Chronic spontaneous urticaria (CSU) considerably alters patients’ quality of life, often for extended periods, due to pruriginous skin lesions, impaired sleep, unexpected development of angioedema, and failure of conventional treatments in properly controlling signs and symptoms. Although the production of specific immunoglobulin E (IgE) antibodies against certain allergens is not a characteristic of the disease, treatment with omalizumab, a monoclonal anti-IgE antibody, proved efficient and safe in patients with moderate to severe chronic spontaneous urticaria uncontrolled by H1-antihistamines. Ligelizumab, a high-affinity monoclonal anti-IgE antibody, may also efficiently relieve symptoms of unresponsive chronic urticaria to standard therapies.
1. Introduction
Chronic idiopathic urticaria or chronic spontaneous urticaria (CSU) is a debilitating disease that significantly impacts the quality of life. It is characterized by the development of wheals (hives), associated or not with angioedema for a period longer than 6 weeks, due to known or unknown apparent cause
[1][2][1,2]. Wheals (hives) are superficial pruritic skin lesions, characterized by central swellings of various sizes, surrounded by reflex erythema, that usually persists for less than 24 h
[1]. Angioedema is defined as an edematous process in the deeper part of the dermis, subcutaneous or mucous tissue that can last for up to 3 days
[1]. It may be perceived as painful rather than itchy
[1]. Unfortunately, the disease generally follows a prolonged course. Identifying a causative factor and finding the most suitable therapeutic option often pose a great challenge for physicians. The patients’ quality of life is considerably altered due to persistent, severe itching, impaired sleep, and associated secondary psychological and social issues
[1][2][1,2].
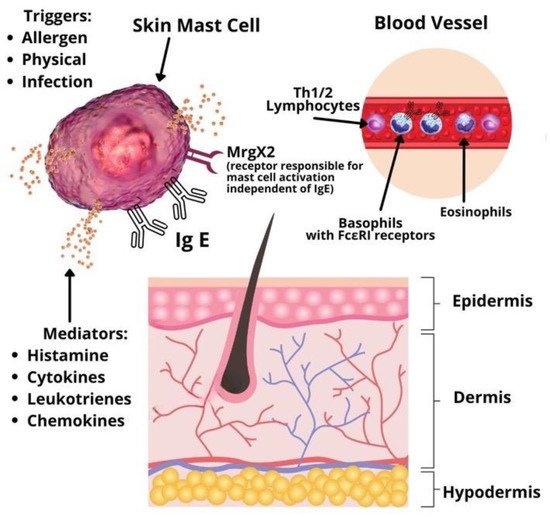
Urticaria is considered a disease driven mainly by mast cells
[1]. Symptoms develop due to mast cell and basophil degranulation, followed by the release of various types of mediators: Preformed (histamine, serotonin, tryptase, proteoglycans, etc.), newly synthesized lipid mediators (prostaglandins, cysteinyl leukotrienes, etc.), cytokines and chemokines (IL-4, IL-5, IL-6, TNF-alpha, TNF-beta, etc.)
[3]. Mast cells and basophils activation may be immunoglobulin E (IgE) or non-IgE mediated. In addition, studies for other infiltrating cells involved in the pathophysiology of CSU, such as lymphocytes and eosinophils, are emerging (
Figure 1). A significant role in type I allergic reactions has the platelet-activating factor (PAF) produced and released by mast cells, eosinophils, basophils, endothelial cells, neutrophils, platelets, fibroblasts, and even the cardiac muscle
[4]. Mast cells can produce and be activated by PAF. When the mast cells are located in the skin, exposure to PAF leads to degranulation of their granules via neuropeptides
[5]. Therefore, PAF plays an essential role in patients with urticaria due to its inflammatory role and chemotactic action. Along with the vascular endothelial growth factor (VEGF), PAF increases the permeability of capillaries in the skin and intensifies the development of urticarial specific lesions, such as wheals and erythema. This effect is especially distinguishable in chronic spontaneous urticaria. Studies on volunteers with CSU revealed that PAF injected subcutaneously induces typical urticarial hives
[3]. Studies on anaphylaxis have shown that PAF is an important mediator in the development of anaphylactic shock. High serum levels of the platelet-activating factor directly influence the severity of systemic reactions
[4][5][4,5].
