Curcumin is a polyphenol extracted from the rhizome of turmeric plant. Beyond its common use as a culinary spice in Eastern Asia, curcumin has been proposed as a therapeutic compound due to its antioxidant, anti-inflammatory and neuroprotective properties. Thus, its efficacy has been evaluated in various inflammatory-based psychiatric disorders, such as schizophrenia, depression or autism. The aim of this study is to review those preclinical and clinical studies carried out in psychiatric disorders whose therapeutic approach has involved the use of curcumin and, therefore, to discern the possible positive effect of curcumin in these disorders.
- curcumin
- psychiatric disorders
- inflammation
- oxidative stress
- schizophrenia
- autism
- depression
- Obsessive Compulsive Disorder
1. Introduction
1.1 History of curcumin
Turmeric (Curcuma longa) is an herbaceous plant widely used in Asia as a dye, culinary spice, and as a traditional natural therapeutic compound [1]. The rhizome of this plant, also called turmeric, is enriched with yellow dyes, the curcuminoids [2]. Within this family of compounds, curcumin is considered one of the most relevant. Curcumin, the active compound of turmeric, is a polyphenol that has also been largely used as a remedy for different pathologies in Asia for several decades due to its healthy and biopharmacological properties, and its lack of adverse effects, even at high doses. Moreover, curcumin has been reported to have anti-inflammatory, antioxidant, neuroprotective, and even anti-aging and antineoplasic properties [3-7][3][4][5][6][7] (Figure 1). Curcumin may exert its anti-inflammatory and antioxidant (anti-IOS) effects by influencing the synthesis of some IOS regulators, such as heme-oxygenase-1 (HO1), glutathione (GSH), catalase (CAT), and superoxide dismutase (SOD) [8]. These properties mcakuse curcumin to have an impact on those diseases in which IOS regulation does not work correctly and are related to the disease appearance. Thus, curcumin hasmay exert a beneficial effect on the immune system, reducing B lymphocyte proliferation by inhibiting B Llymphocyte Sstimulator (BLYS).
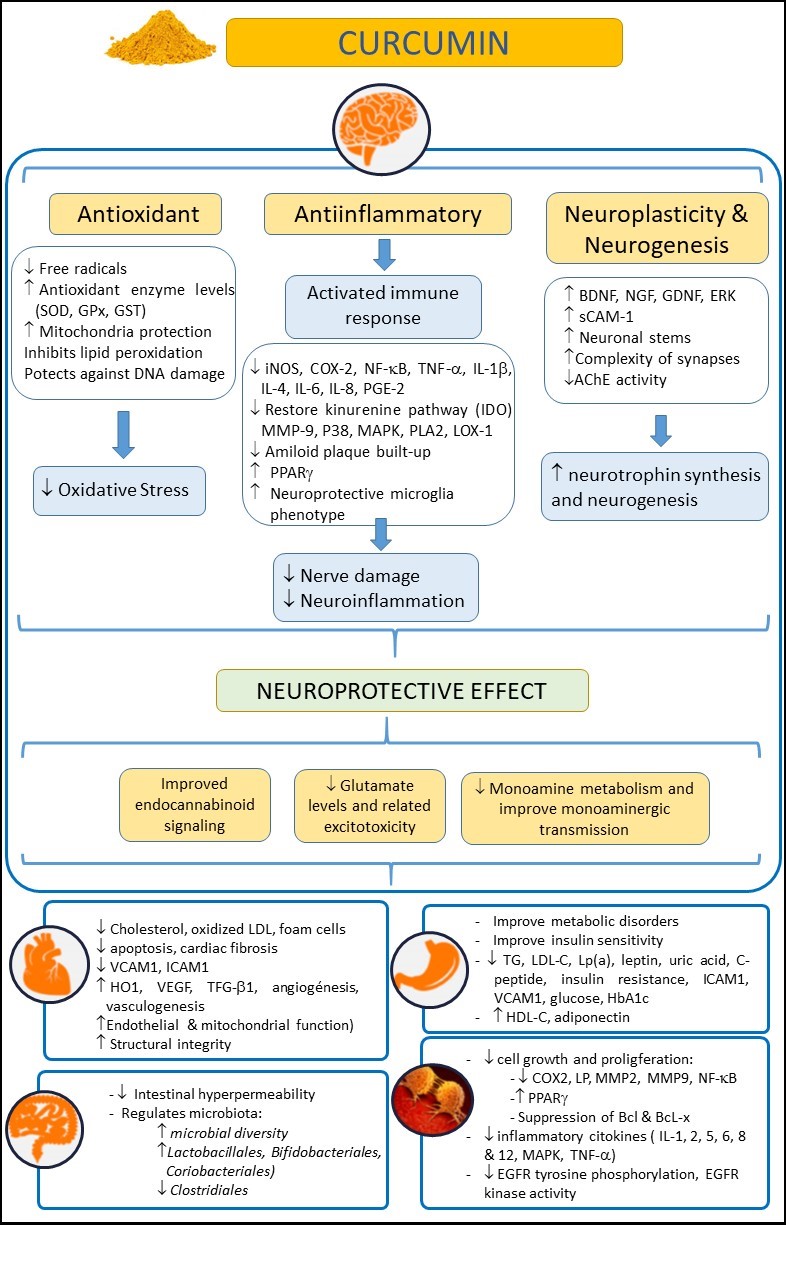
Figure 1. Examining the beneficial effects of curcumin on health, in neurological, cardiovascular, intestinal, metabolic, and oncological disorders. Abbreviations: AChE: Acetylcholinesterase; Bcl: B-cell lymphoma; BDNF: Brain-derived neurotrophic factor; COX-2: Cyclooxygenase 2; EGFR: epidermal growth factor receptor; ERK: Extracellular-regulated kinase9; GDNF: Glial cell line-derived neurotrophic factor; GPx: Glutathione Peroxidase; GSH: Glutathione; GST: Glutathione S-Transferase; HbA1c: glycosylated hemoglobin A1c; HDL-C: high density lipoprotein cholesterol; HO-1: heme-oxygenase-1; ICAM-1: intercellular adhesion molecule-1; IL: Interleukin; iNOS: inducible Nitric Oxide Synthase; IL-10: Interleukin-10; LDL-C: low density lipoprotein cholesterol; LOX-1: Lectin-like oxidized low-density lipoprotein receptor-1; Lp(a): lipoprotein(a); LP: lipooxygenase; MAPK: mitogen-activated protein kinase; MMP-9: Matrix Metalloproteinase 9; NF-κB: nuclear factor kappa-B; NGF: Nerve growth factor; PLA2: Phospholipase A2; P38: p38 MAKP; PGE-2: Prostaglandin E2; PPARγ: Peroxisome proliferator-activated receptor gamma; sCAM-1: soluble Cell Adhesion Molecule 1; TG: Triglycerides; TNF-α: tumor necrosis factor-α; TGF-β1: transforming growth factor; SOD: Superoxide dismutase; VCAM-1: vascular cell adhesion molecule-1; VEGF: Vascular endothelial growth factor.
