Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Laura-Marinela AILIOAIE.
Celiac disease is a chronic inflammatory disease that primarily affects the small intestine following the ingestion of gluten and the related prolamins found in wheat, rye, oats, and barley. It has a prevalence in the general population worldwide of approximately 1%.
- children
- intestinal permeability
- gluten
- larazotide acetate
- zonulin
1. Celiac Disease in Children—General Aspects
Celiac disease is a chronic inflammatory disease that primarily affects the small intestine following the ingestion of gluten and the related prolamins found in wheat, rye, oats, and barley. It has a prevalence in the general population worldwide of approximately 1% [12][1]. Within the last three decades, its prevalence raised due to more accurate diagnostic tests, and the age of diagnosis also increased from under 2 years to 6–9 years [13][2]. The Middle East, North Africa and India, once with low CD rates, have a higher prevalence [14][3] nowadays. CD is often underdiagnosed due to the heterogeneity of the clinical manifestations [15][4]. Most pediatric subjects experience “classic symptoms”: chronic diarrhea, steatorrhea, bloating, abdominal pain, irritability, and other signs of malabsorption, but few patients lack symptoms and are accidentally diagnosed [16][5]. Women are 1.5 times more affected, and gastrointestinal (GI) infections, use of antibiotics or proton pump inhibitors, and age when gluten was introduced into the diet represent environmental risk factors [17,18,19,20,21][6][7][8][9][10].
Genetic factors are recognized in the pathogenesis of CD; the human leukocyte antigen (HLA) class II (HLA)-DQ2 (allele DQA1 * 0501 and haplotypes DQB1 * 0201) and HLA-DQ8 (DQA1 * 0301 and DQB1 * 0302) or other variants are highlighted, but not sufficient to confirm or predict the onset of the disease [13,22,23][2][11][12]. Over 99% of CD patients have the HLA-DQ2 or HLA-DQ8 molecule, compared to only 40% of the general population [24][13].
In addition to genetic predisposition, contact with gluten, prolamins, the gluten-induced innate proinflammatory immune response, the tissue transglutaminase autoantigen (tTG), and other causes such as loss of intestinal barrier function, inadequate adaptive immune response and abnormal intestinal microbiome, may be involved in triggering the autoimmune process of CD. Anti-tissue transglutaminase (anti-tTG), formerly known as anti-tissue transglutaminase 2 (anti-tTG2), are autoantibodies of class IgA and IgG produced by tTG-specific/or tTG2 (old terminology)-specific B cells. In the past, the detection of anti-tissue transglutaminase 2 (anti-tTG2) antibodies in serum as important markers of CD, as well as the presence of other autoimmune phenomena, have included CD in the category of autoimmune diseases. Anti-tTG2 autoantibodies or newer, with the latest terminology—anti-tTG antibodies—are produced in the gut, where they are deposited much earlier before entering the general circulation [25,26,27][14][15][16].
Lack of breastfeeding and gluten intake before the age of six months and GI tract infections may increase the risk of CD [28,29][17][18]. Rotavirus vaccination can reduce the prevalence of CD in children and adolescents [30,31,32][19][20][21] since viral intestinal infections can alter the host’s local immune response for a very long time [33,34,35][22][23][24]. The role of bacteria, such as Clostridium difficile, Helicobacter pylori and Streptococcus pneumoniae, is not fully clarified in the pathophysiology of CD [36,37,38,39][25][26][27][28].
In 1950, Willem Dicke discovered that gluten from wheat is the key determinant of CD symptoms [26,40][15][29]. Over the decades, various theories have suggested that gluten would cause direct toxic damage to the lining of the small intestine [41[30][31][32],42,43], and because of its high resistance to the degradation by intestinal enzymes, it may increase the permeability of the intestinal mucosa [44,45,46,47][33][34][35][36]. Following this process, immunogenic gluten peptides cross the intestinal barrier and reach the general circulation, prolonging the inflammatory processes [48,49,50,51][37][38][39][40]. The penetration of undigested fragments of gluten peptides into intestinal lamina propria leads to their deamidation by the tissue enzyme transglutaminase 2 (TG2). This process of deamidation by TG2 is the cornerstone of CD pathophysiology, and anti-TG2 antibodies are used as biomarkers for positive diagnosis [43,52][32][41].
