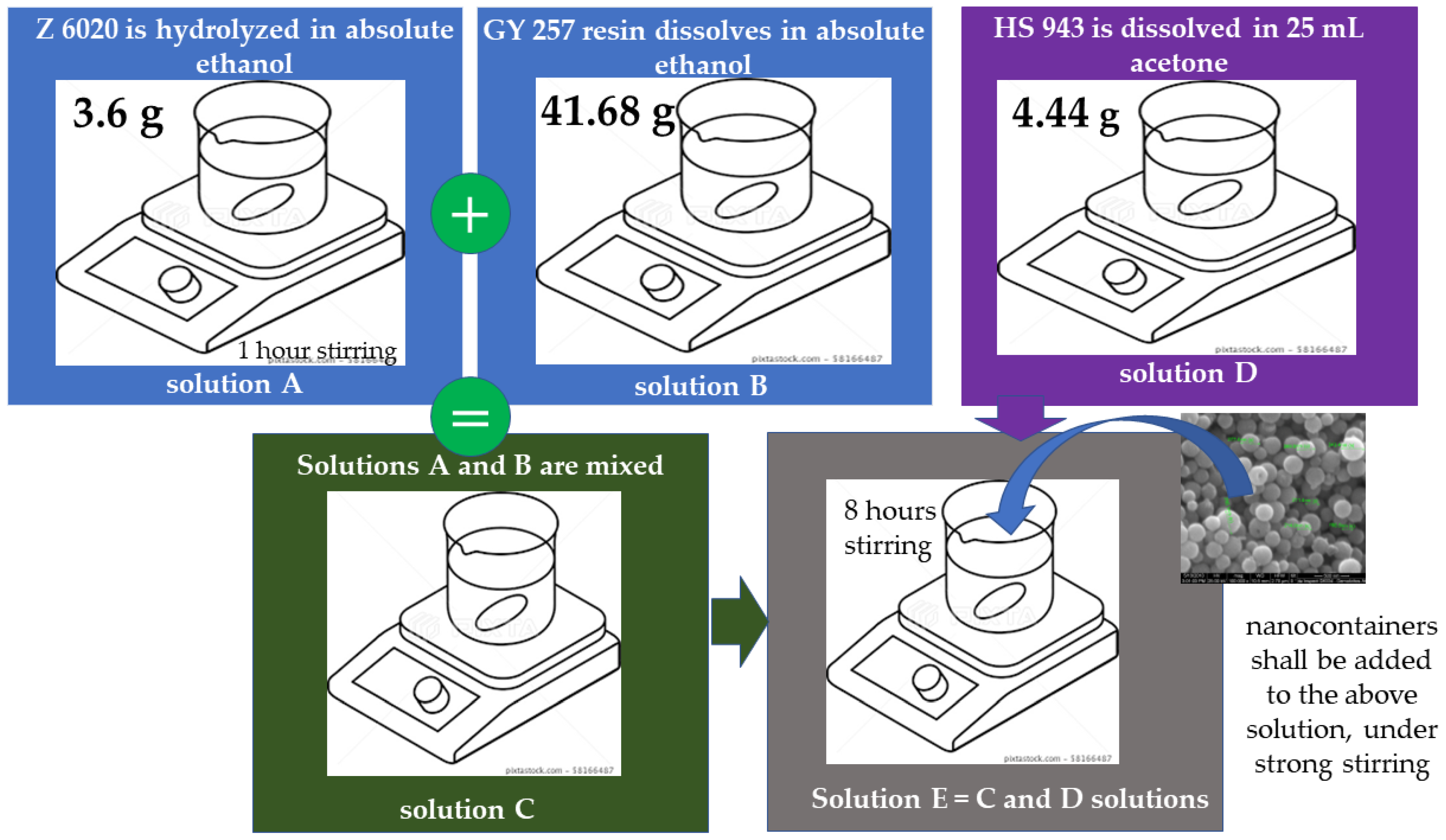
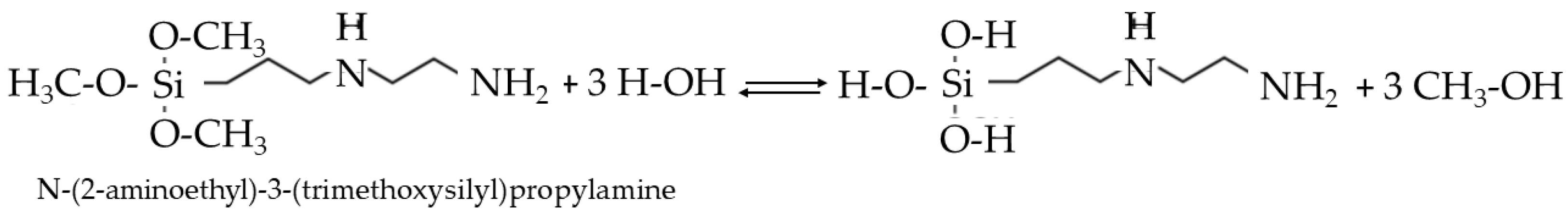
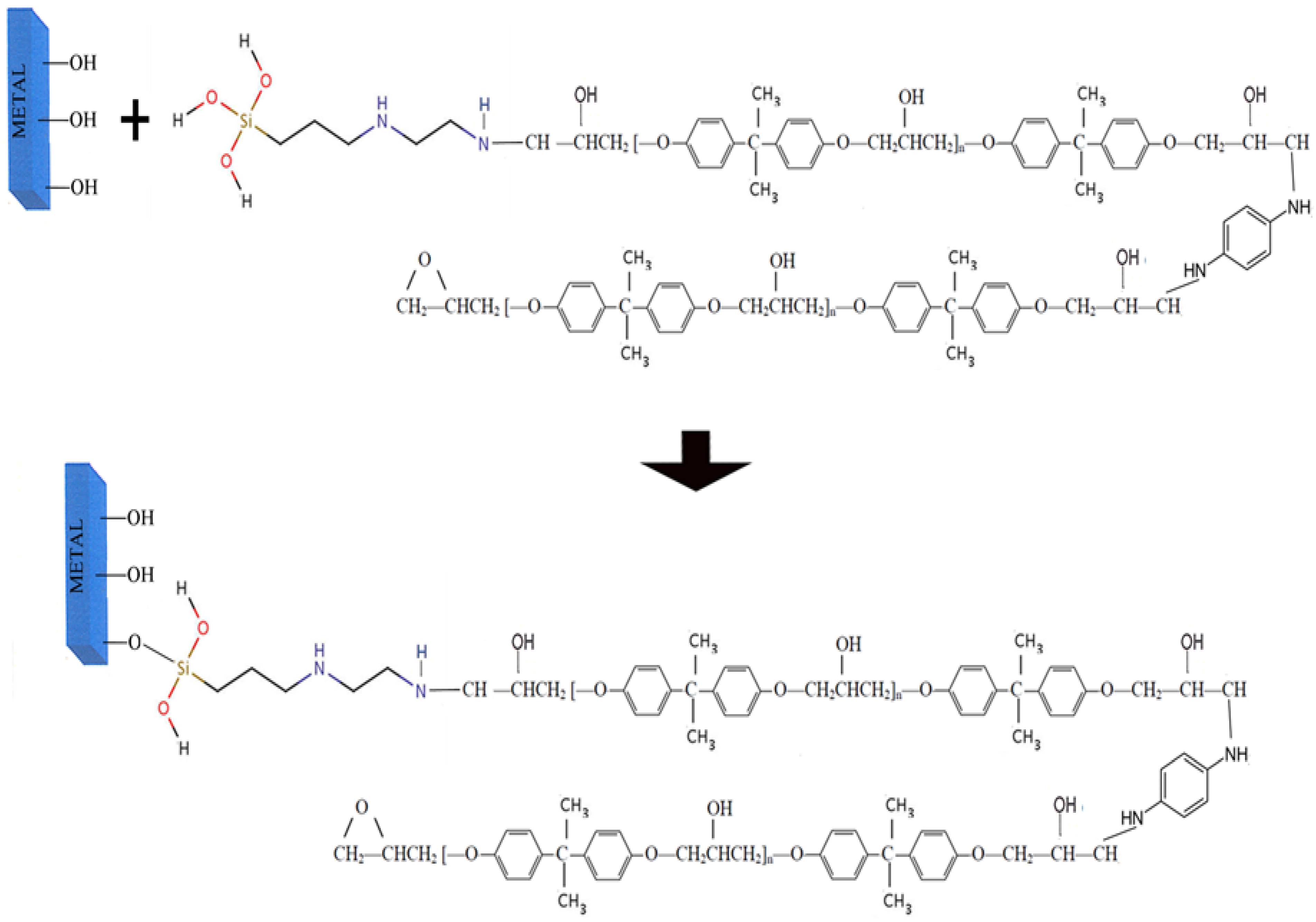

4. Water-Based Commercial Sol-Gel CBCs
References
- Innocenzi, P.C.; Guglielmi, M.; Gobbin, M.; Colombo, P. Coating of metals by the sol-gel dip-coating method. J. Eur. Ceram. Soc. 1992, 10, 431–436.
