Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Vera Von Dossow.
Lung transplantation has a high risk of haemodynamic complications in a highly vulnerable patient population. The effects on the cardiovascular system of the various underlying end-stage lung diseases also contribute to this risk.
- lung transplantation
- circulatory support
- extracorporeal membrane oxygenation
1. Introduction
1. Introduction
Since it was first performed in 1963, lung transplantation has remained a multidisciplinary challenge, especially in the intraoperative setting [1]. Nevertheless, it is the only therapy for patients with end-stage lung disease for whom drug therapy fails. Advanced lung diseases are often associated with pulmonary arterial hypertension (PAH), resulting in right and, secondarily, left heart failure [2][3][4]. Thus, during transplantation in this highly vulnerable patient population, circulatory support through optimal anaesthesiologic and surgical management plays a crucial role [5][6]. A major change in intraoperative management was the use of extracorporeal membrane oxygenation (ECMO) as a closed system as compared to the cardiopulmonary bypass (CPB). With different cannulation strategies (veno-arterial and veno-venous (VA ECMO and VV ECMO), as well as central and peripheral cannulation), it became possible to support both oxygenation and, if needed, circulation with minimal anticoagulation and with reduced invasiveness [7]. Regarding the application of this procedure, different strategies exist depending on the respective transplant centre. ECMO is used in 15–100% of transplant cases [8][9][10]. In addition to the application of mechanical extracorporeal circulatory support, non-mechanical circulatory support, such as the rational use of catecholamines and inhaled pulmonary vasodilators and the management of right ventricular strain on one lung ventilation (OLV) and diastolic left ventricular dysfunction, are crucial to the success of lung transplantation. The administration of intravenous fluids for circulatory support also has an impact on the management of lung transplantation, including the development of reperfusion injury (RI) [11].
Since it was first performed in 1963, lung transplantation has remained a multidisciplinary challenge, especially in the intraoperative setting [1]. Nevertheless, it is the only therapy for patients with end-stage lung disease for whom drug therapy fails. Advanced lung diseases are often associated with pulmonary arterial hypertension (PAH), resulting in right and, secondarily, left heart failure [2,3,4]. Thus, during transplantation in this highly vulnerable patient population, circulatory support through optimal anaesthesiologic and surgical management plays a crucial role [5,6]. A major change in intraoperative management was the use of extracorporeal membrane oxygenation (ECMO) as a closed system as compared to the cardiopulmonary bypass (CPB). With different cannulation strategies (veno-arterial and veno-venous (VA ECMO and VV ECMO), as well as central and peripheral cannulation), it became possible to support both oxygenation and, if needed, circulation with minimal anticoagulation and with reduced invasiveness [7]. Regarding the application of this procedure, different strategies exist depending on the respective transplant centre. ECMO is used in 15–100% of transplant cases [8,9,10]. In addition to the application of mechanical extracorporeal circulatory support, non-mechanical circulatory support, such as the rational use of catecholamines and inhaled pulmonary vasodilators and the management of right ventricular strain on one lung ventilation (OLV) and diastolic left ventricular dysfunction, are crucial to the success of lung transplantation. The administration of intravenous fluids for circulatory support also has an impact on the management of lung transplantation, including the development of reperfusion injury (RI) [11].
Irrespective of the clearly differing approaches to circulatory support between individual lung transplant centres, it can make sense to develop strategies for the management of lung transplantation on a patient-specific basis, depending on the risk for certain pathologies, and to define them at an early stage of treatment. Such an approach, for example within the framework of so-called ERSAS concepts (early risk stratification and strategy), aims at identifying possible risk factors for the occurrence of complications and the corresponding strategies and therapies to avoid these complications (
Irrespective of the clearly differing approaches to circulatory support between individual lung transplant centres, it can make sense to develop strategies for the management of lung transplantation on a patient-specific basis, depending on the risk for certain pathologies, and to define them at an early stage of treatment. Such an approach, for example within the framework of so-called ERSAS concepts (early risk stratification and strategy), aims at identifying possible risk factors for the occurrence of complications and the corresponding strategies and therapies to avoid these complications (
Figure 1
). This is often and especially used in the perioperative setting.