Deamidated native and gliadin peptides are taken up and presented to HLA-DQ2 and DQ8 molecules by dendritic cells (DC) and, via T-helper cells, will initiate an adaptive immune response. At the same time, α-amylase/trypsin inhibitors (ATI) and wheat lectins trigger the body’s innate immune response by stimulating the Toll-like receptor (TLR) 4 on myeloid cells and antigen-presenting cells (dendritic cells, monocytes, macrophages) in the intestinal mucosa, with the release of interleukins 8 (IL-8), 15 (IL-15), tumor necrosis factor-alpha (TNF-α) and monocyte chemoattractant protein-1 (MCP-1) [45,52,53,54,55][34][41][42][43][44].
Stimulated T-helper 1 (Th1) lymphocytes release IL-15, IL-21 and interferon-gamma (IFN-γ), which activate and promote cytotoxic intraepithelial CD8+ lymphocytes (IEL), facilitating the damage of the mucosa and intestinal wall. Activated T-helper type 2 (Th2) lymphocytes participate in the differentiation and activation of B lymphocytes, which stimulate the production of IgM, IgG and IgA, anti-TG2, anti-gliadin and anti-endomysium antibodies [56,57,58][45][46][47]. Some studies suggest that some gliadin peptides bind to TLR2 receptors and will influence the increase in IL-1 production through myeloid differentiation primary response 88 (MYD88), a key protein involved in the release of zonulin after gluten ingestion [59,60][48][49].
Although CD8+ cytotoxic cells and CD4+ Th1 cells are gluten-specific, and they are central exponents by releasing proinflammatory cytokines (IL-1β and IL-18), inflammatory pathways induced by cell death may also be involved in keeping the disease active by delivering proinflammatory molecules, such as the alarmins high-mobility group box-1 (HMGB1), IL-33 and IL-1α [61,62,63][50][51][52]. Helper T-cells produce proinflammatory cytokines (IFN-γ and TNF-α), which will further increase intestinal permeability and, in association with killer T-cells, trigger gluten enteropathy, which allows for intestinal retrotranscytosis of secretory IgA (SIgA)-gliadin complexes to act [64,65][53][54]. In the vast majority of individuals, all manifestations of the immune conflict disappear with a gluten-free diet [66][55].
A positive diagnosis of CD can be achieved through a combination of clinical parameters, immunological parameters (positive serological levels for total IgA and IgA anti-intestinal transglutaminase 2 antibodies (TGA-IgA), IgA anti-endomysium antibodies (EMA-IgA) and IgG deamidated gliadin peptide (DGP) antibodies (DGP-IgG) and/or histological data, obtained by biopsy; in clinical practice, the serologic IgA tissue transglutaminase antibodies have a sensitivity close to 97%, while EMA-IgA antibodies are highly specific markers (approximately 100%) for CD diagnosis [67,68,69,70,71,72,73][56][57][58][59][60][61][62].
In adults, a duodenal biopsy is the current gold standard for CD-positive diagnosis [74][63], whereas, in children, a biopsy is only needed when they have positive anti-tissue transglutaminase IgA antibodies (anti-tTG IgA) but with titers less than 10 times the upper limit of normal. For a positive diagnosis, they should have ≥4 biopsies of the distal duodenum and ≥1 of the duodenal bulb during a gluten-containing diet. A villus/crypt ratio < 2 indicates mucosal damage. In case of uncertain or discordant results between serology (TGA-IgA level) and histopathological appearance, a second opinion of an experienced histopathologist and/or a new biopsy is required [13,75][2][64].
Histopathological aspects (atrophy of the intestinal villi and crypt hyperplasia) are classified according to Marsh-Oberhuber criteria [76,77][65][66].
Current guidelines for positive CD diagnosis require four out of the following five criteria: (1) typical symptoms (diarrhea and malabsorption); (2) antibody positivity; (3) HLA-DQ2 and/or HLA-DQ8 positive; (4) histological intestinal lesions (atrophied villi and minor lesions); and (5) a clinically positive response to GFD [78,79][67][68]. European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) and other recent studies recommend a diagnostic approach without biopsy, especially in children with T1DM and positive serological tests, even in the absence of symptoms [80,81,82][69][70][71].
