Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Christina Breanne Welch.
This interaction between the microbes colonizing the GIT and the immune system impacts organs throughout the host and forms an “axis” that can send signals. Some examples of these axes in cattle include the established gut-brain axis and gut-lung axis and the proposed gut-mammary axis and gut-reproductive axis.
- microbiome-gut-brain axis
- microbiome-gut-mammary axis
- microbiome-gut-reproductive axis
1. Microbiome-Gut-Organ Axes
Recently, there has been a major drive towards understanding the complex synergistic relationship that exists between the gastrointestinal tract (GIT) microbial population and the host. The GIT microbiota interacts with all aspects of the body through the different microbiome-gut-organ axes (MGOA). The metabolites produced by the GIT microbiota send signals throughout the body to different organs, which affect the immune system and host physiology [113,114][1][2]. This interaction between the microbes colonizing the GIT and the immune system impacts organs throughout the host and forms an “axis” that can send signals [115][3]. Some examples of these axes in cattle include the established gut-brain axis and gut-lung axis [116][4] and the proposed gut-mammary axis and gut-reproductive axis (Figure 1).
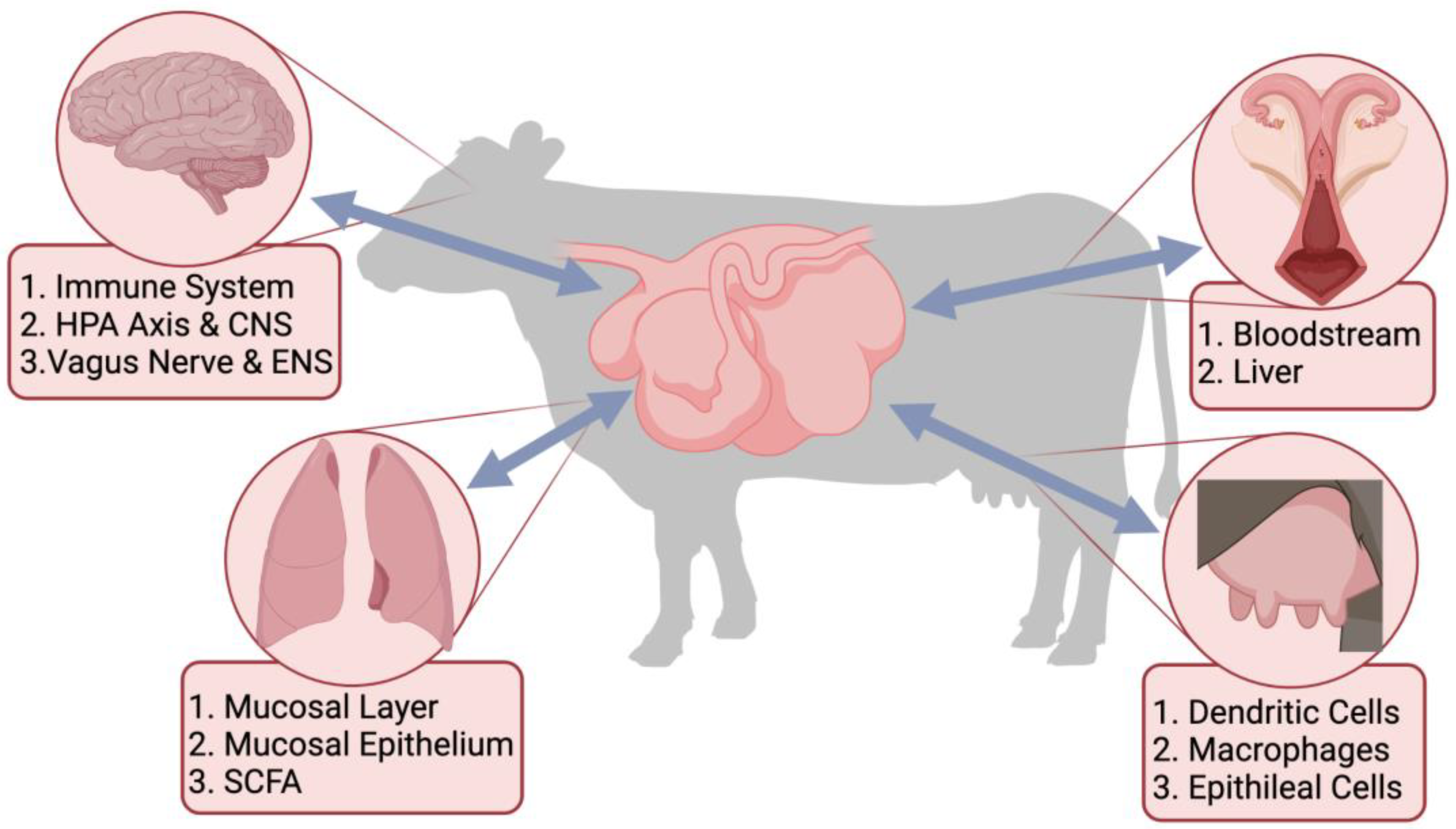
Figure 1. Proposed links between the gastrointestinal tract microbiota and different organ systems through the microbiome-gut-organ axes, including the microbiome-gut-brain axis (MGOA), the microbiome-gut-lung axis, the microbiome-gut-reproductive axis, and the microbiome-gut-mammary axis. Included are pathways, cells, and metabolites important for the bi-directional communication of the MGOA.
The different MGOA enable bidirectional communication between the GIT and different organs that occur through signaling pathways [117,118][5][6]. The metabolites produced in the GIT by the microbes can have a direct impact on the host’s risk of infection [119][7]. Dietary or environmental stressors can alter the species diversity within the GIT, leaving the microbiota susceptible to pathogenic colonization [119,120][7][8]. Ultimately, any alterations within the gut microbial population can have cascading detrimental effects on the health of the host through these different axes. Therefore, it is imperative wpeople understand the mechanisms of the microbiota within the GIT and its communication with other organs to ensure the health and well-being of animals.
