Please note this is a comparison between Version 2 by Conner Chen and Version 1 by James A. McCubrey.
The TP53 gene encodes a tumor suppressor protein. The TP53 gene is one of the most frequently mutated genes in humans. The TP53 protein is a transcription factor. TP53 can also influence the PDAC or CRCpancreatic ductal adenocarcinomas (PDAC) or colorectal cancer (CRC) microenvironment by influencing the expression of many genes.
- TP53
- KRas
- PDAC
- immunotherapy
1. Introduction-Overview of Genes Frequently Mutated in PDAC
There are multiple genes frequently mutated in pancreatic ductal adenocarcinomas (PDAC). These include: TP53, KRAS, cyclin-dependent kinase inhibitor 2A (CDKN2A encodes the p16 (INK4A) and the p14 (ARF) tumor suppressor proteins), and SMAD4 (encodes the small mothers against decapentaplegic homolog 4 protein, which is a transcription factor). These genes have been determined to have mutations, deletions, amplifications or inactivations for quite some time now. There are other genes which may be mutated or expressed abnormally in PDAC. Some genes that are also more frequently mutated in PDAC include: cyclin-dependent kinase inhibitor 2B (CDKN2B encodes p15INK4b tumor suppressor protein) and ARID1A (AT-rich interactive domain-containing protein 1A, which is one component of multiple SWItch/Sucrose Non-Fermentable (SWI/SNF) protein complexes that are involved in chromatin remodeling). Mutations at these genes were detected by next-generation sequencing. The effects of the mutations and/or changes in gene expression on the PDAC microenvironment have been recently reviewed [1].
KRAS is mutated in >95% of PDACs. The KRAS mutations result in the constitutive activation of a critical GTP/GDP GTPase exchange protein which is an important regulator (on/off switch) in multiple signal transduction pathways [2]. KRAS mutations can control various aspects of the tumor microenvironment. KRAS mutations can influence the presence of various immune cells in the inflammatory PDAC tumor microenvironment. KRAS mutations can affect the infiltration of T cells and myeloid-derived suppressive cells (MDSCs) during early stages of pancreatic intraepithelial neoplasia (PanIN) [3]. This can result in changes to pancreatic stellate cells (PSC) and induce mesenchymal-derived cells to form fibroblasts and fibrin remodeling. This results in PDAC remodeling of the microenvironment and progression of the PanIN and the ability of immune cells to infiltrate the PDAC microenvironment [4]. KRAS mutations can also increase the expression many genes associated with the immunosuppressive PDAC microenvironment such as the immune check point regulator programmed death-ligand 1 (PD-L1) [5,6][5][6]. This can result in the differentiation of CD4+CD25− cells into T regulatory (Treg) cells [7] and recruitment in colorectal cancer (CRC), PDAC and other cancers [8]. In addition, KRAS mutations induce growth factors such as interleukin-6 (IL-6) and IL-10, transforming growth factor-β (TGF-β) and sonic hedgehog (Shh) [9].
The TP53 gene encodes a tumor suppressor protein. The TP53 gene is one of the most frequently mutated genes in humans. The TP53 protein is a transcription factor. TP53 can also influence the PDAC or CRC microenvironment by influencing the expression of many genes. Inactivation of wild type (WT) TP53 activity has direct effects on cell cycle progression, apoptosis and senescence. Loss of the normal activities of TP53 changes the immune milieu in the PDAC microenvironment and promotes inflammation which is pro-tumorigenic. Mut-TP53 can alter immunosuppressive properties of the PDAC microenvironment which accelerates tumor progression and metastasis [10]. TP53 can increase the immune response in the PDAC microenvironment by augmenting the levels of T cells, which enhances the effects of dendritic cells (DC) [11]. This was determined by treatment with the mouse double minute 2 homolog (MDM2) inhibitor nutlin-3a. WT TP53 suppresses IL-6 expression while it is detected at higher levels in the PDAC microenvironment in cells with mut-TP53. Increased IL-6 expression is associated with metastasis [12]. Mut-TP53 can also induce NF-κB activity, which in turn induces inflammatory cytokine expression, including IL-6 and tumor necrosis factor-α (TNF-α), and promotes metastasis [13]. In colon cancer models, mutant TP53 can induce the expression of vascular endothelial growth factor (VEGF) which promotes angiogenesis and tumorigenesis [14]. An overview of some of these interactions is presented in Figure 1.