C Surcummin can also reduce the neutrophil recruitment to [9] arary of potential beneficial eas affected by inflammation, and can also increase the phagocytic activity of macrophages [10]. Fs of curcumin on health, in neurological, cardiovascularthermore, curcumin has proven to be an effective modulator of the endocrine system, enhancing the uptake or regulating some hormones, such as insulin [11]. A, intestinal, metabolic, and oncological disorders. Abbreviations: AChE: acetylcholinesterase; Bcl: B-cell tlymphese properties have boosted the interest of researchers in this compound in recent decades. Thus, several preclinical studies and clinical trials have been conducted [12] oma; BDNF: brain-derived neurotrophic factor; COX-2: cyclooxygenase 2; EGFR: epidermal growith the aim of elucidating whether or not curcumin was effective for many different diseases, such as skin [13], cfactor receptor; ERK: extracellular-regulated kinancser, or neurological pathologies [5].
1.2 Applications of curcumin
Re; 9 GDNF: glial centlly, curcumin has also been used in different psychiatric disorders due to the likely involvement of IOS processes in their onset and evolution. In this sense, the above-described role of curcumin as an anti-IOS drug made this compound a good candidate to halt or palliate the course of these diseases. This is especially important, as current therapeutic strategies for many psychiatric disorders have a relatively high failure rate. Thus, the search for new approaches to help address this problem is ongoing.
So fa line-derived neurotrophic factor; GPx: glutathione peroxidase; GSH: glutathione; GST: glutathione S-transferase; HbA1c: glycosylated hemoglobin A1c; HDL-C: high density lipoprotein cholesterol; HO-1: heme-oxygenase-1; ICAM-1: inter, sceveral clinical trials and studies with animal models, which we will detail in depth in the following sections of this work, have reported the efficacy of curcumin in some psychiatric disorders, such as depression, schizophrenia or autism. However, some studies have showed no positive effects of curcumin in neurological diseases. The main and most recommended route of administration of curcumin is oral and, despite considerable high absorption through lipid membranes caused by its lipophilic nature, curcumin has a low bioavailability after being metabolized, accumulating in spleen, liver and intestine and barely absorbed by the rest of organs [8,14,15]. This lllular adhesion molecule-1; IL: interleukin; iNOS: inducible nitric oxide synthase; IL-10: Interleukin-10; LDL-C: low density lipoprotein cholesterol; LOX-1: lectin-like oxidized low-density lipoprotein receptor-1; Lp(a): lipoprotein(a); LP: lipoxygenase; MAPK: mitogen-activated protein kinase; MMP-9: matrix metalloproteinase 9; NF-κB: nuclear factor kappa-B; NGF: Nerve growth absorption by the small intestine and the high metabolism in the liver weaken its oral bioavailability [16],factor; PLA2: phospholipase A2; P38: p38 MAKP; PGE-2: prostaglandin E2; makingPPARγ: necessary the use of high doses of curcumin to reach other target organs such as the brain [16]. Moreover, its apparent ineffectiveness in interacting specifically with a single pharmacological target has prompted the classification of curcumin as a pan assay interference compound (PAINS) and an invalid metabolic panacea (IMPS) [17,18]. Hperoxisome proliferator-activated receptor gamma; sCAM-1: soluble cell adhesion molecule 1; TG: triglycerides; TNF-α: tumor necrosis factowever, despite the poor pharmacokinetics of this compound, the existence of positive results in several studies raises the question of how curcumin could cause a beneficial effect at the brain level despite being barely able to reach this organ-α; TGF-β1: transforming growth factor; SOD: superoxide dismutase; VCAM-1: vascular cell adhesion molecule-1; VEGF: vascular endothelial growth factor. A recent hypothesis explains that c