Children with CD should be monitored in the first six months after diagnosis and then annually [83][72]. GFD helps resolve digestive and extra-digestive manifestations but can induce deficiencies in minerals and vitamins, together with psychological problems [84,85][73][74]. Determining adherence to a GFD can be heterogeneous due to gluten contamination, poor labeling, and restrictive dietary barriers [86,87,88][75][76][77].
In the CD population, there are two types of refractory forms; type 1 should be treated with a strict diet and oral budesonide, steroids in general, or in combination with azathioprine. Type 2 CD also benefits from steroids, associated or not with cyclophosphamide, cladribine, anti-TNF antibodies, and, if possible, stem cell therapy and transplantation. Patients with type 2 refractory form are at risk of developing T-cell lymphoma [79,89,90,91,92][68][78][79][80][81].
Significant progress has been made over the past decade to better understand the pathophysiology of CD, which has guided research directions for possible new treatments targeting junctions between intestinal lumen enterocytes, interfering with the inflammatory cascade to limit mucosal destruction or the invention of digestive enzymes with an intraluminal action and peptide binding agents that turn gluten into a non-toxic food [93][82].
To date, there is no Food and Drug Administration (FDA)-approved drug for the treatment of CD, and the only recommendation for reducing or eliminating the symptoms of this disease is to avoid consuming gluten-based products. There are several ongoing clinical trials testing pharmacological products for CD therapy. Currently, there are two advanced clinical trials testing the drugs: AT-1001 (Larazotide acetate) and IMGX-003 (Latiglutenase; formerly known as ALV003). These products are intended to relieve CD symptoms in two different ways. AT-1001 attempts to close or repair the defect of villi tight junctions, while IMGX-003 works as a gluten endopeptidase that breaks down gluten in the stomach before being absorbed in the small intestine [74][63].
Larazotide acetate is a synthetic peptide with eight amino acids, which works as a regulator of intestinal permeability by the antagonizing action of zonulin, a fundamental protein of the intestinal intercellular junction participating in the pathogenesis of CD. The other enzymatic treatment is IMGX-003 administered orally, which works from the stomach by fragmenting gluten and then in the small intestine, with the effect of improving the quality of life (QOL) and multiple symptoms induced by involuntary consumption of gluten [94][83].
Summarizing the data from the literature on CD management in children and adults, the following observations can be made: —at the current level of knowledge, the treatment and prevention of CD recurrence can be achieved only by suppressing gluten from the patient’s diet for life; —prevention of immune stimulation after absorption of gluten in the small intestine by immunosuppressive drugs (steroids, azathioprine, anti-cytokines, HLA-DQ2 blockers, cathepsin inhibitors, vaccines, etc.); —retention of gluten in the intestinal lumen by various polymers, antibodies, etc.; —prevention of absorption of digested gluten by a zonulin antagonist (e.g., Larazotide acetate); —use of tissue transglutaminase inhibitors; —reducing the immunogenic power of gluten through genetic, thermal, enzymatic engineering techniques, and so on.
2. Celiac Disease in Children during COVID Pandemic
Although COVID-19 primarily affects the respiratory system, many children also have GI symptoms manifested by cramps, abdominal pain, nausea, vomiting and diarrhea. Calitri et al. reviewed the pathophysiological mechanisms, clinical symptoms, diagnosis and management of COVID-19, and the impact of the disease on the digestive tract in children. GI manifestations appear to be more common in COVID-19 in children than in adult patients. The symptoms are usually of short duration and can be resolved by symptomatic treatment. However, in a small number of children, GI involvement precedes severe forms of the multisystem inflammatory syndrome in children (MIS-C). The SARS-CoV-2 virus should be tested in stool samples in sick children by real-time polymerase chain reaction (real-time PCR or RT-PCR) (a laboratory technique of molecular biology based on the polymerase chain reaction). In children at risk, such as those with CD, intestinal inflammation, and chronic liver disease, COVID-19 does not appear to be more severe than in other patients on immunosuppressive therapy. Monitoring CD patients must be adapted to the pandemic to avoid unnecessary endoscopic examinations and duodenal biopsies. Telemedicine can be a good alternative for educating and monitoring chronic patients, keeping away from the risk of unwarranted interventions and viral transmission [95][84].