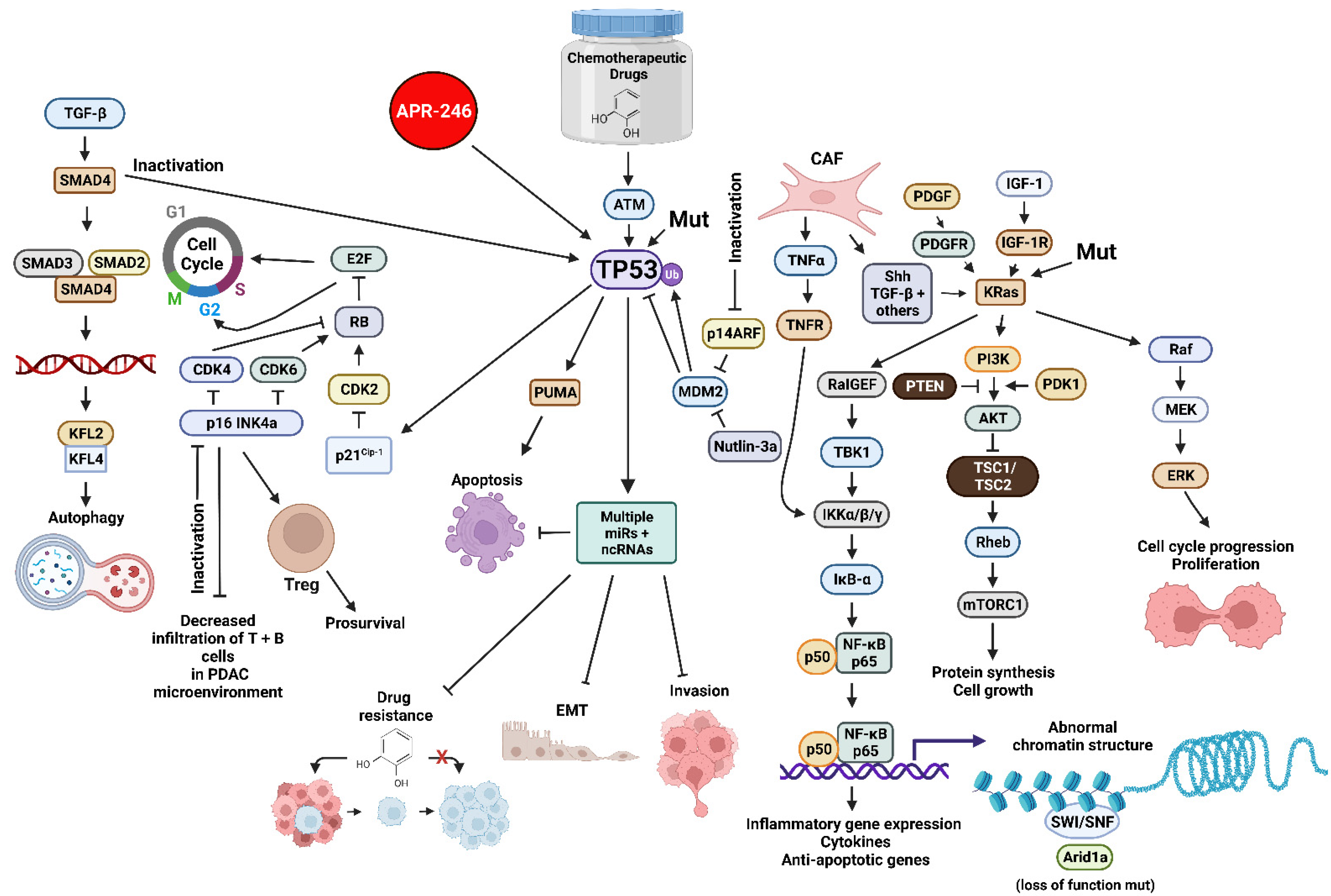
Figure 1. Sites of Interactions of Mutant Genes in PDAC and the Various Pathways which they Effect and also Sites of Interaction of Certain Small Molecule Inhibitors Discussed in this Reviewhere. Abbreviations: transforming growth factor-β (TGF-β), small mothers against decapentaplegic homolog 4 protein (SMAD4), small mothers against decapentaplegic homolog 3 protein (SMAD3), small mothers against decapentaplegic homolog 2 protein (SMAD2), Kruppel-like factor 4 (KLF4), Kruppel-like factor 2 (KLF2), cyclin-dependent kinase 4 (CDK4), cyclin-dependent kinase 2 (CDK2), cyclin-dependent kinase inhibitor 2A encoded by CDKN2A (p16 INK4a) E2F transcription factor (E2F), cyclin-dependent kinase inhibitor 1A encoded by CDKN1A (p21Cip1), small molecule reactivator of mutant TP53 (APR-246), TP53 = tumor suppressor protein 53 KDa, 14 KDa tumor suppressor protein alternate reading frame protein encoded by CDKN2A (p14ARF), p53 upregulated modulator of apoptosis (PUMA), mouse double minute 2 E3 ubiquitin ligase (MDM2), small molecular inhibitor of MDM2 (nutlin-3a), micro RNA (miR), non-coding RNAs including LncRNAs and circRNAs (ncRNA), platelet-derived growth factor receptor (PDGFR), cancer-associated fibroblast (CAF), tumor necrosis factor-α (TNFα), tumor necrosis factor receptor (TNFR), sonic hedgehog growth factor (Shh), Ral guanine nucleotide exchange factor (RalGEF), TANK binding kinase 1 (TBK1), inhibitor of NF-Kappa-B kinase-α/β/γ (IKKα/β/κ), inhibitor of NF-κB-α (IκB-α), p50 KDa subunit of nuclear kappa-κB cells (p50), p65 KDa subunit of nuclear kappa-κB cells (p65), small molecule multi kinase inhibitor (Sorafenib), platelet-derived growth factor (PDGF), phosphatase and tensin homolog (PTEN), insulin-like growth factor-I (IGF-1), insulin-like growth factor-1 receptor-1 (IGF-1R), Kirsten Ras oncogene homolog (KRas), phosphatidylinositol 3-kinase (PI3K) 3-phosphoinositide-dependent protein kinase-1 (PDK1), AKT serine/threonine kinase (AKT), (TSC1 (hamartin) and TSC2 (tuberin) tumor suppressor complex (TSC1/TSC2), Ras Homolog, mTORC1 binding protein (Rheb), mechanistic target of rapamycin kinase (mTORC1), SWItch/Sucrose Non-Fermentable complex (SWI/SNF), AT-rich interactive domain-containing protein 1A (Arid1a)—MAP kinase kinase kinase (Raf), mitogen-activated protein kinase kinase 1 (MEK), ERK = mitogen-activated protein kinase (ERK). We compiled tThe information necessary for all the figures, designed all the figures and their and composition was for all figures were created with BioRender.com.