2. Microbiome-Gut-Brain Axis
One of the most extensively studied gut-organ-axes within all mammalian systems is the microbiome-gut-brain axis (MGBA). This axis serves as a bi-directional link where signals and metabolites can be sent between the brain and the gut. Previous research has revealed the microbes within the GIT play an important role in many processes within the brain of the host, including the development of the brain, neural processes, pain processes, the hypothalamic-pituitary-adrenal (HPA) axis, and behavior [121][9]. Due to its impact on the brain, the MGBA has been increasing in popularity as a tool to modulate brain function and, ultimately, host health.
There are three different pathways that serve as routes of communication for the MGBA [122][10]. The first is the immunoregulatory pathway, where the immune system interacts with the microbiota and affects the production of cytokines, cytokinetic reaction factors, and prostaglandin E2 [123][11], which subsequently alters brain function [13][12]. The second is the neuroendocrine pathway which involves both the HPA axis and the central nervous system (CNS). The intestines of mammals serve as one of the largest endocrine organs in the body by possessing over 20 different types of enteroendocrine cells [124][13]. Additionally, the microbes within the gut regulate the production of many neurotransmitters (e.g., cortisol) through the HPA axis and CNS [125][14]. The last pathway is connected through the vagus nerve and enteric nervous system (ENS). The ENS forms synapses with the vagus nerve, which allows communication between the microbes and the brain [125][14]. Ruminal fermentation produces metabolites that are toxic to the brain (e.g., ammonia and D-lactic acid), which can travel through this pathway to negatively impact brain function and the host’s stress response and quality of sleep [126][15]. Additionally, sensory neurons can send signals through the CNS to control gut motility and hormone secretion [13][12].
The role the MGBA plays in modulating host health has been studied thoroughly in humans; however, research investigating the MGBA has expanded to animals. This communication can occur when there is an infection in the GIT microbiota that negatively impacts the brain and increases sickness behavior or when the GIT microbiota is healthy and promotes brain function. Research utilizing germ-free animals has shown that the inclusion of probiotics and prebiotics results in behavioral changes [127][16]. Throughout livestock production, there are many stressful events (e.g., weaning and transportation) that are unavoidable. Research has shown that after transportation, there is an increase in cortisol, adrenocorticotropic hormone (ACTH), and pro-inflammatory cytokines (i.e., IL-6, TNF-α, IL-1β) in multiple breeds of beef cattle [128][17]. Theisr study also found an abundance of ruminal Lactobacillus that were positively correlated with IL-6 and IL-4. In addition, probiotics or prebiotics can be added to the diet of ruminants to prevent the signaling of the HPA axis to increase anxiety. A study utilizing dairy calves found supplementing the calf’s diet with a multispecies probiotic prior to weaning improved growth, decreased the incidence of diarrhea, affected the fecal microbiota (e.g., increased abundances of Bifidobacterium, Lactobacillus, Collinsella, and Saccharomyces), and reduced serum concentration (i.e., IgA, IgG, and IgM) [129][18]. Ultimately the impact of the GIT microbiota on behavior in animals directly impacts the overall health of the host since the microbes directly impact the immune system of the host [130][19].
In addition to general behavior being affected by the GIT microbiota, feed behavior is directly impacted by the microbial population within the GIT, which directly impacts the feed efficiency of livestock. An increase in pathogenic bacteria within the GIT microbiota results in an increase in sickness behavior followed by a reduction in feed intake [127][16]. When ruminants are fed a high-starch diet, they can experience ruminal acidosis, which can negatively influence feed behavior by decreasing both feed intake and the time spent ruminating [131][20]. This can be reversed by an ruminal fluid transplants (RFT) by introducing healthy microbes back into the GIT microbiota. This increases feed intake while decreasing inflammation, ultimately promoting host health [132][21]. In addition to an RFT, the utilization of a probiotic, S. cerevisiae, can inhibit the negative effects of ruminal acidosis (e.g., reduced pH) by increasing ruminal pH [92,133][22][23]. This influences the MGBA by increasing feed behavior by increasing the time spent ruminating and decreasing the time between feedings [134][24].
3. Microbiome-Gut-Lung Axis
Another MGOA discovered more recently is the microbiome-gut-lung-axis (MGLA). Due to the inability to culture microbes from the lungs of healthy hosts, the lungs were originally thought to be sterile unless an infection was present [135][25]. With the utilization of sequencing technologies (e.g., whole genome sequencing and 16S rRNA gene sequencing), researchers were able to discover that the lungs were inhabited by a community of commensal microbes existing in healthy individuals [136][26]. These microbial communities play a protective role in the respiratory tract, preventing the colonization of bacteria or viruses, which can cause disease [137][27]. Much like the GIT microbiota, the respiratory microbiota plays a role in regulating the activation of both the innate and adaptive immune responses [138,139][28][29].
The respiratory tract is divided into two parts based on location and function: the upper respiratory tract (URT) and the lower respiratory tract (LRT) [140][30]. The URT is comprised of the nasal cavity, paranasal sinuses, nasal passages, nasopharynx, oropharynx, tonsils, and upper portion of the larynx. In contrast, the LRT contains the larynx, trachea, bronchi, bronchioles, and alveoli [141][31]. Much like their function, the URT and LRT are colonized by different microbes [142,143,144][32][33][34] shortly after birth [145][35]. The most abundant phyla of the LRT are Bacteroidetes and Firmicutes, which is similar to the oral cavity microbiota suggesting the oral cavity plays a role in the development of the lung microbiota [136,146,147][26][36][37]. On the other hand, the microbial population of the nasal cavity is mainly comprised of the phyla Firmicutes and Actinobacteria, which more closely resembles the microbial population of the skin [148[38][39][40],149,150], suggesting the development of the nasal cavity microbiota is influenced by the skin microbiota. Due to differences in the suspected sources of colonization of the URT and LRT, researchers need to be cautious when drawing conclusions about the microbiota of one location based on the other. However, research has revealed correlations between microbes within the URT and LRT that can influence both microbial communities [144][34]. Other factors are known to influence the development of the respiratory microbiota, including diet [151][41], genetics, age [145][35], vaccination administration, management, and environment [152,153][42][43].
Although signaling through the MGLA travels bi-directionally, the majority of the communication between the microbial populations of the GIT and respiratory tract travels from the gut to the lungs [135][25]. The specific mechanisms and pathways involved in the MGLA in cattle remain undiscovered. Still, micro-aspiration, inhalation of bacteria, and transfusion of bacteria through mucosal cells play an important role in the communication [154][44]. Additionally, the lymphatic system and bloodstream play an important role in communication between the GIT and respiratory tract by carrying bacteria and bacterial metabolites from the GIT to the lungs [155][45].