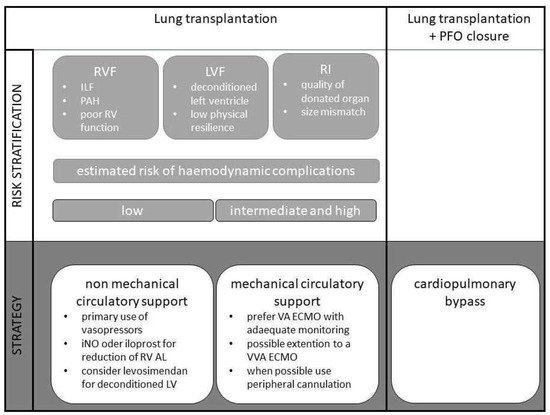
Figure 1. Algorithm for the application of an ERSAS concept for intraoperative circulatory support in lung transplantation.
2. Risk Stratification
In the context of lung transplantation, the occurrence of various haemodynamic complications is possible. These include RVF, LVF, or left ventricular diastolic dysfunction and RI associated with reperfusion oedema, increased pulmonary vascular resistance, and sterile inflammation [6,12,15].
In the context of lung transplantation, the occurrence of various haemodynamic complications is possible. These include RVF, LVF, or left ventricular diastolic dysfunction and RI associated with reperfusion oedema, increased pulmonary vascular resistance, and sterile inflammation [6][12][13].
For none of these entities is there currently a validated scoring system for risk stratification, as is established, for example, in the preoperative risk assessments for the occurrence of cardiac complications in perioperative medicine [16,17]. This is due to the diverse causes for a necessary lung transplantation. In clinical routine, large differences in the operative course can be observed in transplantations due to idiopathic fibrosis with pronounced right heart strain or end-stage chronic obstructive pulmonary disease (COPD) [5]. The analysis of existing databases is also quite difficult, as many centres follow completely different therapeutic strategies during surgery [18]. Hinske et al. developed a score that showed very good predictive power for the need for unplanned mechanical circulatory support. This was achieved in a single-centre study, taking into account the preoperative pulmonary arterial pressure (PAP) and lung allocation scores [19]. However, this risk stratification cannot be used in hospitals that primarily promote the use of ECMO in all transplantations.
For none of these entities is there currently a validated scoring system for risk stratification, as is established, for example, in the preoperative risk assessments for the occurrence of cardiac complications in perioperative medicine [14][15]. This is due to the diverse causes for a necessary lung transplantation. In clinical routine, large differences in the operative course can be observed in transplantations due to idiopathic fibrosis with pronounced right heart strain or end-stage chronic obstructive pulmonary disease (COPD) [5]. The analysis of existing databases is also quite difficult, as many centres follow completely different therapeutic strategies during surgery [16]. Hinske et al. developed a score that showed very good predictive power for the need for unplanned mechanical circulatory support. This was achieved in a single-centre study, taking into account the preoperative pulmonary arterial pressure (PAP) and lung allocation scores [17]. However, this risk stratification cannot be used in hospitals that primarily promote the use of ECMO in all transplantations.
2.1. Right Ventricular Failure
In general, RVF is associated with decreased cardiac output and increased right ventricular filling pressures due to systolic or diastolic dysfunction. In the setting of lung transplantation, pulmonary hypertension is the most important cause of RVF. Other causes, such as pulmonary embolism, myocardial ischaemia, or secondary RVF in left heart failure, are more secondary [20,21].
In general, RVF is associated with decreased cardiac output and increased right ventricular filling pressures due to systolic or diastolic dysfunction. In the setting of lung transplantation, pulmonary hypertension is the most important cause of RVF. Other causes, such as pulmonary embolism, myocardial ischaemia, or secondary RVF in left heart failure, are more secondary [18][19].
In patients scheduled for lung transplantation, the incidence of PAH is up to 40–50% [10]. In about 20–30% of transplant candidates, PAH is even the indication for transplantation [9]. It is not uncommon for systemic to suprasystemic blood pressure values to be measured in the pulmonary circulation [9,10].
In patients scheduled for lung transplantation, the incidence of PAH is up to 40–50% [10]. In about 20–30% of transplant candidates, PAH is even the indication for transplantation [9]. It is not uncommon for systemic to suprasystemic blood pressure values to be measured in the pulmonary circulation [9][10].