CDKN2A is another gene whose expression is frequently altered in PDAC [15]. The CDKN2A locus encodes two proteins, p16 (p16/INK4A) and p14ARF. Altered expression (loss of function) of the CDKN2A locus can occur by many mechanisms, including promoter methylation and gene deletion. p16/INK4A and p14ARF are normally tumor suppressor proteins and they serve to function to inhibit cell cycle progression. p16/INK4a normally inhibits cell cycle progression by suppressing the activity of cyclin-dependent kinase 4 (CDK4) and CDK6. The p14ARF protein induces cell cycle arrest by inhibiting the degradation of MDM2, which results in destabilizing the TP53 protein. CDKN2A mutation (downregulation) has been associated with decreased infiltration of T and B cells in the PDAC microenvironment. This also increased the levels of forkhead box P3 positive (Fox3P) Tregs and was associated with a lower level of survival in PDAC patients [16].
SMAD4 is a tumor suppressor protein. The SMAD4 gene encodes a transcription factor, and it is downstream of TGF-β [17]. Mutation or loss of SMAD4 activity prevents the growth suppressive effects of TGF-β. Loss of SMAD4 activity may result in tumor angiogenesis. Loss of SMAD-4 and WT-TP53 activities were associated with S100A8. The S100A8 protein binds calcium and zinc and regulates various inflammatory and immune reactions. It can induce neutrophil chemotaxis and adhesion [18]. S100A8 is associated with the tumor microenvironment as it increases the secretion of pro-inflammatory cytokines [19].
2. Interactions of TP53 with the Immune System and Fibroblasts in the PDAC Microenvironment
The immune system is key for the prevention of bacterial infections as well as prevention of abnormal growth and tumor development. Although T and B cells are some of the best-known cells in the immune system, especially the adaptive immune response, the innate immune response is also important in regulating tumor growth. The innate immune system consists of macrophages, monocytes, DC, natural killer (NK) and other cells. The PDAC microenvironment consists of tumor-infiltrating T, B and NK lymphocytes as well as myeloid cells including macrophages, monocytes, DC, MDSC and other cells. There are two types of suppressor/regulatory cells in the PDAC microenvironment: Treg cells and type 2 macrophages (M2, MDSC). M2 macrophages can differentiate into tumor-associated macrophages (TAM) cells. These cells promote tumor inflammation and immunosuppression. The extracellular matrix (ECM) and cytokines/chemokine milieu is altered in the PDAC environment. Inflammatory stress is monitored by TP53, and in the absence of WT TP53 this censoring is diminished.