Figure 1.
Pathophysiology of chronic spontaneous urticaria.
Based on studies by Babaie et al. and Grieco et al. IL-6 plays a significant role in the pathogenesis of chronic urticaria by promoting the trans-signaling capacity during the inflammatory response
[6][7][6,7]. IL-6 is released by mast cells, basophils, eosinophils monocytes, activated T cells, and neutrophils, particularly in acute urticaria resistant to conventional antihistamine therapy. Therefore, the disease activity could be explained by this chronic inflammation. Moreover, IL-6 is a major factor for the expression of other pro-inflammatory cytokines, such as TNF-alpha and IL1-beta, as well as for the production of antibodies
[6]. Notably, the release of IL-6 precedes the appearance of antibodies in the serum
[6][7][6,7].
Production of IgE plays a central role in the pathogenesis of allergic diseases. IgE is present both in the peripheral circulation and on the surface of different cell types, bound to low-affinity receptors, CD23 and high-affinity receptors, FcεRIα
[4]. The binding of a particular allergen to specific IgE antibodies attached to high-affinity IgE receptors present on the surface of mast cells and basophils determines cross-linking and cellular degranulation. The released mediators produce cutaneous and, occasionally, systemic allergic manifestations
[1][2][8][1,2,8]. In addition, various autoimmune conditions, including systemic lupus erythematosus, dermatomyositis, polymyositis or rheumatoid arthritis, have been associated with chronic spontaneous urticaria
[9]. One large population study conducted on 12,000 CSU patients in Israel determined that female subjects have a higher incidence of systemic lupus erythematosus, rheumatoid arthritis, celiac disease, type I diabetes, and Sjögren syndrome than female patients without CSU
[10]. The same analysis was performed between male subjects, but the numbers did not reach statistical significance. Further serologic investigations on autoimmune disease markers revealed that patients with CSU had significantly higher levels of antinuclear antibodies (ANA), rheumatoid factor (RF), anti-thyroid peroxidase (anti-TPO) antibodies, anti-parietal cell antibodies, antithyroglobulin antibodies, and anti-transglutaminase IgA antibodies
[10].
2. Management of Chronic Spontaneous Urticaria Using Anti-IgE Antibodies
2.1. Anti-IgE Therapy
Chronic idiopathic urticaria or chronic spontaneous urticaria is a very challenging and frustrating disease for both patients and physicians. Consequently, different biological therapies have been developed as conventional treatments often fail to control the signs and symptoms
[11]. During the past decades, research focused on both IgE antibodies and FcεRIα receptors as achievable targets for the development of new biologic agents that aim to prevent or decrease mast cell and basophil activation
[12]. The correct selection of patients and the choice for the optimal method of investigation are essential for the proper evaluation of the effects of a newly developed treatment in any challenging disease. The objectives of a specific treatment also need to be clearly defined. Therefore, crucial information regarding a particular drug, the target population, and the disease itself can be obtained by evaluating the safety and efficacy of the drug in clinical trials and real-life scenarios. The gained knowledge helps
scholarus in developing solid criteria that enable physicians to formulate strict recommendations for the use of that specific medication.
The first licensed biological treatment for chronic spontaneous urticaria is the monoclonal anti-IgE antibody, omalizumab. It was approved for the treatment of mild to severe and long-lasting allergic asthma in 2003. In 2014, it was approved for the treatment of spontaneous urticaria for patients 12 years and older at a dose of 150 mg every 4 weeks and 300 mg every 4 weeks. This treatment primarily aims to achieve the relief of symptoms, such as pruritus and pain, as well as complete disappearance of the clinical signs in the least possible time frame. In addition, the treatment aims to ensure a better quality of life for chronic urticaria patients
[1].