Pathophysiologically, right heart failure is characterised by dilatation and remodelling of the right ventricle (RV), triggered by an increased right ventricular afterload. Changes in ventricular geometry towards a spherical shape, with displacement of the interventricular septum towards the left ventricle, increased wall stress, and reduced myocardial contractility, are also consequences of the increase in afterload and contribute to the reduction in cardiac output (CO). The decrease in CO is exacerbated by tricuspid regurgitation with corresponding regurgitation volume. Due to reduced left ventricular (LV) filling, ventricular dyssynchrony, and septal kinetic disturbances, the LV is also affected. In advanced stages, end-organ damage such as renal failure, hepatic failure, and intestinal motility disorders can be observed [20,21]. The stress on the right ventricle is further exacerbated by single-lung ventilation. During thoracic surgery, a decrease of RV function up to 25% due to the initiation of OLV and anatomical resection was shown [22]. In principle, the changes in the RV after lung transplantation are reversible and therefore do not represent an absolute contraindication to transplantation even in the case of demonstrable end-organ damage [23].
Pathophysiologically, right heart failure is characterised by dilatation and remodelling of the right ventricle (RV), triggered by an increased right ventricular afterload. Changes in ventricular geometry towards a spherical shape, with displacement of the interventricular septum towards the left ventricle, increased wall stress, and reduced myocardial contractility, are also consequences of the increase in afterload and contribute to the reduction in cardiac output (CO). The decrease in CO is exacerbated by tricuspid regurgitation with corresponding regurgitation volume. Due to reduced left ventricular (LV) filling, ventricular dyssynchrony, and septal kinetic disturbances, the LV is also affected. In advanced stages, end-organ damage such as renal failure, hepatic failure, and intestinal motility disorders can be observed [18][19]. The stress on the right ventricle is further exacerbated by single-lung ventilation. During thoracic surgery, a decrease of RV function up to 25% due to the initiation of OLV and anatomical resection was shown [20]. In principle, the changes in the RV after lung transplantation are reversible and therefore do not represent an absolute contraindication to transplantation even in the case of demonstrable end-organ damage [21].
Predicting the occurrence of acute or acute-on-chronic right heart failure in the setting of lung transplantation is challenging. To our knowledge, no prediction scores exist. Based purely on the intraoperative course of lung transplantation, exacerbation or recurrence of right heart failure is to be expected, primarily before reperfusion of the transplanted lung. Especially in patients with intermediate or severe PAH, immediate positive haemodynamic effects are seen after transplantation. Systolic PAP, mean PAP, and right ventricular end-diastolic volume rapidly decrease. Patients who do not experience these effects are at increased risk for primary graft dysfunction and worse outcomes [24]. Given the link between impaired RV function and outcome, one study investigated the prediction of mortality by echocardiographic parameters in lung transplantation. No pre-transplantation RV parameter predicted all-cause mortality. However, post-transplantation echocardiographic RV parameters (RV strain and systolic PAP) predicted the outcome after lung transplantation [25]. Predicting the occurrence of RVF during lung transplantation also appears difficult because many centres practice liberal use of VA ECMO, which prevents such RVF [10]. Even in centres that favour the selective use of VA ECMO, one of the most important indications for the use of VA ECMO is the existence of severe or intermediate pulmonary hypertension [5,8]. Thus, it is precisely in this risk group that the occurrence of RVF is prevented. Per se, the presence of pulmonary hypertension, idiopathic pulmonary fibrosis, and dilated right ventricle is associated with poor outcomes, even in multivariate analyses [9].
Predicting the occurrence of acute or acute-on-chronic right heart failure in the setting of lung transplantation is challenging. No prediction scores exist. Based purely on the intraoperative course of lung transplantation, exacerbation or recurrence of right heart failure is to be expected, primarily before reperfusion of the transplanted lung. Especially in patients with intermediate or severe PAH, immediate positive haemodynamic effects are seen after transplantation. Systolic PAP, mean PAP, and right ventricular end-diastolic volume rapidly decrease. Patients who do not experience these effects are at increased risk for primary graft dysfunction and worse outcomes [22]. Given the link between impaired RV function and outcome, one study investigated the prediction of mortality by echocardiographic parameters in lung transplantation. No pre-transplantation RV parameter predicted all-cause mortality. However, post-transplantation echocardiographic RV parameters (RV strain and systolic PAP) predicted the outcome after lung transplantation [23]. Predicting the occurrence of RVF during lung transplantation also appears difficult because many centres practice liberal use of VA ECMO, which prevents such RVF [10]. Even in centres that favour the selective use of VA ECMO, one of the most important indications for the use of VA ECMO is the existence of severe or intermediate pulmonary hypertension [5][8]. Thus, it is precisely in this risk group that the occurrence of RVF is prevented. Per se, the presence of pulmonary hypertension, idiopathic pulmonary fibrosis, and dilated right ventricle is associated with poor outcomes, even in multivariate analyses [9].