In studies with TP53 knock-out mice (TP53-null), enhanced levels of IL-1, IL-6 and IL-12 were observed in the macrophages. Increased levels of these proinflammatory cytokines altered macrophage function in the PDAC microenvironment [20]. Inhibition of normal TP53 function led to T cell differentiation into T helper Th17 cells [21]. Loss of WT-TP53 activity altered Treg differentiation and led to inflammation [22,23][22][23]. Restoration of WT-TP53 activity suppressed inflammation and autoimmunity [20].
In addition, the PDAC microenvironment has cancer-associated fibroblasts (CAF). CAFs alter the pancreatic cancer microenvironment by the secretion of growth factors such as C-X-C motif chemokine ligand 1 (CXCL1), CXCL12, C-C motif chemokine ligand 8 (CCL8), stromal cell-derived factor-1 (SDF-1), IL-6, IL-11, VEGF and others [24,25,26][24][25][26]. The CAFs can have important effects on immunosuppression, angiogenesis and metastasis in the tumor microenvironment by secreting multiple growth factors. Likewise, primary pancreatic cancers can modulate the tumor microenvironment by secreting various factors in exosomes which favor colonization in metastatic sites [27].
References
- Sun, H.; Zhang, B.; Li, H. The roles of frequently mutated genes of pancreatic cancer in regulation of tumor microenvironment. Technol. Cancer Res. Treat. 2020, 19, 1533033820920969.
- Buscail, L.; Bournet, B.; Cordelier, P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 153–168.
- Steele, C.W.; Jamieson, N.B.; Evans, T.R.; McKay, C.J.; Sansom, O.J.; Morton, J.P.; Carter, C.R. Exploiting inflammation for therapeutic gain in pancreatic cancer. Br. J. Cancer 2013, 108, 997–1003.
- Clark, C.E.; Hingorani, S.R.; Mick, R.; Combs, C.; Tuveson, D.A.; Vonderheide, R.H. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007, 67, 9518–9527.
- Hashimoto, S.; Furukawa, S.; Hashimoto, A.; Tsutaho, A.; Fukao, A.; Sakamura, Y.; Parajuli, G.; Onodera, Y.; Otsuka, Y.; Handa, H.; et al. ARF6 and AMAP1 are major targets of KRAS and TP53 mutations to promote invasion, PD-L1 dynamics, and immune evasion of pancreatic cancer. Proc. Nat. Acad. Sci. USA 2019, 116, 17450–17459.
- Chen, N.; Fang, W.; Lin, Z.; Peng, P.; Wang, J.; Zhan, J.; Hong, S.; Huang, J.; Liu, L.; Sheng, J.; et al. KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol. Immunother. 2017, 66, 1175–1187.
- Zdanov, S.; Mandapathil, M.; Abu Eid, R.; Adamson-Fadeyi, S.; Wilson, W.; Qian, J.; Carnie, A.; Tarasova, N.; Mkrtichyan, M.; Berzofsky, J.A.; et al. Mutant KRAS conversion of conventional T cells into regulatory T cells. Cancer Immunol. Res. 2016, 4, 354–365.
- Liao, W.; Overman, M.J.; Boutin, A.T.; Shang, X.; Zhao, D.; Dey, P.; Li, J.; Wang, G.; Lan, Z.; Li, J.; et al. KRAS-IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell 2019, 35, 559–572.e7.
- Hafezi, S.; Saber-Ayad, M.; Abdel-Rahman, W.M. Highlights on the role of KRAS mutations in reshaping the microenvironment of pancreatic adenocarcinoma. Int. J. Mol. Sci. 2021, 22, 10219.
- Cui, Y.; Guo, G. Immunomodulatory function of the tumor suppressor p53 in host immune response and the tumor microenvironment. Int. J. Mol. Sci. 2016, 17, 1942.
- Gasparini, C.; Tommasini, A.; Zauli, G. The MDM2 inhibitor Nutlin-3 modulates dendritic cell-induced T cell proliferation. Hum. Immunol. 2012, 73, 342–345.
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324.
- Cooks, T.; Pateras, I.S.; Tarcic, O.; Solomon, H.; Schetter, A.J.; Wilder, S.; Lozano, G.; Pikarsky, E.; Forshew, T.; Rosenfeld, N.; et al. Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell 2013, 23, 634–646.