- Wang, D.; Bierwagen, G.P. Sol-gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338.
- Deflorian, F.; Rossi, S.; Fedel, M.; Ecco, L.G.; Paganica, R.; Bastarolo, M.; Mathiazhagan, A.; Joseph, R.; Chen, X.; Hong, R.; et al. Corrosion protection properties of organofunctional silanes—An overview. Prog. Org. Coat. 2005, 51, 259–269.
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic–inorganic hybrid sol–gel coatings for metal corrosion protection: A review of recent progress. J. Coat. Technol. Res. 2015, 12, 1–35.
- Palomino, L.M.; Suegama, P.H.; Aoki, I.V.; Fatima Montemor, M.; De Melo, H.G. Electrochemical study of modified non-functional bis-silane layers on Al alloy 2024-T3. Corros. Sci. 2008, 50, 1258–1266.
- Montemor, M.F.; Cabral, A.M.; Zheludkevich, M.L.; Ferreira, M.G.S. The corrosion resistance of hot dip galvanized steel pretreated with Bis-functional silanes modified with microsilica. Surf. Coat. Technol. 2006, 200, 2875–2885.
- Franquet, A.; Biesemans, M.; Willem, R.; Terryn, H.; Vereecken, J. Multinuclear 1D- and 2D-NMR study of the hydrolysis and condensation of bis-1,2-(triethoxysilyl)ethane. J. Adhes. Sci. Technol. 2004, 18, 765–778.
- Palanivel, V.M. Modified Silane Thin Films as an Aternative to Chromates for Corrosion Protection of AA2024-T3 Alloy. Ph.D. Thesis, University of Cincinnati, Cincinnatim, OH, USA, 1957. Volume 9.
- Figueira, R.B.; Fontinha, I.R.; Silva, C.J.R.; Pereira, E.V.; Yang, K.; van Ooij, W.J.; Seth, A.; Mugada, T.; Pan, G.; Schaefer, D.W.; et al. Hybrid sol-gel coatings: Smart and green materials for corrosion mitigation. Prog. Org. Coat. 2016, 63, 283–292.
- Trabelsi, W.; Dhouibi, L.; Triki, E.; Ferreira, M.G.S.; Montemor, M.F.; Cabral, A.M.; Duarte, R.G.; Montemor, M.F.; Zheludkevich, M.L.; Ferreira, M.G.S.; et al. Corrosion protection properties of organofunctional silanes—An overview. Prog. Org. Coat. 2005, 48, 639–664.
- Mirabedini, S.M.; Thompson, G.E.; Moradian, S.; Scantlebury, J.D. Corrosion performance of powder coated aluminium using EIS. Prog. Org. Coat. 2003, 46, 112–120.
- Franquet, A.; Terryn, H.; Vereecken, J. IRSE study on effect of thermal curing on the chemistry and thickness of organosilane films coated on aluminium. Appl. Surf. Sci. 2003, 211, 259–269.
- Aïssa, B.; Therriault, D.; Haddad, E.; Jamroz, W. Self-healing materials systems: Overview of major approaches and recent developed technologies. Adv. Mater. Sci. Eng. 2012, 2012, 854203.
- Ji, W.G.; Hu, J.M.; Liu, L.; Zhang, J.Q.; Cao, C.N. Improving the corrosion performance of epoxy coatings by chemical modification with silane monomers. Surf. Coat. Technol. 2007, 201, 4789–4795.
- Kim, H.; Jang, J. Corrosion protection and adhesion promotion for polyimide/copper system using silane-modified polymeric materials. Polymer 2000, 41, 6553–6561.
- Chen, M.A.; Xie, X.; Zhang, X.M. Interactions of BTESPT silane and maleic anhydride grafted polypropylene with epoxy and application to improve adhesive durability between epoxy and aluminium sheet. Prog. Org. Coat. 2009, 66, 40–51.
- Cabral, A.; Duarte, R.G.; Montemor, M.F.; Zheludkevich, M.L.; Ferreira, M.G.S. Analytical characterisation and corrosion behaviour of bis-tetrasulphide pre-treated AA2024-T3. Corros. Sci. 2005, 47, 869–881.