Within the respiratory tract, the nasopharyngeal mucosal layer serves as the first line of defense against pathogenic colonization by capturing particles that are inhaled through the respiratory tract and moving them back up into nasal and oral cavities [141][31]. The mucus contains immune cells, including antimicrobial peptides, glycoproteins, and IgA, that help maintain homeostasis in the respiratory microbiota [156,157][46][47]. The second line of defense is the mucosal epithelium which produces molecules that trigger innate and adaptive immune responses to improve barrier function [158,159,160,161][48][49][50][51]. Not only do the respiratory tract epithelial cells, but luminal and mucosal surface macrophages and dendritic cells express innate patter-recognition receptors that work to identify and clear pathogenic microorganisms [161,162][51][52]. The commensal microbes comprising the microbiota of the respiratory tract, mucosal epithelium, and the immune system communicate to promote respiratory health, reduce inflammation, and maintain a functioning microbiota [141][31].
One of the main ways the GIT microbiota influences the immune system and, thus, the respiratory tract through the MGLA is with short chain SCFAfatty acid (SCFA) [135][25]. SCFA are important for maintaining intestinal integrity and preventing inflammation in both the gut and the respiratory tract [163,164,165][53][54][55]. SCFA can enhance the intestinal epithelial barrier function within the gut by increasing mucus production by goblet cells [166,167][56][57] and strengthening tight junctions [168][58]. Additionally, SCFA increase IgA production by enhancing plasma B cells metabolism to ensure the intestines are protected from inflammation [169,170][59][60]. An individual SCFA, butyrate, aids the intestinal epithelium in suppressing inflammation to maintain homeostasis [171][61]. SCFA also promote intestinal homeostasis through a positive feedback loop directing the metabolism of intestinal cells toward increased fatty acid ß-oxidation [172][62]. Butyrate supplementation has the ability to improve epithelial integrity while also increasing the host’s defense mechanisms [173][63].
Due to the numerous stressors calves are exposed to during weaning, it serves as one of the most influential times for respiratory microbiota development [151,152,174,175][41][42][64][65]. The composition of the URT is majorly affected by the first 40 days after arrival at the feedlot [176][66]. This is due to the stress, exposure to diseases, and dietary changes that occur when calves arrive at the feedlot that can result in dysbiosis in the URT, which weakens a calf’s immune response and allow pathogens in the URT to migrate into the LRT [146,154,177,178][36][44][67][68]. In dairy calves experiencing illness, there was an increase in Mannheimia, Moraella, and Mycoplasma in their URT compared to that of healthy calves [179][69]. Additionally, the URT of calves that later developed pneumonia was inhabited by a greater number of bacteria at three days of age than calves that remained healthy.
Bovine raspatory disease (BRD) is one of the most significant health concerns that can occur in weaned calves or feedlot cattle shortly after transportation [180][70]. After years of research to eradicate the disease, BRD remains one of the leading causes of morbidity, mortality, welfare issues, and economic losses within beef production [181][71]. It is influenced by a combination of factors, including the host, environment, and management [175,180,182][65][70][72]. The bacteria causing BRD are common commensals (e.g., Mycoplasma, Mannheimia, Histophilus, and Pasteurella) within the URT microbiota of healthy and sick cattle that can translocate from the URT to the LRT through inhalation after the host undergoes stress resulting in the development of pneumonia [177,179,183,184,185][67][69][73][74][75]. One example of how these commensals cause disease is in the case of Mannheimia haemolytica. After the stressors occurring after arrival at a feedlot occur and cause dysbiosis, M. haemolytica rapidly proliferates within the URT [186][76]. It then travels to the bronchial epithelial cells, where it damages tight junction proteins, causes lesions in the lungs, releases leukotoxins and lipopolysaccharides, which cause further damage to the respiratory tract, and triggers the host’s immune response causing inflammation. Due to this disease occurring as a result of a stressful event to the host, the MGLA may play a role in the development of this disease; therefore, the GIT microbiota may aid producers in helping eradicate the disease.
Several studies have shown that employing management strategies can positively impact the nasopharyngeal microbial diversity in calves post-weaning [141][31]. Management strategies can thus help the immune system maintain respiratory health during stressful times throughout the production cycle that leaves the host susceptible to diseases. Research has shown that dietary changes that influence the GIT microbiota shortly after weaning can also have an impact on the respiratory microbiota [151][41]. One example is preconditioning weaned calves for nine weeks prior to entry into a feedlot with selenium-fortified alfalfa hay can positively impact the microbial population in the nasal cavity [151][41]. This suggests fortifying the GIT microbiota prior to stress can help prevent respiratory disease through the MGLA.
4. Microbiome-Gut-Mammary Axis
As research on the GIT microbiota continues to progress, it becomes evident that the microbial population plays a major role in the functioning and disease prevention in other organs besides the brain and the lungs. Within cattle production, the mammary gland is an organ with major importance not only for cattle in general but also for producers in terms of milk production. Much like the GIT and the respiratory tract, the mammary system is also inhabited by a community of microbes [187][77]. Research into the milk microbiota has begun to suggest communication between the GIT microbiota and the mammary microbiota through a microbiome-gut-mammary axis (MGMA).
The microbiota of the milk is influenced by direct and indirect contact. Direct contact comes from contact with the surface of the teat from milking machines or other dairy equipment [188][78]. Indirect contact stems from environmental factors, including bedding, feces, forage, drinking water, washing water, air, and the milker itself [189,190][79][80]. A study of healthy Holstein Friesian and Rendena cows showed the milk microbiota was influenced by breed [188][78]. Although there were differences present, Firmicutes was the most abundant phylum, with Streptococcus being the most abundant genus. Milk from cows infected with mastitis was dominated by the order Enterobacteriales, followed by Pseudomonadales, Bacillales, and Lactobacillales [191][81]. In the purebred cattle, E. coli was the most abundant genus, followed by Pseudomonas aeruginosa, P. mendocina, Shigella flexneri, and Bacillus cereus; whereas, in the crossbred cattle, the most dominant genus was Staphylococcus aureus followed by Klebsiella pneumoniae, S. epidermidis, and E. coli. Although the majority of the bacteria detected are pathogenic, there are still other commensal strains present. Previous studies have found some of the commensals can come from direct contact with skin or can travel from the intestinal lumen to the mammary gland through the communication of the MGMA [187,192][77][82].