Curcumin could be acting on the gut microbiota [19] sincn also reduce intestine and liver are primary sites of metabolism for curcumin [16], rhe neutrophil recruitmeducing intestinal inflammation and, hence, functioning as a neuroprotective agent due to the likely involvement of neuroinft to areas affected by inflammation in[9], many psychiatric disorders in which alterations of the gut-brain axis play an important roled can also increase the phagocytic activity of macrophages [2,20][10]. Furthermore, in order to address the low bioavailability of the curcumin, new formulations of this compound are being synthetized to improve its pharmacokinetics and achieve stable curcumin that can reach the brain in higher concentration. Some of them are based on conjugating curcumin with lipids or co-tre has proven to be an effective modulator of the endocrine system, enhancing the uptake or regulating curcumin with piperine, a bio-enhancer that improves the absorption of curcumsome hormones, such as insulin [8,21][11]. All recent promising strategy, consisting on the use of curcumin carriers as a drug delivery system using liposomes, exosomes, magnetic particles or ultrasound bubbles, has emerged in the nano-pharmaceutical scenario to improve its bioavailability in the brain [22]these properties have boosted the interest of researchers in this compound in recent decades. Moreover, some of tThese exosomes have anti-inflammatory capacity [23] whus, several preclinich would enhance the anti-inflammatory capacity of curcumin in the brain and other organs of interest. Of note, oral and intra-nasal administration of nanoparticles are also being explore, which would increase drugl studies and clinical trials have been conducted absorption[12] win the brain, representing a great advantage in brain disorders [24,25].
Tth the aim of elucidating wherefore, the aim of this work is to review the current literature on the effect of curcumin and its derivatives in the field of psychiatricther or not curcumin was effective for many different diseases, such as skin disorders[13], cand to discern whether the initial enthusiasm for this compound icer, or neurological pathologies well-founded [21,26][5].
2. Current status of curcumin in neurpopsychiatric disorders
1.2 Applications of curcumin
2. Current Status of Curcumin in Neurpopsychiatric Disorders
2.1 Schizophrenia
2.2 Depression
2.3 Autism Spectrum Disorder (ASD)
2.4 Obsessive Compulsive Disorder (OCD)
2.4. Obsessive Compulsive Disorder (OCD)
3. Pros and consCons of cCurcumin in nNeuropsychiatric dDisorders
4. Conclussion4. Conclusion
References
- Ceremuga, T.E.; Helmrick, K.; Kufahl, Z.; Kelley, J.; Keller, B.; Philippe, F.; Golder, J.; Padron, G. Investigation of the Anxiolytic and Antidepressant Effects of Curcumin, a Compound From Turmeric (Curcuma longa), in the Adult Male Sprague-Dawley Rat. Holist. Nurs. Pract. 2017, 31, 193–203.
- Sanmukhani, J.; Satodia, V.; Trivedi, J.; Patel, T.; Tiwari, D.; Panchal, B.; Goel, A.; Tripathi, C.B. Efficacy and safety of curcumin in major depressive disorder: A randomized controlled trial. Phytother. Res.: PTR 2014, 28, 579–585.
- Liu, Z.; Cui, C.; Xu, P.; Dang, R.; Cai, H.; Liao, D.; Yang, M.; Feng, Q.; Yan, X.; Jiang, P. Curcumin Activates AMPK Pathway and Regulates Lipid Metabolism in Rats Following Prolonged Clozapine Exposure. Front. Neurosci. 2017, 11, 558.
- Ong, W.Y.; Farooqui, T.; Kokotos, G.; Farooqui, A.A. Synthetic and natural inhibitors of phospholipases A2: Their importance for understanding and treatment of neurological disorders. ACS Chem. Neurosci. 2015, 6, 814–831.
- Zhang, W.Y.; Guo, Y.J.; Han, W.X.; Yang, M.Q.; Wen, L.P.; Wang, K.Y.; Jiang, P. Curcumin relieves depressive-like behaviors via inhibition of the NLRP3 inflammasome and kynurenine pathway in rats suffering from chronic unpredictable mild stress. Int. Immunopharmacol. 2019, 67, 138–144.
- Ramaholimihaso, T.; Bouazzaoui, F.; Kaladjian, A. Curcumin in Depression: Potential Mechanisms of Action and Current Evidence-A Narrative Review. Front. Psychiatry 2020, 11, 572533.
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637.
- Baell, J.; Walters, M.A. Chemistry: Chemical con artists foil drug discovery. Nature 2014, 513, 481–483.
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127.
- Abdollahi, E.; Momtazi, A.A.; Johnston, T.P.; Sahebkar, A. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: A nature-made jack-of-all-trades? J. Cell. Physiol. 2018, 233, 830–848.
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and Type 2 Diabetes Mellitus: Prevention and Treatment. Nutrients 2019, 11, 1837.