- Montoya, P.; Martins, C.R.; De Melo, H.G.; Aoki, I.V.; Jaramillo, F.; Calderón, J.A. Synthesis of polypyrrole-magnetite/silane coatings on steel and assessment of anticorrosive properties. Electrochim. Acta 2014, 124, 100–108.
- Nguyen Thi Le, H.; Bernard, M.C.; Garcia-Renaud, B.; Deslouis, C. Raman spectroscopy analysis of polypyrrole films as protective coatings on iron. Synth. Met. 2004, 140, 287–293.
- Herrasti, P.; Recio, F.J.; Ocón, P.; Fatás, E. Effect of the polymer layers and bilayers on the corrosion behaviour of mild steel: Comparison with polymers containing Zn microparticles. Prog. Org. Coat. 2005, 54, 285–291.
- Van Schaftinghen, T.; Deslouis, C.; Hubin, A.; Terryn, H. Influence of the surface pre-treatment prior to the film synthesis, on the corrosion protection of iron with polypyrrole films. Electrochim. Acta 2006, 51, 1695–1703.
- Giacomelli, C.; Giacomelli, F.C.; Baptista, J.A.A.; Spinelli, A. The effect of oxalic acid on the corrosion of carbon steel. Anti-Corros. Methods Mater. 2004, 51, 105–111.
- Asan, A.; Kabasakaloglu, M. Electrochemical and corrosion behaviors of mild steel coated with polypyrrole. Mater. Sci. 2003, 39, 643–651.
- Rahman, S.U. Corrosion protection of steel by catalyzed polypyrrole films. Surf. Coat. Technol. 2011, 205, 3035–3042.
- Koene, L.; Hamer, W.J.; De Wit, J.H.W. Electrochemical behaviour of poly(pyrrole) coatings on steel. J. Appl. Electrochem. 2006, 36, 545–556.
- Grgur, B.N.; Krstajić, N.V.; Vojnović, M.V.; Lačnjevac, Č.; Gajić-Krstajić, L. The influence of polypyrrole films on the corrosion behavior of iron in acid sulfate solutions. Prog. Org. Coat. 1998, 33, 1–6.
- Grgur, B.N.; Živković, P.; Gvozdenović, M.M. Kinetics of the mild steel corrosion protection by polypyrrole-oxalate coating in sulfuric acid solution. Prog. Org. Coat. 2006, 56, 240–247.
- Ocón, P.; Cristobal, A.B.; Herrasti, P.; Fatas, E. Corrosion performance of conducting polymer coatings applied on mild steel. Corros. Sci. 2005, 47, 649–662.
- Roy, I. Synthesis, Surface Modification, Characterization, and Biomedical In Vitro Applications of Organically Modified Silica (ORMOSIL) Nanoparticles. In Nanoparticles in Biology and Medicine: Methods and Protocols; Soloviev, M., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; Volume 906, pp. 365–379. ISBN 9781617799532.
- Kordas, G. Nanocontainers Against Biofouling and Corrosion Degradation of Materials: A Short Review With Prospects. Front. Nanotechnol. 2022, 4, 813908.
- Kordas, G. Nanocontainers (CeO2): Synthesis, Characterization, Properties, and Anti-corrosive Application. ACS Symp. Ser. 2021, 1404, 177–185.
- Kordas, G. Nanocontainers for Enhanced Protection of HDG Steel Used in Concrete. Materials 2022, 2, 1–12.
- Metroke, T.L.; Apblett, A. Effect of solvent dilution on corrosion protective properties of Ormosil coatings on 2024-T3 aluminum alloy. Prog. Org. Coat. 2004, 51, 36–46.
- Metroke, T.L.; Kachurina, O.; Knobbe, E.T. Spectroscopic and corrosion resistance characterization of GLYMO-TEOS Ormosil coatings for aluminum alloy corrosion inhibition. Prog. Org. Coat. 2002, 44, 295–305.
- Program, E.E.S.T.C. Non-Chromate Aluminum Pretreatments Phase II Interim Report. Available online: http://www.iaindy.com/Documents/ncapii.pdf (accessed on 18 April 2022).
- Chung, Y.J.; Jeanjaquet, S.L.; Kendig, M.W. Normal Probability Function. US Patent 6,579,472 B2, 17 June 2003.