The mechanisms by which the mammary gland and the GIT microbiota communicate with each other has yet to be fully elucidated. The current knowledge of the route of communication between these two organs is from research done in humans and mice. A previous study reported that bacteria travel from the GIT to the mammary system via dendritic cells and macrophages [193][83]. These cell types, which are in the gut-associated mucosal tissue (GALT), can internalize bacteria from the GIT microbiota and translocate it to a different location, such as the mammary gland [194,195][84][85]. This suggests an infection within the mammary system may affect the integrity of the epithelial cells allowing translocation of pathogenic bacteria through the MGMA.
Within dairy production, mastitis is the most impactful disease for dairy producers [196][86]. It causes a major economic loss worldwide by decreasing both the quality and quantity of milk [197][87]. A study of healthy and infected dairy cows revealed the microbiota of milk from infected quarters has more variation compared to the milk microbiota of healthy quarters, suggesting the milk microbiota experiences dysbiosis when infection occurs [198][88]. When a quarter is infected, the microbial community can become dominant by a specific microbial taxon. When comparing milk and skin microbiotas from cows with mastitis, Staphylococcus spp. and Debaryomyces spp. were shared between them [198][88], indicating a link between the skin and milk microbiota.
There is debate on the validity of mammary microbiota, with some theories speculating bacteria sequenced from the mammary gland being contamination introduced during sample collection, sample processing, or DNA extraction [199,200][89][90]. When determining if bacteria isolated from the mammary gland is part of a community of microorganisms or a pathogen infecting a sterile environment, researchers need to objectively evaluate the validity of their results to determine if the differences are biological in nature, if the relationship is causative, what the mechanism of action is, how effective the experimental design was at answering the hypothesis, and if any potential factors could be contributing to the experimental differences [201][91]. Research has shown sequencing samples using different methods can result in differences [202][92], so it is imperative to maintain proper sterile techniques to ensure accuracy when performing microbiome studies [107][93]. However, despite this debate and the many theories surrounding it, the mammary gland can still be impacted by the GIT microbiota through the MGMA. This impact may be direct by influencing the colonization of the mammary gland or indirect by the GIT causing systemic inflammation after gut dysbiosis [68][94], which can lead to inflammation in the mammary system and potential for disease.
5. Microbiome-Gut-Reproductive Axis
Like the MGMA, the relationship that exists between the GIT microbiota and the reproductive system remains relatively unexplored. Much like the GIT microbial population, the reproductive microbiota can experience dysbiosis where a disease state occurs. Research has begun to explore the interactions that exist between the GIT microbiota and the reproductive microbiota, which can be referred to as the microbiome-gut-reproductive axis (MGRA), and its role in preventing pathogenic colonization and promoting host health. With the importance of reproductive health for both beef and dairy production, the MGRA could majorly impact the reproductive efficiency of cattle.
For a long period of time, much like the lungs, the reproductive tract of healthy females was considered sterile [203,204,205][95][96][97]. With recent advances in sequencing technologies, there has been increasing evidence that the reproductive tract of humans and animals is inhabited by a resident microbiota [206][98]. This microbial population begins to colonize the reproductive tract shortly after birth. At birth, the barrier of the cervix is compromised, which can allow microbes to travel from the vagina, the environment, feces, or skin into the reproductive tract [207,208][99][100].
The different sections of the reproductive tract have distinct microbial populations; however, they can still influence each other. The vaginal niche is mainly comprised of the phyla Firmicutes, Bacteroidetes, and Proteobacteria [18,209][101][102]. A study of Bos Indicus breeds found the most abundant bacterial genera in the vagina were Aeribacillus, Bacillus, Clostridium, Bacteroides, and Ruminococcus [210][103]. Very similarly, the most dominant phyla in the cervix have been found to be Proteobacteria, Bacteroidetes, and Firmicutes [211][104]. Meanwhile, the uterus has been found to be colonized by the phyla Proteobacteria, Tenericutes, Firmicutes, Bacteroidetes, Fusobacteria, and Actinobacteria [212][105].
Previous research has highlighted the bloodstream as a major route of communication for the MGRA [213][106]. Theisr study found the bloodstream contained a microbiota that was colonized with the main pathogens within the uterine microbiota, such as Bacteroides, Porphyromonas, and Fusobacterium, which are found in the blood shortly after calving, suggesting the bloodstream has a role in bacteria translocation. Additionally, some bacteria which cause liver abscesses in cattle, T. pyogenes and F. necrophorum, have been isolated in the uterus, suggesting these bacteria travel from the liver to the uterus through the bloodstream [214][107]. The hematogenous pathway can be utilized to carry bacteria from the GIT microbiota to the reproductive tract microbiota; thus, the GIT microbial population influences the composition of the vaginal and uterine microbial populations [18][101]. For example, Bacteroides heparinolyticus, identified in both the feces and blood of dairy cows, is assumed to be part of the uterine microbiota.
Through the MGRA, the GIT microbiota plays a major role in the functioning of the reproductive system. Research in dairy cows found a genetic similarity in strains of E. coli found in the GIT and uterus, suggesting the GIT aids in the colonization of the uterine microbiota through ascending colonization of bacteria by the lower genital tract [215][108]. Butyrate supplementation in postpartum cows positively influenced breeding capacity by re-establishing estrous and restoring ovarian function earlier than in supplemented cows [216][109]. The ratio of circulating follicle-stimulating hormone (FSH)/luteinizing hormone (LH) has been positively correlated with systemic lipopolysaccharide (LPS) and the bacterial genera Actinobacteria, Bacteroides, and Streptococcus [217][110]. A study in male sheep found that supplementing the diet with SCFA, including acetate, propionate, and butyrate, increased the secretion of LH and FSH [218][111]. Previous research in dairy cows found the uterine and vaginal microbiotas were most similar in composition seven days postpartum [219][112]. Additionally, cows that later developed endometritis at 21 days postpartum had a delay in uterine and vaginal microbiota differentiation and a decrease in bacterial diversity at seven days postpartum. The presence of Bacteroides, Poryphomonas, and Fusobacteria was associated with metritis occurring after a decrease in bacterial richness, causing uterine dysbiosis [220][113]. This indicates these two environments may indicate future reproductive diseases. Specific bacterial families (Lachnospiraceae and Rikenellaceae) and genera (Acinetobacter, Bacillus, Oscillospira, CF231, and 5–7NS) have been identified as an indication of a healthy vaginal microbiota and have the potential to be a therapeutic target [221][114]. Research has demonstrated that the MGRA may be a valuable tool to improve reproductive efficiency in cattle herds by preventing reproductive diseases and increasing hormone secretion.