Because a CD outburst has been seen as a hallmark after SARS-CoV-2 infection, Cakir et al. pursued to study of the impact of the COVID-19 pandemic on the incidence and the clinical symptoms of CD. Researchers divided CD patients into two groups [diagnosed in pre-pandemic (January 2008–February 2020) and in pandemic period (March 2020–June 2021)] and compared them so as to reveal the differences between the groups regarding the clinical and the histological data. Supplementary information was gathered concerning the second subgroup (n = 22) diagnosed with CD and COVID-19 during the pandemic. It came out that the number of patients per year (12.1–37.6) and the percentage of patients who were diagnosed with CD increased during the pandemic (2.2% vs. 10%). T1DM has been reported in 17% of patients with CD, compared with only 4% before the pandemic. The incidence of moderate-severe mucosal lesions has been reduced by almost half in the pandemic (42.4% vs. 81.7%). More than one-third (36.3%) of patients diagnosed with CD during the pandemic had a previous severe infection with SARS-CoV-2, reflected by the biological markers and clinical symptoms. AutResearchoers accept as true that the incidence of CD and its association with T1DM increased in children during the COVID-19 pandemic [96][85].
In a recently published article on COVID-19 and CD, Trovato et al. reveal a new pathogenetic hypothesis regarding the outbreak of CD in the current pandemic, highlighting the role of COVID-19 as a potential trigger for celiac disease in predisposed patients. The context cited by the reseauthorchers is the growing body of available information corroborated with the gut tropism with ciliated cells and intestinal enterocytes as ideal targets for the SARS-CoV-2 virus through high levels of angiotensin-converting enzyme 2 (ACE2) receptors and transmembrane serine protease 2 (TMPRSS2) expression. The virus can easily enter the cells by binding to ACE2, followed by its priming by TMPRSS2 and the activation and increase of inflammation locally. As an extra factor, priming of the spike protein by the serine protease TMPRSS2 in ciliated cells and the brush border of gut enterocytes is essential for SARS-CoV-2 to invade the cells of the intestinal lining, provoking mucosal deterioration, and conducting to increased permeability due to damage of the gut barrier. Further consequences are the movement of microbes, including microbial-associated molecular patterns (MAMPs), generating an inflammatory immune response by especially the macrophages and adipocytes as TLR-expressing cells of the mesentery fat, which on this path can extend into the systemic circulation. This complex portrayal reinforces the assumption that gut cells could contribute to an increase in the presence of the SARS-CoV-2 virus in the blood (higher viremia). A consequence of damage to the intestinal epithelium is a greater barrier permeability, which grants permission to the gliadin to pass into the intestinal lamina. However, loss of intestinal barrier function is very important in the pathogenesis of CD because it is a systemic autoimmune disease acquired by genetically predisposed subjects due to gliadin, which passes from the intestinal lumen to the lamina propria—either by crossing the barrier or by transcellular transfer. This transition is the first step toward disease progression because the binding of DGP to antigen-presenting cells (APC) takes place in the lamina propria. Based on these pairs of pathogenetic implications that may precede the onset of CD, the authoresearchers concluded that genetically predisposed patients are more likely to develop CD after SARS CoV-2 infection, so the current pandemic could be a potential trigger for an outburst of CD in the very near future [97][86].
Asri et al. examined the levels of genes that influence immune homeostasis and are related to inflammation [IL-6, CD4, CD25 and forkhead box P3 (FOXP3)] in peripheral fresh whole blood samples from 60 newly diagnosed CD people (mean age 35.40 ± 24.12 yrs.), 30 patients with severe COVID-19 (mean age 59.67 ± 17.22 yrs.), and 60 healthy subjects (mean age 35.6 ± 13.02 yrs.), enrolled for a period of 6 months in 2020. RNA expression levels of the aforementioned genes were evaluated applying real-time quantitative RT-PCR and performant statistical analysis. Higher expression of CD4, CD25 and FOXP3 was determined in patients with CD compared to the control group and the COVID-19 group, the latter having lower expression levels compared to controls. However, a higher expression of IL-6 was observed in both groups of patients compared to controls. AutResearchoers concluded that due to the high expression of IL-6, patients with untreated CD may be at higher risk of developing severe COVID-19, but the increased expression of anti-inflammatory markers may be salutary for them, possible through diminishing the gravity of COVID-19, aspects to be scientifically proved in time to come research on CD patients contaminated with SARS-CoV-2 [98][87].