- Salehi, B.; Stojanovic-Radic, Z.; Matejic, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545.
- Barbalho, S.M.; de Sousa Gonzaga, H.F.; de Souza, G.A.; de Alvares Goulart, R.; de Sousa Gonzaga, M.L.; de Alvarez Rezende, B. Dermatological effects of Curcuma species: A systematic review. Clin. Exp. Dermatol. 2021, 46, 825–833.
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702.
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18.
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147.
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637.
- Baell, J.; Walters, M.A. Chemistry: Chemical con artists foil drug discovery. Nature 2014, 513, 481–483.
- Dey, P. Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol. Res. 2019, 147, 104367.
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216.
- Witika, B.A.; Makoni, P.A.; Matafwali, S.K.; Mweetwa, L.L.; Shandele, G.C.; Walker, R.B. Enhancement of Biological and Pharmacological Properties of an Encapsulated Polyphenol: Curcumin. Molecules 2021, 26, 4244.
- Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review. Molecules 2020, 25, 689.
- Tran, T.H.; Mattheolabakis, G.; Aldawsari, H.; Amiji, M. Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin. Immunol. 2015, 160, 46–58.
- Bonaccorso, A.; Gigliobianco, M.R.; Pellitteri, R.; Santonocito, D.; Carbone, C.; Di Martino, P.; Puglisi, G.; Musumeci, T. Optimization of Curcumin Nanocrystals as Promising Strategy for Nose-to-Brain Delivery Application. Pharmaceutics 2020, 12, 476.
- Don, T.M.; Chang, W.J.; Jheng, P.R.; Huang, Y.C.; Chuang, E.Y. Curcumin-laden dual-targeting fucoidan/chitosan nanocarriers for inhibiting brain inflammation via intranasal delivery. Int. J. Biol. Macromol. 2021, 181, 835–846.
- Shireen, E. Experimental treatment of antipsychotic-induced movement disorders. J. Exp. Pharmacol. 2016, 8, 1–10.
- Pillai, A.; Parikh, V.; Terry, A.V., Jr.; Mahadik, S.P. Long-term antipsychotic treatments and crossover studies in rats: Differential effects of typical and atypical agents on the expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J. Psychiatr. Res. 2007, 41, 372–386.
- Naserzadeh, P.; Hafez, A.A.; Abdorahim, M.; Abdollahifar, M.A.; Shabani, R.; Peirovi, H.; Simchi, A.; Ashtari, K. Curcumin loading potentiates the neuroprotective efficacy of Fe3O4 magnetic nanoparticles in cerebellum cells of schizophrenic rats. Biomed. Pharmacother. 2018, 108, 1244–1252.
- Moghaddam, A.H.; Maboudi, K.; Bavaghar, B.; Sangdehi, S.R.M.; Zare, M. Neuroprotective effects of curcumin-loaded nanophytosome on ketamine-induced schizophrenia-like behaviors and oxidative damage in male mice. Neurosci. Lett. 2021, 765, 136249.
- Wynn, J.K.; Green, M.F.; Hellemann, G.; Karunaratne, K.; Davis, M.C.; Marder, S.R. The effects of curcumin on brain-derived neurotrophic factor and cognition in schizophrenia: A randomized controlled study. Schizophr. Res. 2018, 195, 572–573.
- Hosseininasab, M.; Zarghami, M.; Mazhari, S.; Salehifar, E.; Moosazadeh, M.; Fariborzifar, A.; Babaeirad, S.; Hendouei, N. Nanocurcumin as an Add-on to Antipsychotic Drugs for Treatment of Negative Symptoms in Patients With Chronic Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Psychopharmacol. 2021, 41, 25–30.
- Kucukgoncu, S.; Guloksuz, S.; Tek, C. Effects of Curcumin on Cognitive Functioning and Inflammatory State in Schizophrenia: A Double-Blind, Placebo-Controlled Pilot Trial. J. Clin. Psychopharmacol. 2019, 39, 182–184.
- Miodownik, C.; Lerner, V.; Kudkaeva, N.; Lerner, P.P.; Pashinian, A.; Bersudsky, Y.; Eliyahu, R.; Kreinin, A.; Bergman, J. Curcumin as Add-On to Antipsychotic Treatment in Patients With Chronic Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Study. Clin. Neuropharmacol. 2019, 42, 117–122.
- Wang, R.; Xu, Y.; Wu, H.L.; Li, Y.B.; Li, Y.H.; Guo, J.B.; Li, X.J. The antidepressant effects of curcumin in the forced swimming test involve 5-HT1 and 5-HT2 receptors. Eur. J. Pharmacol. 2008, 578, 43–50.
- Zhang, L.; Xu, T.; Wang, S.; Yu, L.; Liu, D.; Zhan, R.; Yu, S.Y. Curcumin produces antidepressant effects via activating MAPK/ERK-dependent brain-derived neurotrophic factor expression in the amygdala of mice. Behav. Brain Res. 2012, 235, 67–72.
- Zhang, L.; Xu, T.; Wang, S.; Yu, L.; Liu, D.; Zhan, R.; Yu, S.Y. NMDA GluN2B receptors involved in the antidepressant effects of curcumin in the forced swim test. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 40, 12–17.