References
- Forkosh, E.; Ilan, Y. The heart-gut axis: New target for atherosclerosis and congestive heart failure therapy. Open Heart 2019, 6, e000993.
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079.
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267.
- Ahlawat, S.; Sharma, K.K. Gut–organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021, 72, 636–668.
- El Aidy, S.; Dinan, T.G.; Cryan, J.F. Gut microbiota: The conductor in the orchestra of immune-neuroendocrine communication. Clin. Ther. 2015, 37, 954–967.
- Wells, J.M.; Rossi, O.; Meijerink, M.; Van Baarlen, P. Epithelial crosstalk at the microbiota-mucosal interface. Proc. Natl. Acad. Sci. USA 2011, 108, 4607–4614.
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270.
- Conlon, M.; Bird, A. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2015, 7, 17–44.
- Bienenstock, J.; Kunze, W.; Forsythe, P. Microbiota and the gut-brain axis. Nutr. Rev. 2015, 73, 28–31.
- Bercik, P. The microbiota-gut-brain axis: Learning from intestinal bacteria? Gut 2011, 60, 288–290.
- Feng, Q.; Chen, W.D.; Wang, Y.D. Gut microbiota: An integral moderator in health and disease. Front. Microbiol. 2018, 9, 151.
- Das, P.; Ranjan, R. Role of Gut Microbiome in Improving Animal Health and Productivity. Indian J. Anim. Health 2020, 59, 146–155.
- Raybould, H.E. Gut chemosensing: Interactions between gut endocrine cells and visceral afferents. Auton. Neurosci. 2010, 153, 41–46.
- Powley, T.L.; Wang, X.Y.; Fox, E.A.; Phillips, R.J.; Liu, L.W.C.; Huizinga, J.D. Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol. Motil. 2008, 20, 69–79.
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12.
- Kraimi, N.; Dawkins, M.; Gebhardt-Henrich, S.G.; Velge, P.; Rychlik, I.; Volf, J.; Creach, P.; Smith, A.; Colles, F.; Leterrier, C. Influence of the microbiota-gut-brain axis on behavior and welfare in farm animals: A review. Physiol. Behav. 2019, 210, 112658.
- Li, F.; Shah, A.M.; Wang, Z.; Peng, Q.; Hu, R.; Zou, H.; Tan, C.; Zhang, X.; Liao, Y.; Zeng, L.; et al. Effects of Land Transport Stress on Variations in Ruminal Microbe Diversity and Immune Functions in Different Breeds of Cattle. Animals 2019, 9, 599.
- Wu, Y.; Wang, L.; Luo, R.; Chen, H.; Nie, C.; Niu, J.; Chen, C.; Xu, Y.; Li, X.; Zhang, W. Effect of a Multispecies Probiotic Mixture on the Growth and Incidence of Diarrhea, Immune Function, and Fecal Microbiota of Pre-weaning Dairy Calves. Front. Microbiol. 2021, 12, 681014.
- Han, Z.; Willer, T.; Li, L.; Pielsticker, C.; Rychlik, I.; Velge, P.; Kaspers, B.; Rautenschlein, S. Rautenschlein. Influence of the gut microbiota composition on Campylobacter jejuni colonization in chickens. Infect. Immun. 2017, 85, e00380-17.
- Nagata, R.; Kim, Y.-H.; Ohkubo, A.; Kushibiki, S.; Ichijo, T.; Sato, S. Effects of repeated subacute ruminal acidosis challenges on the adaptation of the rumen bacterial community in Holstein bulls. J. Dairy Sci. 2018, 101, 4424–4436.
- Thomson, R.G. Rumenitis in cattle. Can. Vet. J. 1967, 8, 189–192.
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS ONE 2013, 8, e76993.
- Desnoyers, M.; Giger-Reverdin, S.; Sauvant, D.; Bertin, G.; Duvaux-Ponter, C. The influence of acidosis and live yeast (Saccharomyces cerevisiae) supplementation on time-budget and feeding behaviour of dairy goats receiving two diets of differing concentrate proportion. Appl. Anim. Behav. Sci. 2009, 121, 108–119.
- Devries, T.; Chevaux, E. Modification of the feeding behavior of dairy cows through live yeast supplementation. J. Dairy Sci. 2014, 97, 6499–6510.
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol. 2019, 12, 843–850.
- Morris, A.; Beck, J.M.; Schloss, P.D.; Campbell, T.B.; Crothers, K.; Curtis, J.L.; Flores, S.C.; Fontenot, A.P.; Ghedin, E.; Huang, L.; et al. Comparison of the respiratory microbiome in healthy non-smokers and smokers. Am. J. Respir. Crit. Care Med. 2013, 187, 1067–1075.
- Schuijt, T.J.; Lankelma, J.M.; Scicluna, B.P.; e Melo, F.D.S.; Roelofs, J.J.; de Boer, J.D.; Hoogendijk, A.J.; de Beer, R.; de Vos, A.; Belzer, C.; et al. The gut microbiota plays a protective role in the host defense against pneumococcal pneumonia. Gut 2016, 65, 575–583.
- Chen, L.W.; Chen, P.H.; Hsu, C.M. Commensal microflora contribute to host defense against Escherichia coli pneumonia through Toll-like receptors. Shock 2011, 36, 67–75.
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359.
- Beers, M.H. The Merck Manual of Diagnosis and Therapy; Merck Research Laboratories: Whitehouse Station, NJ, USA, 2001.