In accordance with the current international EAACI/GA
2LEN/EuroGuiDerm/APAAACI guidelines for the management of chronic spontaneous urticaria, a four-step treatment algorithm is recommended. Following an inadequate response to the first and second lines of treatment with H1-antihistamines at licensed dose or up to 4 times the licensed dose, respectively, patients may be offered omalizumab as the third line of treatment
[1]. Immunosuppressants, such as cyclosporine are administered to patients that are nonresponsive to omalizumab due to a higher incidence of adverse effects. Studies comparing the efficacy of omalizumab to cyclosporine showed the superiority of the first
[13]. Moreover, in contrast to cyclosporine or montelukast, omalizumab is a licensed treatment for chronic urticaria
[11].
2.2. Management of Chronic Spontaneous Urticaria with Omalizumab and Ligelizumab in Clinical Practice
At present, few questions still remain unanswered. The discrepancies between clinical trial results and those seen in everyday practice are vital for practitioners. For example, in some instances, a better response to omalizumab has been observed in real-life clinical settings compared with pivotal randomized controlled trials
[14][15][16][14,15,16].
Real-life practical evidence has demonstrated that patients with chronic spontaneous urticaria may exhibit a rapid response to omalizumab. A retrospective evaluation of patients who underwent treatment with omalizumab outside of clinical trials showed a quick control of the symptoms, with 57% of patients achieving complete clinical remission within a week
[17][37]. In addition, our personal experience and reports in the medical literature are in accordance with the mentioned study results conducted by Metz et al. [38]. Furthermore, relapses of chronic urticaria in patients formerly treated with omalizumab can be successfully managed with the same biologic agent. A retrospective study showed that most of the relapses occurred within 2 to 8 weeks after discontinuation of omalizumab treatment
[18][39]. Once again, all patients achieved complete remission of urticaria signs and symptoms within the initial 4 weeks after reinitiating omalizumab at a dose ranging between 150 and 600 mg per month, with no adverse effects
[18][39].
2.3. Potential Biomarkers for the Effectiveness of Chronic Urticaria Treatment
The prediction of variations in the disease activity in response to treatment can be very helpful in improving treatment algorithms. Therefore, identifying biomarkers that can be used to predict the course of the disease and the extent of variation in the activity of chronic urticaria upon administration of a particular treatment would represent a significant advancement in providing an optimal individualized therapeutic approach. This is applicable for all the drugs currently used to treat chronic urticaria, such as omalizumab, leukotriene receptor antagonists, cyclosporine, and H1-antihistamines.
Among patients treated with cyclosporine, a positive serum basophil histamine release assay suggests that these patients are more inclined to respond to cyclosporine treatment than patients with a negative test
[19][52].
For the moment, data on which and how biomarkers may anticipate the result of the other treatments are lacking, but research on the subject is ongoing. An example of the interest in the field is a recent retrospective study that included 41 patients with chronic refractory urticaria and concluded that the absence of basophil CD203c upregulation activity in the patients’ serum was correlated with the clinical response to omalizumab
[20][57]. The upregulation activity of CD203c was present in 18 out of 41 patients
[20][57]. Interestingly, only 50% of these 18 patients recorded clinical improvement with omalizumab
[20][57]. On the other hand, 87% of the 23 patients who did not display upregulation activity of CD203c had a positive clinical response to omalizumab treatment
[20][57].
3. Conclusions
Chronic spontaneous urticaria has a significant medical impact worldwide due to its increasing prevalence in a general, age-dependent population, the potential to associate systemic symptoms and life-threatening angioedema, and due to the unpredictable evolution, particularly in patients with uncontrolled disease. Therefore, extending the therapeutical possibilities in monotherapy or integrative approaches is a priority. In the last decades, research focused on developing monoclonal anti-IgE antibodies. The first monoclonal anti-IgE antibody was omalizumab, which is now recommended by EAACI guidelines for patients with refractory urticaria. A second monoclonal anti-IgE antibody is ligelizumab, possibly with a higher IgE affinity than omalizumab. Additional recent agents are still waiting for evaluation by several ongoing trials.