- He, X.; Yang, L.; Wang, M.; Zhuang, X.; Huang, R.; Zhu, R.; Wang, S. Targeting the Endocannabinoid/CB1 Receptor System For Treating Major Depression Through Antidepressant Activities of Curcumin and Dexanabinol-Loaded Solid Lipid Nanoparticles. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 42, 2281–2294.
- Choi, G.Y.; Kim, H.B.; Hwang, E.S.; Lee, S.; Kim, M.J.; Choi, J.Y.; Lee, S.O.; Kim, S.S.; Park, J.H. Curcumin Alters Neural Plasticity and Viability of Intact Hippocampal Circuits and Attenuates Behavioral Despair and COX-2 Expression in Chronically Stressed Rats. Mediat. Inflamm. 2017, 2017, 6280925.
- Abd-Rabo, M.M.; Georgy, G.S.; Saied, N.M.; Hassan, W.A. Involvement of the serotonergic system and neuroplasticity in the antidepressant effect of curcumin in ovariectomized rats: Comparison with oestradiol and fluoxetine. Phytother. Res. PTR 2019, 33, 387–396.
- Mohammed, H.S.; Khadrawy, Y.A.; El-Sherbini, T.M.; Amer, H.M. Electrocortical and Biochemical Evaluation of Antidepressant Efficacy of Formulated Nanocurcumin. Appl. Biochem. Biotechnol. 2019, 187, 1096–1112.
- He, X.L.; Yang, L.; Wang, Z.J.; Huang, R.Q.; Zhu, R.R.; Cheng, L.M. Solid lipid nanoparticles loading with curcumin and dexanabinol to treat major depressive disorder. Neural Regen. Res. 2021, 16, 537–542.
- Khadrawy, Y.A.; Hosny, E.N.; Magdy, M.; Mohammed, H.S. Antidepressant effects of curcumin-coated iron oxide nanoparticles in a rat model of depression. Eur. J. Pharmacol. 2021, 908, 174384.
- Kulkarni, S.K.; Bhutani, M.K.; Bishnoi, M. Antidepressant activity of curcumin: Involvement of serotonin and dopamine system. Psychopharmacology 2008, 201, 435–442.
- Bhutani, M.K.; Bishnoi, M.; Kulkarni, S.K. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol. Biochem. Behav. 2009, 92, 39–43.
- He, X.; Zhu, Y.; Wang, M.; Jing, G.; Zhu, R.; Wang, S. Antidepressant effects of curcumin and HU-211 coencapsulated solid lipid nanoparticles against corticosterone-induced cellular and animal models of major depression. Int. J. Nanomed. 2016, 11, 4975–4990.
- Hurley, L.L.; Akinfiresoye, L.; Nwulia, E.; Kamiya, A.; Kulkarni, A.A.; Tizabi, Y. Antidepressant-like effects of curcumin in WKY rat model of depression is associated with an increase in hippocampal BDNF. Behav. Brain Res. 2013, 239, 27–30.
- Kulkarni, S.K.; Akula, K.K.; Deshpande, J. Evaluation of antidepressant-like activity of novel water-soluble curcumin formulations and St. John’s wort in behavioral paradigms of despair. Pharmacology 2012, 89, 83–90.
- Xu, Y.; Ku, B.S.; Yao, H.Y.; Lin, Y.H.; Ma, X.; Zhang, Y.H.; Li, X.J. Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol. Biochem. Behav. 2005, 82, 200–206.
- Borre, Y.E.; Panagaki, T.; Koelink, P.J.; Morgan, M.E.; Hendriksen, H.; Garssen, J.; Kraneveld, A.D.; Olivier, B.; Oosting, R.S. Neuroprotective and cognitive enhancing effects of a multi-targeted food intervention in an animal model of neurodegeneration and depression. Neuropharmacology 2014, 79, 738–749.
- Rinwa, P.; Kumar, A.; Garg, S. Suppression of neuroinflammatory and apoptotic signaling cascade by curcumin alone and in combination with piperine in rat model of olfactory bulbectomy induced depression. PLoS ONE 2013, 8, e61052.
- Jiang, H.; Wang, Z.; Wang, Y.; Xie, K.; Zhang, Q.; Luan, Q.; Chen, W.; Liu, D. Antidepressant-like effects of curcumin in chronic mild stress of rats: Involvement of its anti-inflammatory action. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 47, 33–39.
- Lin, Z.; Shi, L.; Lu, J.; Li, J.; Hu, H.; Zuo, C.; Tang, W.; Lu, Y.; Bao, A.; Xu, L. Effects of curcumin on glucose metabolism in the brains of rats subjected to chronic unpredictable stress: A 18 F-FDG micro-PET study. BMC Complementary Altern. Med. 2013, 14, 202.
- Liu, D.; Wang, Z.; Gao, Z.; Xie, K.; Zhang, Q.; Jiang, H.; Pang, Q. Effects of curcumin on learning and memory deficits, BDNF, and ERK protein expression in rats exposed to chronic unpredictable stress. Behav. Brain Res. 2014, 271, 116–121.
- Chang, X.R.; Wang, L.; Li, J.; Wu, D.S. Analysis of anti-depressant potential of curcumin against depression induced male albino wistar rats. Brain Res. 2016, 1642, 219–225.