- Zeineldin, M.; Lowe, J.; Aldridge, B. Contribution of the mucosal microbiota to bovine respiratory health. Trends Microbiol. 2019, 27, 753–770.
- Goddard, A.F.; Staudinger, B.J.; Dowd, S.E.; Joshi-Datar, A.; Wolcott, R.D.; Aitken, M.L.; Fligner, C.L.; Singh, P.K. Direct sampling of cystic fibrosis lungs indicates that DNA- based analyses of upper-airway specimens can misrepresent lung microbiota. Proc. Natl. Acad. Sci. USA 2012, 109, 13769–13774.
- Nicola, I.; Cerutti, F.; Grego, E.; Bertone, I.; Gianella, P.; D’Angelo, A.; Peletto, S.; Bellino, C. Characterization of the upper and lower respiratory tract microbiota in Piedmontese calves. Microbiome 2017, 5, 152.
- Zeineldin, M.M.; Lowe, J.F.; Grimmer, E.D.; De Godoy, M.R.C.; Ghanem, M.M.; El-Raof, Y.M.A.; Aldridge, B.M. Relationship between nasopharyngeal and bronchoalveolar microbial communities in clinically healthy feedlot cattle. BMC Microbiol. 2017, 17, 138.
- Woldehiwet, Z.; Mamache, B.; Rowan, T.G. Effects of age, environmental temperature and relative humidity on the colonization of the nose and trachea of calves by Mycoplasma spp. Br. Vet. J. 1990, 146, 419–424.
- Dickson, R.P.; Erb-Downward, J.R.; Martinez, F.J.; Huffnagle, G.B. The microbiome and the respiratory tract. Annu. Rev. Physiol. 2016, 78, 481–504.
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017.
- Lemon, K.P.; Klepac-Ceraj, V.; Schiffer, H.K.; Brodie, E.L.; Lynch, S.V.; Kolter, R. Comparative analyses of the bacterial microbiota of the human nostril and oropharynx. mBio 2010, 1, e00129-10.
- Rasmussen, T.T.; Kirkeby, L.P.; Poulsen, K.; Reinholdt, J.; Kilian, M. Resident aerobic microbiota of the adult human nasal cavity. APMIS 2000, 108, 663–675.
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253.
- Hall, J.A.; Isaiah, A.; Estill, C.T.; Pirelli, G.J.; Suchodolski, J.S. Weaned beef calves fed selenium-biofortified alfalfa hay have an enriched nasal microbiota compared with healthy controls. PLoS ONE 2017, 12, e0179215.
- Timsit, E.; Holman, D.B.; Hallewell, J.; Alexander, T.W. The nasopharyngeal microbiota in feedlot cattle and its role in respiratory health. Anim. Front. 2016, 6, 44–50.
- McMullen, C.; Orsel, K.; Alexander, T.W.; van der Meer, F.; Plastow, G.; Timsit, E. Evolution of the nasopharyngeal bacterial microbiota of beef calves from spring processing to 40 days after feedlot arrival. Vet. Microbiol. 2018, 225, 139–148.
- Bassis, C.M.; Erb-Downward, J.R.; Dickson, R.P.; Freeman, C.M.; Schmidt, T.M.; Young, V.B.; Beck, J.M.; Curtis, J.L.; Huffnagle, G.B. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio 2015, 6, e00037-15.
- He, Y.; Wen, Q.; Yao, F.; Xu, D.; Huang, Y.; Wang, J. Gut- lung axis: The microbial contributions and clinical implications. Crit. Rev. Microbiol. 2017, 43, 81–95.
- Krishnamoorthy, N.; Khare, A.; Oriss, T.B.; Raundhal, M.; Morse, C.; Yarlagadda, M.; Wenzel, S.E.; Moore, M.L.; Peebles, R.S.; Ray, A.; et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat. Med. 2012, 18, 1525–1530.
- Roy, M.G.; Livraghi-Butrico, A.; Fletcher, A.A.; McElwee, M.M.; Evans, S.E.; Boerner, R.M.; Alexander, S.N.; Bellinghausen, L.K.; Song, A.S.; Petrova, Y.M.; et al. Muc5b is required for airway defense. Nature 2014, 505, 412–416.
- Eisele, N.A.; Anderson, D.M. Host defense and the airway epithelium: Frontline responses that protect against bacterial invasion and pneumonia. J. Pathog. 2011, 2011, 249802.
- Vareille, M.; Kieninger, E.; Edwards, M.R.; Regamey, N. The airway epithelium: Soldier in the fight against respiratory viruses. Clin. Microbiol. Rev. 2011, 24, 210–229.
- Osman, R.; Malmuthuge, N.; Gonzalez-Cano, P.; Griebel, P. Development and function of the mucosal immune system in the upper respiratory tract of neonatal calves. Annu. Rev. Anim. Biosci. 2018, 6, 141–155.
- Uehara, A.; Fujimoto, Y.; Fukase, K.; Takada, H. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce anti-microbial peptides, but not proinflammatory cytokines. Mol. Immunol. 2007, 44, 3100–3111.
- Ackermann, M.R.; Derscheid, R.; Roth, J.A. Innate immunology of bovine respiratory disease. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 215–228.
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286.
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547.
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166.
- Gaudier, E.; Jarry, A.; Blottière, H.M.; de Coppet, P.; Buisine, M.P.; Aubert, J.P.; Laboisse, C.; Cherbut, C.; Hoebler, C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1168–G1174.
- Willemsen, L.E.M.; Koetsier, M.A.; Van Deventer, S.J.H.; Van Tol, E.A.F. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447.
- Ohata, A.; Usami, M.; Miyoshi, M. Short-chain fatty acids alter tight junction permeability in intestinal monolayer cells via lipoxygenase activation. Nutrition 2005, 21, 838–847.
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut Microbial metabolites fuel host antibody responses. Cell Host Microbe 2016, 20, 202–214.
- Wu, W.; Sun, M.; Chen, F.; Cao, A.T.; Liu, H.; Zhao, Y.; Huang, X.; Xiao, Y.; Yao, S.; Zhao, Q.; et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017, 10, 946–956.
- Donohoe, D.R.; Collins, L.B.; Wali, A.; Bigler, R.; Sun, W.; Bultman, S.J. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell 2012, 48, 612–626.