Renzo et al. managed an online analysis of many Italian pediatric centers engaged in GI endoscopy in order to assess the adjustments of this medical branch in the COVID-19 pandemic during high viral transmission. Facts and statistics collected for analysis were compared in two selected periods. Findings of the study from 24 pediatric endoscopy units that responded highlighted a marked decrease in GI endoscopy procedures with a total reduction of 37.2% in 2020 compared to 2019, consistent with another new survey conducted in 12 European centers in April 2020. There has been a dramatic drop from 621 to 279 (55.1%) from 2019 to 2020 in the procedures for prepositive CD, with the longest waiting lists for the new onset of CD. All centers had to suspend or reschedule the GI endoscopies due to the outbreak of SARS-CoV-2 infections, especially since mid-March’20. Therefore, it could be concluded that the effect of COVID-19 on the practice of GI endoscopy in Italian children was important [99][88].
Although SARS-CoV-2 virus infection is transmitted mainly by tiny drops, in addition to respiratory manifestations, patients often have GI symptoms and liver damage. Concas et al. published an updated practical review for gastroenterologists facing many patients with COVID-19 and chronic GI disease (inflammatory bowel disease, celiac disease, chronic liver disease). Rapid collecting of valuable information from the latest publications for improved standards in medical consultation and patient care regarding COVID-19 is essential. The reseauthorchers explored all available medical references, deepened the origin and pathophysiological mechanisms of COVID-19, examined the clinical manifestations of GI involvement, introduced the latest guidelines on main practical GI procedures and recommended immunosuppressive therapy, and emphasized the importance of maintaining social distance. Particular attention should be paid to fecal-oral transmission and intestinal microbiota in COVID-19. In general, patients with CD are not usually considered immunocompromised, except for those with an extremely poor diet and weight loss, refractory CD type 2, immunosuppressive drugs, or other serious illnesses that could develop severe COVID-19, and they must be under medical supervision. However, the authoe researchers pointed out that as of the time of their analysis, no previous research had shown that patients with CD would be at increased risk of developing severe COVID-19, but important data are collected continuously in an international registry (SECURE-Celiac) in which clinicians worldwide are asked to report all cases of COVID-19 in celiac patients, regardless of the severity of the disease. A review of studies conducted by authoresearchers on publications from China, Italy, the United States, and the United Kingdom, having samples from children and adolescents in the relationship between celiac disease and SARS-CoV-2 infection, showed that a small percentage did COVID-19, with possible arguments the active resistance of children to the virus or multiple unrecognized asymptomatic cases. Although with a mild course of COVID-19 compared to adults, preschool children (under five years) had a higher load of viral RNA in the nasopharynx than other ages and adults. Regarding the presence of GI symptoms in children with COVID-19, in any case of the severity of the disease, it is essential to note that stool samples and rectal swabs may be positive for viral RNA for several days after infection, and children appear to eliminate the virus a longer time than adults, which makes them possible essential viral transmitters [100][89].
In a research correspondence on the results of COVID-19 in CD, Uche-Anya et al. conducted an investigation on the International Registry of Celiac Disease Patients, SECURE-CELIAC registry, and they found 12% hospitalization and 2.5% mortality rates, reflecting the low risk of admission to hospital for treatment, or death. In conclusion, according to this research, patients with CD do not have an increased risk of hospitalization or death due to COVID-19, but old age and new GI symptoms during SARS-CoV-2 infection may trigger an unfavorable course [101][90].