- Cui, M.; Li, Q.; Zhang, M.; Zhao, Y.J.; Huang, F.; Chen, Y.J. Long-term curcumin treatment antagonizes masseter muscle alterations induced by chronic unpredictable mild stress in rats. Arch. Oral Biol. 2014, 59, 258–267.
- Haider, S.; Naqvi, F.; Batool, Z.; Tabassum, S.; Sadir, S.; Liaquat, L.; Naqvi, F.; Zuberi, N.A.; Shakeel, H.; Perveen, T. Pretreatment with curcumin attenuates anxiety while strengthens memory performance after one short stress experience in male rats. Brain Res. Bull. 2015, 115, 1–8.
- Zhang, L.; Luo, J.; Zhang, M.; Yao, W.; Ma, X.; Yu, S.Y. Effects of curcumin on chronic, unpredictable, mild, stress-induced depressive-like behaviour and structural plasticity in the lateral amygdala of rats. Int. J. Neuropsychopharmacol. 2014, 17, 793–806.
- Demir, E.A.; Oz, M.; Alp, M.I.; Gergerlioglu, H.S.; Nurullahoglu, K.E.; Yerlikaya, F.H. Co-administration of cisplatin and curcumin does not alter mood-associated behaviors. Bratisl. Lek. Listy 2016, 117, 106–111.
- Vasileva, L.V.; Saracheva, K.E.; Ivanovska, M.V.; Petrova, A.P.; Marchev, A.S.; Georgiev, M.I.; Murdjeva, M.A.; Getova, D.P. Antidepressant-like effect of salidroside and curcumin on the immunoreactivity of rats subjected to a chronic mild stress model. Food Chem. Toxicol. 2018, 121, 604–611.
- Lee, B.; Lee, H. Systemic Administration of Curcumin Affect Anxiety-Related Behaviors in a Rat Model of Posttraumatic Stress Disorder via Activation of Serotonergic Systems. Evid.-Based Complementary Altern. Med. 2018, 2018, 9041309.
- Liao, D.; Lv, C.; Cao, L.; Yao, D.; Wu, Y.; Long, M.; Liu, N.; Jiang, P. Curcumin Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-Like Behaviors via Restoring Changes in Oxidative Stress and the Activation of Nrf2 Signaling Pathway in Rats. Oxidative Med. Cell. Longev. 2020, 2020, 9268083.
- Saied, N.M.; Georgy, G.S.; Hussien, R.M.; Hassan, W.A. Neuromodulatory effect of curcumin on catecholamine systems and inflammatory cytokines in ovariectomized female rats. Clin. Exp. Pharmacol. Physiol. 2021, 48, 337–346.
- Mohammad Abu-Taweel, G.; Al-Fifi, Z. Protective effects of curcumin towards anxiety and depression-like behaviors induced mercury chloride. Saudi J. Biol. Sci. 2021, 28, 125–134.
- da Silva Marques, J.G.; Antunes, F.T.T.; da Silva Brum, L.F.; Pedron, C.; de Oliveira, I.B.; de Barros Falcao Ferraz, A.; Martins, M.I.M.; Dallegrave, E.; de Souza, A.H. Adaptogenic effects of curcumin on depression induced by moderate and unpredictable chronic stress in mice. Behav. Brain Res. 2021, 399, 113002.
- Rubab, S.; Naeem, K.; Rana, I.; Khan, N.; Afridi, M.; Ullah, I.; Shah, F.A.; Sarwar, S.; Din, F.U.; Choi, H.I.; et al. Enhanced neuroprotective and antidepressant activity of curcumin-loaded nanostructured lipid carriers in lipopolysaccharide-induced depression and anxiety rat model. Int. J. Pharm. 2021, 603, 120670.
- Huang, Z.; Zhong, X.M.; Li, Z.Y.; Feng, C.R.; Pan, A.J.; Mao, Q.Q. Curcumin reverses corticosterone-induced depressive-like behavior and decrease in brain BDNF levels in rats. Neurosci. Lett. 2011, 493, 145–148.
- Li, Y.C.; Wang, F.M.; Pan, Y.; Qiang, L.Q.; Cheng, G.; Zhang, W.Y.; Kong, L.D. Antidepressant-like effects of curcumin on serotonergic receptor-coupled AC-cAMP pathway in chronic unpredictable mild stress of rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 435–449.
- Wang, Z.; Zhang, Q.; Yuan, L.; Wang, S.; Liu, L.; Yang, X.; Li, G.; Liu, D. The effects of curcumin on depressive-like behavior in mice after lipopolysaccharide administration. Behav. Brain Res. 2014, 274, 282–290.
- Fan, C.; Song, Q.; Wang, P.; Li, Y.; Yang, M.; Liu, B.; Yu, S.Y. Curcumin Protects Against Chronic Stress-induced Dysregulation of Neuroplasticity and Depression-like Behaviors via Suppressing IL-1beta Pathway in Rats. Neuroscience 2018, 392, 92–106.