- Hayashi, Y.; Tsujii, M.; Kodama, T.; Akasaka, T.; Kondo, J.; Hikita, H.; Inoue, T.; Tsujii, Y.; Maekawa, A.; Yoshii, S.; et al. p53 functional deficiency in human colon cancer cells promotes fibroblast-mediated angiogenesis and tumor growth. Carcinogenesis 2016, 37, 972–984.
- Cremin, C.; Howard, S.; Le, L.; Karsan, A.; Schaeffer, D.F.; Renouf, D.; Schrader, K.A. CDKN2A founder mutation in pancreatic ductal adenocarcinoma patients without cutaneous features of familial atypical multiple mole melanoma (FAMMM) syndrome. Hered. Cancer Clin. Pract. 2018, 16, 7.
- Wartenberg, M.; Cibin, S.; Zlobec, I.; Vassella, E.; Eppenberger-Castori, S.; Terracciano, L.; Eichmann, M.D.; Worni, M.; Gloor, B.; Perren, A.; et al. Integrated genomic and immunophenotypic classification of pancreatic cancer reveals three distinct subtypes with prognostic/predictive significance. Clin. Cancer Res. 2018, 24, 4444–4454.
- Zhao, M.; Mishra, L.; Deng, C.X. The role of TGF-β/SMAD4 signaling in cancer. Int. J. Bio. Sci. 2018, 14, 111–123.
- Sheikh, A.A.; Vimalachandran, D.; Thompson, C.C.; Jenkins, R.E.; Nedjadi, T.; Shekouh, A.; Campbell, F.; Dodson, A.; Prime, W.; Crnogorac-Jurcevic, T.; et al. The expression of S100A8 in pancreatic cancer-associated monocytes is associated with the Smad4 status of pancreatic cancer cells. Proteomics 2007, 7, 1929–1940.
- Nedjadi, T.; Evans, A.; Sheikh, A.; Barerra, L.; Al-Ghamdi, S.; Oldfield, L.; Greenhalf, W.; Neoptolemos, J.P.; Costello, E. S100A8 and S100A9 proteins form part of a paracrine feedback loop between pancreatic cancer cells and monocytes. BMC Cancer 2018, 18, 1255.
- Zheng, S.J.; Lamhamedi-Cherradi, S.E.; Wang, P.; Xu, L.; Chen, Y.H. Tumor suppressor p53 inhibits autoimmune inflammation and macrophage function. Diabetes 2005, 54, 1423–1428.
- Okuda, Y.; Okuda, M.; Bernard, C.C. Regulatory role of p53 in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2003, 135, 29–37.
- Park, J.S.; Lim, M.A.; Cho, M.L.; Ryu, J.G.; Moon, Y.M.; Jhun, J.Y.; Byun, J.K.; Kim, E.K.; Hwang, S.Y.; Ju, J.H.; et al. p53 controls autoimmune arthritis via STAT-mediated regulation of the Th17 cell/Treg cell balance in mice. Arthritis Rheumatol. 2013, 65, 949–959.
- Kawashima, H.; Takatori, H.; Suzuki, K.; Iwata, A.; Yokota, M.; Suto, A.; Minamino, T.; Hirose, K.; Nakajima, H. Tumor suppressor p53 inhibits systemic autoimmune diseases by inducing regulatory T cells. J. Immunol. 2013, 191, 3614–3623.
- Louault, K.; Li, R.R.; DeClerck, Y.A. Cancer-associated fibroblasts: Understanding their heterogeneity. Cancers 2020, 12, 3108.
- Addadi, Y.; Moskovits, N.; Granot, D.; Lozano, G.; Carmi, Y.; Apte, R.N.; Neeman, M.; Oren, M. p53 status in stromal fibroblasts modulates tumor growth in an SDF1-dependent manner. Cancer Res. 2010, 70, 9650–9658.
- Huang, C.; Li, Z.; Li, N.; Li, Y.; Chang, A.; Zhao, T.; Wang, X.; Wang, H.; Gao, S.; Yang, S.; et al. Interleukin 35 expression correlates with microvessel density in pancreatic ductal adenocarcinoma, recruits monocytes, and promotes growth and angiogenesis of xenograft tumors in mice. Gastroenterology 2018, 154, 675–688.
- Mlecnik, B.; Bindea, G.; Kirilovsky, A.; Angell, H.K.; Obenauf, A.C.; Tosolini, M.; Church, S.E.; Maby, P.; Vasaturo, A.; Angelova, M.; et al. The tumor microenvironment and immunoscore are critical determinants of dissemination to distant metastasis. Sci. Transl. Med. 2016, 8, 327ra26.
More
 Encyclopedia
Encyclopedia