- Byndloss, M.X.; Olsan, E.E.; Rivera-Chávez, F.; Tiffany, C.R.; Cevallos, S.A.; Lokken, K.L.; Torres, T.P.; Byndloss, A.J.; Faber, F.; Gao, Y.; et al. Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 2017, 357, 570–575.
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384.
- Meale, S.J.; Li, S.; Azevedo, P.; Derakhshani, H.; Plaizier, J.C.; Steele, M.; Khafipour, E. Does weaning age affect the development of ruminal and fecal microbiomes in dairy calves? J. Anim. Sci. 2016, 94, 785.
- Zeineldin, M.; Lowe, J.; de Godoy, M.; Maradiaga, N.; Ramirez, C.; Ghanem, M.; Abd El-Raof, Y.; Al-dridge, B. Disparity in the nasopharyngeal microbiota between healthy cattle on feed, at entry processing and with respiratory disease. Vet. Microbiol. 2017, 208, 30–37.
- Timsit, E.; Workentine, M.; Schryvers, A.B.; Holman, D.B.; van der Meer, F.; Alexander, T.W. Evolution of the nasopharyngeal microbiota of beef cattle from weaning to 40 days after arrival at a feedlot. Vet. Microbiol. 2016, 187, 75–81.
- Caswell, J.L. Failure of respiratory defenses in the pathogenesis of bacterial pneumonia of cattle. Vet. Pathol. 2014, 51, 393–409.
- Lyte, M. The effect of stress on microbial growth. Anim. Health Res. Rev. 2014, 15, 172–174.
- Lima, S.F.; Teixeira, A.G.V.; Higgins, C.H.; Lima, F.S.; Bicalho, R.C. The upper respiratory tract microbiome and its potential role in bovine respiratory disease and otitis media. Sci. Rep. 2016, 6, 29050.
- Buckham Sporer, K.R.; Weber, P.S.D.; Burton, J.L.; Earley, B.; Crowe, M.A. Transportation of young beef bulls alters circulating physiological parameters that may be effective biomarkers of stress. J. Anim. Sci. 2008, 86, 1325–1334.
- Apley, M. The clinical syndrome of BRD: What it is and what it is not. Anim. Health Res. Rev. 2014, 15, 135–137.
- Zeineldin, M.M.; El-Raof, Y.M.A.; El-attar, H.A.; Ghanem, M.M. Lung ultrasonography and computer-aided scoring system as a diagnostic aid for bovine respiratory disease in feedlot cattle. Glob. Vet. 2016, 17, 588–594.
- Alexander, T.W.; Plaizier, J.C. The importance of microbiota in ruminant production. Anim. Front. 2016, 6, 4.
- Rice, J.A.; Carrasco-Medina, L.; Hodgins, D.C.; Shewen, P.E. Mannheimia haemolytica and bovine respiratory disease. Anim. Health Res. Rev. 2007, 8, 117–128.
- Timsit, E.; Workentine, M.; van der Meer, F.; Alexander, T. Distinct bacterial metacommunities inhabit the upper and lower respiratory tracts of healthy feedlot cattle and those diagnosed with bronchopneumonia. Vet. Microbiol. 2018, 221, 105–113.
- Chai, J.; Capik, S.F.; Kegley, B.; Richeson, J.; Powell, J.; Zhao, J. Bovine respiratory microbiota of feedlot cattle and its association with disease. Vet. Res. 2022, 53, 4.
- Addis, M.F.; Tanca, A.; Uzzau, S.; Oikonomou, G.; Bicalho, R.C.; Moroni, P. The bovine milk microbiota: Insights and perspectives from -omics studies. Mol. BioSyst. 2016, 12, 2359–2372.
- Cremonesi, P.; Ceccarani, C.; Curone, G.; Severgnini, M.; Pollera, C.; Bronzo, V.; Riva, F.; Addis, M.F.; Filipe, J.; Amadori, M.; et al. Milk microbiome diversity and bacterial group prevalence in a comparison between healthy Holstein Friesian and Rendena cows. PLoS ONE 2018, 13, e0205054.
- Montel, M.C.; Buchin, S.; Mallet, A.; Delbes-Paus, C.; Vuitton, D.A.; Desmasures, N.; Berthier, F. Traditional cheeses: Rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 2014, 177, 136–154.
- Quigley, L.; O’Sullivan, O.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. Molecular approaches to analysing the microbial composition of raw milk and raw milk cheese. Int. J. Food Microbiol. 2011, 150, 81–94.
- Bhatt, V.; Ahir, V.; Koringa, P.; Jakhesara, S.; Rank, D.; Nauriyal, D.; Kunjadia, A.; Joshi, C. Milk microbiome signatures of subclinical mastitis-affected cattle analysed by shotgun sequencing. J. Appl. Microbiol. 2012, 112, 639–650.
- Young, W.; Hine, B.C.; Wallace, O.A.; Callaghan, M.; Bibiloni, R. Transfer of intestinal bacterial components to mammary secretions in the cow. PeerJ 2015, 3, e888.
- Rodriguez, J.M. The origin of human milk bacteria: Is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv. Nutr. 2014, 5, 779–784.
- Roux, M.E.; Mcwilliams, M.; Phillips-Quaglita, J.M.; Weisz-Carrington, P.; Lamm, M.E. Origin of IgA-secreting plasma cells in the mammary gland. J. Exp. Med. 1977, 146, 1311–1322.
- Macpherson, A.J.; Uhr, T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 2004, 303, 1662–1665.
- Hu, X.; Guo, J.; Zhao, C.; Jiang, P.; Maimai, T.; Yanyi, L.; Cao, Y.; Fu, Y.; Zhang, N. The gut microbiota contributes to the development of Staphylococcus aureus-induced mastitis in mice. ISME J. 2020, 14, 1897–1910.
- Melchior, M.; Vaarkamp, H.; Fink-Gremmels, J. Biofilms: A role in recurrent mastitis infections? Vet. J. 2006, 171, 398–407.
- Andrews, T.; Neher, D.A.; Weicht, T.R.; Barlow, J.W. Mammary microbiome of lactating organic dairy cows varies by time, tissue site, and infection status. PLoS ONE 2019, 14, e0225001.