- Fan, C.; Song, Q.; Wang, P.; Li, Y.; Yang, M.; Yu, S.Y. Neuroprotective Effects of Curcumin on IL-1beta-Induced Neuronal Apoptosis and Depression-Like Behaviors Caused by Chronic Stress in Rats. Front. Cell. Neurosci. 2018, 12, 516.
- Madiha, S.; Haider, S. Curcumin restores rotenone induced depressive-like symptoms in animal model of neurotoxicity: Assessment by social interaction test and sucrose preference test. Metab. Brain Dis. 2019, 34, 297–308.
- Shen, J.D.; Wei, Y.; Li, Y.J.; Qiao, J.Y.; Li, Y.C. Curcumin reverses the depressive-like behavior and insulin resistance induced by chronic mild stress. Metab. Brain Dis. 2017, 32, 1163–1172.
- Wang, Z.; Ren, W.; Zhao, F.; Han, Y.; Liu, C.; Jia, K. Curcumin amends Ca(2+) dysregulation in microglia by suppressing the activation of P2 × 7 receptor. Mol. Cell. Biochem. 2020, 465, 65–73.
- Zhang, L.; Ma, Z.; Wu, Z.; Jin, M.; An, L.; Xue, F. Curcumin Improves Chronic Pain Induced Depression Through Regulating Serum Metabolomics in a Rat Model of Trigeminal Neuralgia. J. Pain Res. 2020, 13, 3479–3492.
- Zhang, W.Y.; Guo, Y.J.; Han, W.X.; Yang, M.Q.; Wen, L.P.; Wang, K.Y.; Jiang, P. Curcumin relieves depressive-like behaviors via inhibition of the NLRP3 inflammasome and kynurenine pathway in rats suffering from chronic unpredictable mild stress. Int. Immunopharmacol. 2019, 67, 138–144.
- Afzal, A.; Batool, Z.; Sadir, S.; Liaquat, L.; Shahzad, S.; Tabassum, S.; Ahmad, S.; Kamil, N.; Perveen, T.; Haider, S. Therapeutic Potential of Curcumin in Reversing the Depression and Associated Pseudodementia via Modulating Stress Hormone, Hippocampal Neurotransmitters, and BDNF Levels in Rats. Neurochem. Res. 2021, 46, 3273–3285.
- Xu, Y.; Ku, B.; Tie, L.; Yao, H.; Jiang, W.; Ma, X.; Li, X. Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res. 2006, 1122, 56–64.
- Yohn, S.E.; Gorka, D.; Mistry, A.; Collins, S.; Qian, E.; Correa, M.; Manchanda, A.; Bogner, R.H.; Salamone, J.D. Oral Ingestion and Intraventricular Injection of Curcumin Attenuates the Effort-Related Effects of the VMAT-2 Inhibitor Tetrabenazine: Implications for Motivational Symptoms of Depression. J. Nat. Prod. 2017, 80, 2839–2844.
- Lian, L.; Xu, Y.; Zhang, J.; Yu, Y.; Zhu, N.; Guan, X.; Huang, H.; Chen, R.; Chen, J.; Shi, G.; et al. Antidepressant-like effects of a novel curcumin derivative J147: Involvement of 5-HT1A receptor. Neuropharmacology 2018, 135, 506–513.
- Xu, Y.; Ku, B.S.; Yao, H.Y.; Lin, Y.H.; Ma, X.; Zhang, Y.H.; Li, X.J. The effects of curcumin on depressive-like behaviors in mice. Eur. J. Pharmacol. 2005, 518, 40–46.
- Yusuf, M.; Khan, M.; Khan, R.A.; Maghrabi, I.A.; Ahmed, B. Polysorbate-80-coated, polymeric curcumin nanoparticles for in vivo anti-depressant activity across BBB and envisaged biomolecular mechanism of action through a proposed pharmacophore model. J. Microencapsul. 2016, 33, 646–655.
- Zhao, X.; Wang, C.; Zhang, J.F.; Liu, L.; Liu, A.M.; Ma, Q.; Zhou, W.H.; Xu, Y. Chronic curcumin treatment normalizes depression-like behaviors in mice with mononeuropathy: Involvement of supraspinal serotonergic system and GABAA receptor. Psychopharmacology 2014, 231, 2171–2187.
- Fidelis, E.M.; Savall, A.S.P.; da Luz Abreu, E.; Carvalho, F.; Teixeira, F.E.G.; Haas, S.E.; Bazanella Sampaio, T.; Pinton, S. Curcumin-Loaded Nanocapsules Reverses the Depressant-Like Behavior and Oxidative Stress Induced by beta-Amyloid in Mice. Neuroscience 2019, 423, 122–130.
- Li, J.; Chen, L.; Li, G.; Chen, X.; Hu, S.; Zheng, L.; Luria, V.; Lv, J.; Sun, Y.; Xu, Y.; et al. Sub-Acute Treatment of Curcumin Derivative J147 Ameliorates Depression-Like Behavior Through 5-HT1A-Mediated cAMP Signaling. Front. Neurosci. 2020, 14, 701.
- Pan, X.; Chen, L.; Xu, W.; Bao, S.; Wang, J.; Cui, X.; Gao, S.; Liu, K.; Avasthi, S.; Zhang, M.; et al. Activation of monoaminergic system contributes to the antidepressant- and anxiolytic-like effects of J147. Behav. Brain Res. 2021, 411, 113374.