Mehtab et al. assessed the consequences of lockdown and limited mobility during the COVID-19 pandemic on the accurate adhesion to GFD, symptom management and QOL in CD patients from northern India. Researchers sent a web-based questionnaire to 3130 patients on WhatsApp and contacted telephonically 68 patients, who were not present on any social network, and finally included in the analysis 505 fully responders. The questionnaire comprised both specific purpose and certified questions, introduced after the review of the medical literature, discussions and seminars with experts, and included the CD adherence test, the celiac symptom index score and CD-relevant QOL. Of the 505 patients finally included, 6.7% had had poor GFD compliance before the pandemic, but their number nearly doubled during the pandemic. In addition, almost 5% were diagnosed with CD when tested for SARS-CoV-2 infection. About two-thirds of patients liked the online consultation more than in person. Most usual problems to overcome during the lockdown were high delivery prices for gluten-free (GF) food at home (54.4%), an increase in prices for regular GF food (43.1%) and long-distance travel to get GF food (44.9%). In conclusion, as a positive effect, the pandemic paved the way for teleconsultation for patients with CD but negatively affected the GFD, symptom management, and the QOL due to lack of money at home, high costs for the purchase and delivery of GF food at home, sometimes very difficult to find. Future steps should be taken to maintain GF food supply chains, online consultation and monitoring of CD patients in case of regional lockdowns or across the country [102][91].
Falcomer et al. investigated the effects of the pandemic as an extra burden on long-term celiac disease (CD), which further compromised the QOL of patients diagnosed with this condition in Brazil. The purpose of this research was to assess the QOL of Brazilian patients with CD during the current pandemic caused by the SAR-Cov-2 epidemic and its very fast dissemination around the globe, subsequent restrictions and lockdowns, and the overlapping dietary restrictions and other overloads on CD patients. The restudyearch was conducted online across the country through a previously validated questionnaire in Brazilian and Portuguese to investigate the QOL of patients diagnosed with CD. The answers to the sent and self-administered questionnaire were received from 674 patients with CD and revealed the following aspects: QOL of people with CD in Brazil has not been negatively affected by the current pandemic; GI manifestations had the greatest influence, followed by social ones; unlike psychological, mental suffering or affective, with no effect on the QOL of CD people in Brazil; all other issues related to profession, age, gender, marital status, children, or even a positive test for COVID-19 did not affect QOL in CD subjects; most dramatic influence on QOL had non-compliance to GFD and the use of drugs to prevent or relieve mental depression in CD patients. The authoresearchers believe that further research is needed to extend these results to the post-pandemic COVID-19 era [103][92].
In another article published by Monzani et al., the reseauthorchers studied GFD adherence during COVID-19 lockdown in Italian patients with CD (adults and children/adolescents) using an online survey. Out of the total of 1983 replies, 369 (18.6%) were for children/adolescents with CD (answers given by their parents or caregivers), and the remaining 81.4% for adults with CD. GFD adherence was unchanged in 70% of children (69% of adults, respectively) and improved for 29% in both age groups. The authoresearchers reported that the particularities that increased the likelihood of reporting better adherence in adults were the constant appearance of CD symptoms in the last year before lockdown, but also usual partial compliance and testing of new natural, gluten-free formulas with more ingredients than usual. In the case of children or adolescents with CD, the critical factors were the existence of CD symptoms in the last year, CD antibodies yet positive, and also the existence of other family members diagnosed with CD. The conclusion was that the lockdown resulted in improved compliance to GFD in 33% of participants, but especially in those with poorer disease control, slaughtering sources of contamination or deviation, and new confidence in naturally gluten-free products [104][93].
In a recently published study, Temsah et al. analyzed the success of facts, information and skills acquired by parents and caregivers, as well as their views on unintended and preventable injuries, the well-being and safety of their children or adolescents during the COVID-19 pandemic lockdowns. Pre-post investigative research with predetermined assessments, such as questions about the socio-demographic status and knowledge acquisition before and after participating in a security campaign on 308 volunteer parents in Saudi Arabia, showed an improved score from 36.2 to 79.3, as well as an increased perception of the general expertise and accomplishments towards the security of children and adolescents, so that during lockdowns, additional training tools, programs and other pertinent information are justified to promote safe and harmless practices to parents, nurses, social workers and others [105][94].
Barschkett et al. compared the number of outpatient visits of children in the German national registry before and during the first wave of the COVID-19 pandemic, i.e., between January 2019 and June 2020. There was an 18% decrease in the number of outpatient visits per child during the first wave of the pandemic, with a significant decrease (51%) in intercurrent infections, especially in young children under five years, but for chronic diseases, the outpatient visits diminished only to a minor degree, for example, T1DM (to 92%), CD (to 86%) and hay fever (to 95%), as well as for mental and behavioral conditions have shown only insignificant differences. The reseauthorchers concluded that lack of contact between children seems to reduce the transmission of infections. Future targeted educational and counseling measures, as well as adequate preventive measures to reduce stress and improve the QOL in children, are necessary and welcome, including for parents who have lost precious time away from work [106][95].