In summary, no clear specific parameters exist to predict RVF during lung transplantation. Patients at particular risk appear to be those who already have severe or intermediate pulmonary hypertension, have pulmonary fibrosis, or do not show recovery of right ventricular function after transplantation.
In 2021, the international consensus recommendations for anaesthesiologic and intensive care management in lung transplantation were published [14]. It was again emphasised that patients with relevant pulmonary hypertension are one of the most challenging patient populations for lung transplantation. The ever-present risk of RVF was also pointed out. However, with weak evidence, no recommendation for a specific risk stratification could be provided here either. It is recommended that a preoperative right heart catheterisation should be performed to assess pulmonary hypertension and right ventricular function. A pragmatic approach is to clinically assess haemodynamic changes and response to inotropics during transplantation after clamping of the pulmonary artery and to derive from this an appropriate risk stratification regarding the occurrence of RVF, and later also to develop an appropriate strategy.
In 2021, the international consensus recommendations for anaesthesiologic and intensive care management in lung transplantation were published [24]. It was again emphasised that patients with relevant pulmonary hypertension are one of the most challenging patient populations for lung transplantation. The ever-present risk of RVF was also pointed out. However, with weak evidence, no recommendation for a specific risk stratification could be provided here either. It is recommended that a preoperative right heart catheterisation should be performed to assess pulmonary hypertension and right ventricular function. A pragmatic approach is to clinically assess haemodynamic changes and response to inotropics during transplantation after clamping of the pulmonary artery and to derive from this an appropriate risk stratification regarding the occurrence of RVF, and later also to develop an appropriate strategy.
2.2. Left Ventricular Failure/Diastolic Dysfunction
The previously described frequent right ventricular dysfunction in patients with chronic lung disease who are scheduled for transplantation results in a decreased left ventricular preload. If prolonged, this results in atrophy of left ventricular cardiomyocytes, which may be associated with both diastolic dysfunction and a reduction in myocardial contractility [26]. This also results in a reduced left ventricular cardiac output and increased filling pressures. Especially in the context of transplantation, the occurrence and extent of pulmonary oedema in the sense of left ventricular congestion can be relevantly increased. Diastolic dysfunction as a so-called deconditioning of the left ventricle is common in patients with PAH. In the total collective of lung transplant candidates, an occurrence of around 30% has been described, depending on the centre, and almost all patients with PAH are affected [9,26]. This also applies to the postoperative course. Therefore, monitoring of LV function is crucial [27].
The previously described frequent right ventricular dysfunction in patients with chronic lung disease who are scheduled for transplantation results in a decreased left ventricular preload. If prolonged, this results in atrophy of left ventricular cardiomyocytes, which may be associated with both diastolic dysfunction and a reduction in myocardial contractility [25]. This also results in a reduced left ventricular cardiac output and increased filling pressures. Especially in the context of transplantation, the occurrence and extent of pulmonary oedema in the sense of left ventricular congestion can be relevantly increased. Diastolic dysfunction as a so-called deconditioning of the left ventricle is common in patients with PAH. In the total collective of lung transplant candidates, an occurrence of around 30% has been described, depending on the centre, and almost all patients with PAH are affected [9][25]. This also applies to the postoperative course. Therefore, monitoring of LV function is crucial [26].
Porteous et al. investigated the relationship between diastolic left ventricular dysfunction and primary graft dysfunction (PGD) [28]. For the first time, a clear correlation between impaired left ventricular filling and PGD was shown. Correspondingly, evidence between poor outcome and diastolic dysfunction as measured by the ratio between early transmitral flow (E) and mitral annular velocity (e′), which is independent on loading conditions, was also obtained. The association between the presence of diastolic dysfunction and survival after lung transplantation in patients with pulmonary hypertension as the pathophysiological cause of left ventricular diastolic dysfunction was also shown [9,29]. Similarly, in this patient group, the use of ECMO was more frequent and the duration of ventilation was longer in the presence of diastolic dysfunction [9].