- Qi, X.J.; Liu, X.Y.; Tang, L.M.; Li, P.F.; Qiu, F.; Yang, A.H. Anti-depressant effect of curcumin-loaded guanidine-chitosan thermo-sensitive hydrogel by nasal delivery. Pharm. Dev. Technol. 2020, 25, 316–325.
- Choudhary, K.M.; Mishra, A.; Poroikov, V.V.; Goel, R.K. Ameliorative effect of Curcumin on seizure severity, depression like behavior, learning and memory deficit in post-pentylenetetrazole-kindled mice. Eur. J. Pharmacol. 2013, 704, 33–40.
- Ceremuga, T.E.; Helmrick, K.; Kufahl, Z.; Kelley, J.; Keller, B.; Philippe, F.; Golder, J.; Padron, G. Investigation of the Anxiolytic and Antidepressant Effects of Curcumin, a Compound From Turmeric (Curcuma longa), in the Adult Male Sprague-Dawley Rat. Holist. Nurs. Pract. 2017, 31, 193–203.
- Xu, Y.; Ku, B.; Cui, L.; Li, X.; Barish, P.A.; Foster, T.C.; Ogle, W.O. Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain Res. 2007, 1162, 9–18.
- Arora, V.; Kuhad, A.; Tiwari, V.; Chopra, K. Curcumin ameliorates reserpine-induced pain-depression dyad: Behavioural, biochemical, neurochemical and molecular evidences. Psychoneuroendocrinology 2011, 36, 1570–1581.
- Sanmukhani, J.; Satodia, V.; Trivedi, J.; Patel, T.; Tiwari, D.; Panchal, B.; Goel, A.; Tripathi, C.B. Efficacy and safety of curcumin in major depressive disorder: A randomized controlled trial. Phytother. Res.: PTR 2014, 28, 579–585.
- Bergman, J.; Miodownik, C.; Bersudsky, Y.; Sokolik, S.; Lerner, P.P.; Kreinin, A.; Polakiewicz, J.; Lerner, V. Curcumin as an add-on to antidepressive treatment: A randomized, double-blind, placebo-controlled, pilot clinical study. Clin. Neuropharmacol. 2013, 36, 73–77.
- Lopresti, A.L.; Maes, M.; Maker, G.L.; Hood, S.D.; Drummond, P.D. Curcumin for the treatment of major depression: A randomised, double-blind, placebo controlled study. J. Affect. Disord. 2014, 167, 368–375.
- Lopresti, A.L.; Maes, M.; Meddens, M.J.; Maker, G.L.; Arnoldussen, E.; Drummond, P.D. Curcumin and major depression: A randomised, double-blind, placebo-controlled trial investigating the potential of peripheral biomarkers to predict treatment response and antidepressant mechanisms of change. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2015, 25, 38–50.
- Panahi, Y.; Badeli, R.; Karami, G.R.; Sahebkar, A. Investigation of the efficacy of adjunctive therapy with bioavailability-boosted curcuminoids in major depressive disorder. Phytother. Res. 2015, 29, 17–21.
- Yu, J.J.; Pei, L.B.; Zhang, Y.; Wen, Z.Y.; Yang, J.L. Chronic Supplementation of Curcumin Enhances the Efficacy of Antidepressants in Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J. Clin. Psychopharmacol. 2015, 35, 406–410.
- Lopresti, A.L.; Drummond, P.D. Efficacy of curcumin, and a saffron/curcumin combination for the treatment of major depression: A randomised, double-blind, placebo-controlled study. J. Affect. Disord. 2017, 207, 188–196.
- Kanchanatawan, B.; Tangwongchai, S.; Sughondhabhirom, A.; Suppapitiporn, S.; Hemrunrojn, S.; Carvalho, A.F.; Maes, M. Add-on Treatment with Curcumin Has Antidepressive Effects in Thai Patients with Major Depression: Results of a Randomized Double-Blind Placebo-Controlled Study. Neurotox. Res. 2018, 33, 621–633.
- Bhandari, R.; Kuhad, A. Neuropsychopharmacotherapeutic efficacy of curcumin in experimental paradigm of autism spectrum disorders. Life Sci. 2015, 141, 156–169.
- Al-Askar, M.; Bhat, R.S.; Selim, M.; Al-Ayadhi, L.; El-Ansary, A. Postnatal treatment using curcumin supplements to amend the damage in VPA-induced rodent models of autism. BMC Complementary Altern. Med. 2017, 17, 259.
- Zhong, H.; Xiao, R.; Ruan, R.; Liu, H.; Li, X.; Cai, Y.; Zhao, J.; Fan, X. Neonatal curcumin treatment restores hippocampal neurogenesis and improves autism-related behaviors in a mouse model of autism. Psychopharmacology 2020, 237, 3539–3552.
- Jayaprakash, P.; Isaev, D.; Shabbir, W.; Lorke, D.E.; Sadek, B.; Oz, M. Curcumin Potentiates alpha7 Nicotinic Acetylcholine Receptors and Alleviates Autistic-Like Social Deficits and Brain Oxidative Stress Status in Mice. Int. J. Mol. Sci. 2021, 22, 7251.