- Hillerton, J.E. Whatever happened to mastitis pathogenesis? J. Dairy Res. 2020, 87, 273–276.
- Rainard, P. Mammary microbiota of dairy ruminants: Fact or fiction? Vet. Res. 2017, 48, 25.
- Hanage, W.P. Microbiology: Microbiome science needs a healthy dose of scepticism. Nature 2014, 512, 247–248.
- Taponen, S.; McGuinness, D.; Hiitiö, H.; Simojoki, H.; Zadoks, R.; Pyorala, S. Bovine milk microbiome: A more complex issue than expected. Vet. Res. 2019, 50, 44.
- Lourenco, J.M.; Welch, C.B. Using microbiome information to understand and improve animal performance. Ital. J. Anim. Sci. 2022, 21, 899–913.
- Marchiando, A.M.; Graham, W.V.; Turner, J.R. Epithelial barriers in homeostasis and disease. Annu. Rev. Pathol. 2010, 5, 119–144.
- Schoenmakers, S.; Steegers-Theunissen, R.; Faas, M. The matter of the reproductive microbiome. Obstet. Med. 2019, 12, 107–115.
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875.
- Scolari, F.; Attardo, G.M.; Aksoy, E.; Weiss, B.; Savini, G.; Takac, P.; Abd-Alla, A.; Parker, A.G.; Aksoy, S.; Malacrida, A.R. Symbiotic microbes affect the expression of male reproductive genes in Glossina m. morsitans. BMC Microbiol. 2018, 18, 169.
- Appiah, M.; Wang, J.; Lu, W. Microflora in the reproductive tract of cattle: A review. Agriculture 2020, 10, 232.
- Piersanti, R.L.; Bromfield, J.J. The Consequence of Postpartum Uterine Disease on Dairy Cow Fertility. EDIS 2019, 2019, 107174.
- Sheldon, I.M.; Dobson, H. Postpartum uterine health in cattle. Anim. Reprod. Sci. 2004, 82–83, 295–306.
- Laguardia-Nascimento, M.; Branco, K.M.G.R.; Gasparini, M.R.; Giannattasio-Ferraz, S.; Leite, L.R.; Araujo, F.M.G.; Salim, A.C.D.M.; Nicoli, J.R.; De Oliveira, G.C.; Barbosa-Stancioli, E.F. Vaginal microbiome characterization of nellore cattle using metagenomic analysis. PLoS ONE 2015, 10, e0143294.
- Nesengani, L.T.; Wang, J.; Yang, Y.; Yang, L.; Lu, W. Unravelling vaginal microbial genetic diversity and abundance between Holstein and Fleckvieh cattle. RSC Adv. 2017, 7, 56137–56143.
- Giannattasio-Ferraz, S.; Laguardia-Nascimento, M.; Gasparini, M.R.; Leite, L.R.; Araujo, F.M.G.; de Matos Salim, A.C.; de Oliveira, A.P.; Nicoli, J.R.; de Oliveira, G.C.; da Fonseca, F.G.; et al. A common vaginal microbiota composition among breeds of Bos taurus indicus (Gyr and Nellore). Braz. J. Microbiol. 2019, 50, 1115–1124.
- Wang, Y.; Wang, J.; Li, H.; Fu, K.; Pang, B.; Yang, Y.; Liu, Y.; Tian, W.; Cao, R. Characterization of the cervical bacterial community in dairy cows with metritis and during different physiological phases. Theriogenology 2018, 108, 306–313.
- Santos, T.M.; Bicalho, R.C. Diversity and succession of bacterial communities in the uterine fluid of postpartum metritic, endometritic and healthy dairy cows. PLoS ONE 2012, 7, e53048.
- Jeon, S.J.; Cunha, F.; Vieira-Neto, A.; Bicalho, R.C.; Lima, S.; Bicalho, M.L.; Galvão, K.N. Blood as a route of transmission of uterine pathogens from the gut to the uterus in cows. Microbiome 2017, 5, 109.
- Nagaraja, T.; Lechtenberg, K.F. Liver abscesses in feedlot cattle. Vet. Clin. N. Am. Food Anim. Pract. 2007, 23, 351–369.
- Jones, K.; Cunha, F.; Jeon, S.J.; Pérez-Báez, J.; Casaro, S.; Fan, P.; Liu, T.; Lee, S.; Jeong, K.C.; Yang, Y.; et al. Tracing the source and route of uterine colonization by exploring the genetic relationship of Escherichia coli isolated from the reproductive and gastrointestinal tract of dairy cows. Vet. Microbiol. 2020, 266, 109355.
- Ulfina, G.G.; Kimothi, S.P.; Oberoi, P.S.; Baithalu, R.K.; Kumaresan, A.; Mohanty, T.K.; Imtiwati, P.; Dang, A.K. Modulation of post-partum reproductive performance in dairy cows through supplementation of long- or short-chain fatty acids during transition period. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1056–1064.
- Wang, T.; Sha, L.; Li, Y.; Zhu, L.; Wang, Z.; Li, K.; Lu, H.; Bao, T.; Guo, L.; Zhang, X.; et al. Dietary alinolenic acid-rich flaxseed oil exerts beneficial effects on polycystic ovary syndrome through sex steroid hormones—microbiota—inflammation Axis in rats. Front. Endocrinol. 2020, 11, 284.
- Boukhliq, R.; Martin, G.B. Administration of fatty acids and gonadotrophin secretion in the mature ram. Anim. Reprod. Sci. 1997, 49, 143–159.
- Miranda-CasoLuengo, R.; Lu, J.; Williams, E.J.; Miranda-CasoLuengo, A.A.; Carrington, S.D.; Evans, A.C.; Meijer, W.G. Delayed differentiation of vaginal and uterine microbiomes in dairy cows developing postpartum endometritis. PLoS ONE 2019, 14, e0200974.
- Galvão, K.N.; Bicalho, R.C.; Jeon, S.J. Symposium review: The uterine microbiome associated with the development of uterine disease in dairy cows. J. Dairy Sci. 2019, 102, 11786–11797.
- Moreno, C.G.; Luque, A.T.; Galvão, K.N.; Otero, M.C. Bacterial communities from vagina of dairy healthy heifers and cows with impaired reproductive performance. Res. Vet. Sci. 2022, 142, 15–23.
More