Porteous et al. investigated the relationship between diastolic left ventricular dysfunction and primary graft dysfunction (PGD) [27]. For the first time, a clear correlation between impaired left ventricular filling and PGD was shown. Correspondingly, evidence between poor outcome and diastolic dysfunction as measured by the ratio between early transmitral flow (E) and mitral annular velocity (e′), which is independent on loading conditions, was also obtained. The association between the presence of diastolic dysfunction and survival after lung transplantation in patients with pulmonary hypertension as the pathophysiological cause of left ventricular diastolic dysfunction was also shown [9][28]. Similarly, in this patient group, the use of ECMO was more frequent and the duration of ventilation was longer in the presence of diastolic dysfunction [9].
For the risk stratification of patients, not only the right ventricular but also the left ventricular function should be assessed, especially regarding diastolic dysfunction, already at the time of listing and preoperatively. The diastolic dysfunction can be excellently detected by echocardiography and also monitored intra- and post-operatively [30,31].
For the risk stratification of patients, not only the right ventricular but also the left ventricular function should be assessed, especially regarding diastolic dysfunction, already at the time of listing and preoperatively. The diastolic dysfunction can be excellently detected by echocardiography and also monitored intra- and post-operatively [29][30].
A specific evaluation of this pathology is not mentioned in the international recommendations. A cardiac assessment according to the recommendations of the American College of Cardiology (ACC) and the European Society of Cardiology (ESC) is recommended. This generally advises echocardiographic examinations including assessment of diastolic function in high-risk interventions and patients with evidence of clinically relevant cardiac disease, which includes the patient population in lung transplantation [14,16,17].
A specific evaluation of this pathology is not mentioned in the international recommendations. A cardiac assessment according to the recommendations of the American College of Cardiology (ACC) and the European Society of Cardiology (ESC) is recommended. This generally advises echocardiographic examinations including assessment of diastolic function in high-risk interventions and patients with evidence of clinically relevant cardiac disease, which includes the patient population in lung transplantation [14][15][24].
2.3. Reperfusion Injury
Post lung transplant oedema was described early as a complication after transplantation [32]. There is not always a correlation between radiological evidence of oedema and the severity of symptoms [33,34,35]. More recent views interpret reperfusion oedema with increased pulmonary vascular resistance alongside microvascular permeability disturbance, endothelial cell dysfunction, and sterile inflammation as RI [15]. Molecular and cellular mechanisms of this multi-layered complex syndrome are described in detail elsewhere [36]. Clinically, RI results in primary graft dysfunction (PGD) with fatal consequences for short- and long-term survival and morbidity after lung transplantation [37]. Pathophysiologically, it is now known that RI can be mitigated by the use of intraoperative circulatory support. As mentioned above, left ventricular deconditioning can cause diastolic dysfunction, which in turn exacerbates RI. The use of a VA ECMO intra- and post-operatively can provide the opportunity to unload the left ventricle and allow it to readapt stepwise to a normal CO [26]. Early analyses show an occurrence of oedema in more than half of the patients. Later data with all current therapeutic measures in both donors and recipients as well as in organ preservation suggest that an occurrence of RI in the context of PGD can be between 16% and 22%, also depending on the severity of the dysfunction [38,39].
Post lung transplant oedema was described early as a complication after transplantation [31]. There is not always a correlation between radiological evidence of oedema and the severity of symptoms [32][33][34]. More recent views interpret reperfusion oedema with increased pulmonary vascular resistance alongside microvascular permeability disturbance, endothelial cell dysfunction, and sterile inflammation as RI [13]. Molecular and cellular mechanisms of this multi-layered complex syndrome are described in detail elsewhere [35]. Clinically, RI results in primary graft dysfunction (PGD) with fatal consequences for short- and long-term survival and morbidity after lung transplantation [36]. Pathophysiologically, it is now known that RI can be mitigated by the use of intraoperative circulatory support. As mentioned above, left ventricular deconditioning can cause diastolic dysfunction, which in turn exacerbates RI. The use of a VA ECMO intra- and post-operatively can provide the opportunity to unload the left ventricle and allow it to readapt stepwise to a normal CO [25]. Early analyses show an occurrence of oedema in more than half of the patients. Later data with all current therapeutic measures in both donors and recipients as well as in organ preservation suggest that an occurrence of RI in the context of PGD can be between 16% and 22%, also depending on the severity of the dysfunction [37][38].
Risk factors for the occurrence of RI with PGD and thus also reperfusion oedema can be divided into donor- and recipient-related factors. Nicotine abuse was found to be the most important donor risk factor. In addition, pre-mortem hypoxaemia, hypotension, aspiration, and prolonged mechanical ventilation are also known risk factors. Race, gender, and age also play a role. Recipient body mass index (BMI) and size mismatch are two of the most important receiver-related risk factors. In addition, high FiO
2 during reperfusion, need for transfusion, and use of the heart-lung machine are noted to increase the risk for RI as intraoperative factors [40].
during reperfusion, need for transfusion, and use of the heart-lung machine are noted to increase the risk for RI as intraoperative factors [39].
The consensus-based recommendations highlight the role of anaesthesiologists in the perioperative setting to avoid PGD, including reperfusion oedema. A general identification of modifiable risk factors that may influence the outcome after lung transplantation is recommended. Specific assessments for the identification of risk factors for PGD are not mentioned [14].
The consensus-based recommendations highlight the role of anaesthesiologists in the perioperative setting to avoid PGD, including reperfusion oedema. A general identification of modifiable risk factors that may influence the outcome after lung transplantation is recommended. Specific assessments for the identification of risk factors for PGD are not mentioned [24].
References
- Panchabhai, T.S.; Chaddha, U.; McCurry, K.R.; Bremner, R.M.; Mehta, A.C. Historical perspectives of lung transplantation: Connecting the dots. J. Thorac. Dis. 2018, 10, 4516–4531.
- Glanville, A.R. Inhaled nitric oxide after lung transplantation: No more cosmesis? Am. J. Respir. Crit. Care Med. 2003, 167, 1463–1464.
- Ohsumi, A.; Date, H. Perioperative circulatory support for lung transplantation. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 631–637.
- Yoo, Y.C. Anesthetic considerations for lung transplantation. Anesth Pain Med. 2019, 14, 241–248.
- Ius, F.; Tudorache, I.; Warnecke, G. Extracorporeal support, during and after lung transplantation: The history of an idea. J. Thorac. Dis. 2018, 10, 5131–5148.
- Kiziltug, H.; Falter, F. Circulatory support during lung transplantation. Curr. Opin. Anaesthesiol. 2020, 33, 37–42.
- Faccioli, E.; Terzi, S.; Pangoni, A.; Lomangino, I.; Rossi, S.; Lloret, A.; Cannone, G.; Marino, C.; Catelli, C.; Dell’Amore, A. Extracorporeal membrane oxygenation in lung transplantation: Indications, techniques and results. World J. Transplant. 2021, 11, 290–302.
- Hoechter, D.J.; von Dossow, V.; Winter, H.; Müller, H.-H.; Meiser, B.; Neurohr, C.; Behr, J.; Guenther, S.; Hagl, C.; Schramm, R. The Munich Lung Transplant Group: Intraoperative Extracorporeal Circulation in Lung Transplantation. Thorac. Cardiovasc. Surg. 2015, 63, 706–714.
- Ius, F.; Sommer, W.; Tudorache, I.; Avsar, M.; Siemeni, T.; Salman, J.; Molitoris, U.; Gras, C.; Juettner, B.; Puntigam, J.; et al. Five-year experience with intraoperative extracorporeal membrane oxygenation in lung transplantation: Indications and midterm results. J. Heart Lung Transplant. 2016, 35, 49–58.
- Hoetzenecker, K.; Benazzo, A.; Stork, T.; Sinn, K.; Schwarz, S.; Schweiger, T.; Klepetko, W. Bilateral lung transplantation on intraoperative extracorporeal membrane oxygenator: An observational study. J. Thorac. Cardiovasc. Surg. 2020, 160, 320–327.e1.
- Geube, M.A.; Perez-Protto, S.E.; McGrath, T.L.; Yang, D.; Sessler, D.I.; Budev, M.M.; Kurz, A.; McCurry, K.R.; Duncan, A.E. Increased Intraoperative Fluid Administration Is Associated with Severe Primary Graft Dysfunction After Lung Transplantation. Anesth. Analg. 2016, 122, 1081–1088.
- Buckwell, E.; Vickery, B.; Sidebotham, D. Anaesthesia for lung transplantation. BJA Educ. 2020, 20, 368–376.
- den Hengst, W.A.; Gielis, J.F.; Lin, J.Y.; van Schil, P.E.; de Windt, L.J.; Moens, A.L. Lung ischemia-reperfusion injury: A molecular and clinical view on a complex pathophysiological process. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1283–H1299.
- Fleisher, L.A.; Fleischmann, K.E.; Auerbach, A.D.; Barnason, S.A.; Beckman, J.A.; Bozkurt, B.; Davila-Roman, V.G.; Gerhard-Herman, M.D.; Holly, T.A.; Kane, G.C.; et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 130, e278–e333.
- Kristensen, S.D.; Knuuti, J.; Saraste, A.; Anker, S.; Bøtker, H.E.; de Hert, S.; Ford, I.; Gonzalez-Juanatey, J.R.; Gorenek, B.; Heyndrickx, G.R.; et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: Cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: Cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur. Heart J. 2014, 35, 2383–2431.
- Gelzinis, T.A. Anesthetic Management of Lung Transplantation: Center Specific Practices and Geographical and Centers Size Differences. J. Cardiothorac. Vasc. Anesth. 2018, 32, 70–72.
- Hinske, L.C.; Hoechter, D.J.; Schröeer, E.; Kneidinger, N.; Schramm, R.; Preissler, G.; Tomasi, R.; Sisic, A.; Frey, L.; von Dossow, V.; et al. Predicting the Necessity for Extracorporeal Circulation During Lung Transplantation: A Feasibility Study. J. Cardiothorac. Vasc. Anesth. 2017, 31, 931–938.
- Taimeh, Z. Assessment and treatment of the failing right heart: Considerations for transplantation referral. J. Thorac. Dis. 2019, 11, S1817–S1820.
- Hoeper, M.M.; Benza, R.L.; Corris, P.; de Perrot, M.; Fadel, E.; Keogh, A.M.; Kühn, C.; Savale, L.; Klepetko, W. Intensive care, right ventricular support and lung transplantation in patients with pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801906.
- Gelzinis, T.; Assaad, S.; Perrino, A.C. Right ventricular function during and after thoracic surgery. Curr. Opin. Anaesthesiol. 2020, 33, 27–36.
- Gorter, T.M.; Verschuuren, E.A.M.; van Veldhuisen, D.J.; Hoendermis, E.S.; Erasmus, M.E.; Bogaard, H.J.; Vonk Noordegraaf, A.; Berger, R.M.F.; van Melle, J.P.; Willems, T.P. Right ventricular recovery after bilateral lung transplantation for pulmonary arterial hypertension. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 890–897.
- Katz, W.E.; Gasior, T.A.; Quinlan, J.J.; Lazar, J.M.; Firestone, L.; Griffith, B.P.; Gorcsan, J. Immediate effects of lung transplantation on right ventricular morphology and function in patients with variable degrees of pulmonary hypertension. J. Am. Coll. Cardiol. 1996, 27, 384–391.
- Kusunose, K.; Tsutsui, R.S.; Bhatt, K.; Budev, M.M.; Popović, Z.B.; Griffin, B.P.; Bolen, M.A. Prognostic value of RV function before and after lung transplantation. JACC Cardiovasc. Imaging 2014, 7, 1084–1094.
- Marczin, N.; de Waal, E.E.C.; Hopkins, P.M.A.; Mulligan, M.S.; Simon, A.; Shaw, A.D.; van Raemdonck, D.; Neyrinck, A.; Gries, C.J.; Algotsson, L.; et al. International consensus recommendations for anesthetic and intensive care management of lung transplantation. An EACTAIC, SCA, ISHLT, ESOT, ESTS, and AST approved document. J. Heart Lung Transplant. 2021, 40, 1327–1348.
- Tudorache, I.; Sommer, W.; Kühn, C.; Wiesner, O.; Hadem, J.; Fühner, T.; Ius, F.; Avsar, M.; Schwerk, N.; Böthig, D.; et al. Lung transplantation for severe pulmonary hypertension—Awake extracorporeal membrane oxygenation for postoperative left ventricular remodelling. Transplantation 2015, 99, 451–458.
- Ohsumi, A.; Aoyama, A.; Kinoshita, H.; Yoneda, T.; Yamazaki, K.; Tanaka, S.; Nakajima, D.; Ikeda, T.; Minatoya, K.; Date, H. New strategy to resume and taper epoprostenol after lung transplant for pulmonary hypertension. Gen. Thorac. Cardiovasc. Surg. 2022, 70, 372–377.
- Porteous, M.K.; Ky, B.; Kirkpatrick, J.N.; Shinohara, R.; Diamond, J.M.; Shah, R.J.; Lee, J.C.; Christie, J.D.; Kawut, S.M. Diastolic Dysfunction Increases the Risk of Primary Graft Dysfunction after Lung Transplant. Am. J. Respir. Crit. Care Med. 2016, 193, 1392–1400.
- Avriel, A.; Klement, A.H.; Johnson, S.R.; de Perrot, M.; Granton, J. Impact of Left Ventricular Diastolic Dysfunction on Lung Transplantation Outcome in Patients with Pulmonary Arterial Hypertension. Am. J. Transplant. 2017, 17, 2705–2711.
- Smiseth, O.A. Evaluation of left ventricular diastolic function: State of the art after 35 years with Doppler assessment. J. Echocardiogr. 2018, 16, 55–64.
- Kossaify, A.; Nasr, M. Diastolic Dysfunction and the New Recommendations for Echocardiographic Assessment of Left Ventricular Diastolic Function: Summary of Guidelines and Novelties in Diagnosis and Grading. J. Diagn. Med. Sonogr. 2019, 35, 317–325.
- Khan, S.U.; Salloum, J.; O’Donovan, P.B.; Mascha, E.J.; Mehta, A.C.; Matthay, M.A.; Arroliga, A.C. Acute pulmonary edema after lung transplantation: The pulmonary reimplantation response. Chest 1999, 116, 187–194.
- Hacking, C.; Weerakkody, Y. Post Lung Transplantation Pulmonary Oedema. Available online: https://radiopaedia.org/articles/post-lung-transplantation-pulmonary-oedema (accessed on 3 July 2022).
- Marom, E.M.; Choi, Y.W.; Palmer, S.M.; DeLong, D.M.; Stuart, M.D.; McAdams, H.P. Reperfusion edema after lung transplantation: Effect of daclizumab. Radiology 2001, 221, 508–514.
- Kundu, S.; Herman, S.J.; Winton, T.L. Reperfusion edema after lung transplantation: Radiographic manifestations. Radiology 1998, 206, 75–80.
- Chen-Yoshikawa, T.F. Ischemia-Reperfusion Injury in Lung Transplantation. Cells 2021, 10, 1333.
- Snell, G.I.; Yusen, R.D.; Weill, D.; Strueber, M.; Garrity, E.; Reed, A.; Pelaez, A.; Whelan, T.P.; Perch, M.; Bag, R.; et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading—A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2017, 36, 1097–1103.
- Diamond, J.M.; Lee, J.C.; Kawut, S.M.; Shah, R.J.; Localio, A.R.; Bellamy, S.L.; Lederer, D.J.; Cantu, E.; Kohl, B.A.; Lama, V.N.; et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am. J. Respir. Crit. Care Med. 2013, 187, 527–534.
- Kreisel, D.; Krupnick, A.S.; Puri, V.; Guthrie, T.J.; Trulock, E.P.; Meyers, B.F.; Patterson, G.A. Short- and long-term outcomes of 1000 adult lung transplant recipients at a single center. J. Thorac. Cardiovasc. Surg. 2011, 141, 215–222.
- Talaie, T.; DiChiacchio, L.; Prasad, N.K.; Pasrija, C.; Julliard, W.; Kaczorowski, D.J.; Zhao, Y.; Lau, C.L. Ischemia-reperfusion Injury in the Transplanted Lung: A Literature Review. Transplant. Direct 2021, 7, e652.
More