In a recently published paper, Dipasquale et al. investigated the effects and consequences of the COVID-19 pandemic in highlighting the nature and all the complications of the GI diseases in children and adolescents for a correct diagnosis and management in the COVID era. In this revisewarch, the authoresearchers turned their attention to pediatric gastroenterologists to assist in the correct diagnosis and management so that researchers presented evidence of digestive and clinical involvement of COVID-19; they highlighted the effects of COVID-19 on the clinical approach in children and adolescents with pre-existing disease or in an early stage from onset; and they also focused on the duty and restricted access to instrumental investigations, for example, endoscopy of the digestive tract in the coronavirus pandemic. It is currently unknown whether immunosuppressive therapy for inflammatory bowel disease (IBD) or chronic hepatitis is at risk and may cause adverse reactions in some subjects. In the case of patients in remission, outpatient follow-up consultations may be postponed, but telemedicine is especially recommended in the absence of any risk of infection. Any new therapies must be individualized, and there will be presented not only the benefits but especially the risks to each patient and the family. Psychological counseling therapy should be initiated for all children with chronic diseases and for their parents or caregivers. All endoscopic procedures that are not urgently needed or optional may be suspended while minimizing the risk of viral spread. Social distance and the use of individual safeguarding equipment should be further recommended, as well as SARS-CoV-2 vaccination [107][96].
Bükülmez et al. investigated the influence of COVID-19 on pediatric patients diagnosed with CD in Turkey. The reseauthorchers tried to sound the alarm for the parents and caregivers of children diagnosed with CD to make them aware of the need for necessary measures to be taken in the current coronavirus pandemic for their children. COVID-19 has caused unpredictable changes in life through restrictions, lockdowns, severe complications and death in some cases. Several patients with CD have asplenism or hyposplenism and a higher risk of pneumococcal sepsis in the latter case. The authoresearchers designed and conducted a cross-sectional study between May and July 2020 through an online survey of a sample of 73 parents whose children with a mean age of 11.36 ± 4.36 years had confirmed CD diagnostic at a university hospital in Turkey. The most important results were that 90.4% of participants responded that SARS-CoV-2 infection was transmitted through small droplets from the respiratory tract released by sneezing, coughing, speaking, or after contact with virus-infected surfaces followed by touching the face. The vast majority (78.1%) said they had no problem following the GFD because they found all the GF foods needed. Parents of children with CD did not know that the risk of infection with this virus in their children may differ from that of healthy children, so the studyresearch accentuated that these parents should have been better informed about COVID-19. Parents also noticed the increase in the level of anxiety in their children during this pandemic, as well as the fact that they gained several extra pounds in lockdown, with a negative effect on their health, well-being and keeping up a healthy way of living [108][97].
In a letter to the editor of Clinical Gastroenterology and Hepatology, Lionetti et al. expressed interest in investigating whether the risk of coronavirus disease 2019 (COVID-19) is increased in children and adolescents with CD including the morbidity and mortality in these patients. The reseauthorchers pointed out that Italy had a sudden spread of SARS-CoV-2 infection and that the crisis had hit the country hard, especially in the central part where there was an important center for children with CD, so the researchers took the opportunity to study the prevalence and the severity of COVID-19 in children with CD and compared them with general population data. AuthoResearchers who signed the letter investigated between February and June 2020, through a telephone survey, using a questionnaire with 26 questions, the prevalence and clinical characteristics of SARS-CoV-2 infection in CD patients. The researchers included all children diagnosed with CD according to ESPGHAN criteria in a CD group who tested positive for SARS-CoV-2 infection. The control group was like this group but consisted of patients with possible COVID-19-related symptoms but untested. For the prevalence of COVID-19 in children and adolescents in the Marche region during the same period, the reports of the Italian National Institutes of Health and of the regional government were used. Of the initial 419 patients with CD contacted, only 387 had a positive response, of which 37% were males, with a mean age of 9.9 (1–16 years) and a mean age at CD diagnosis of 7.5 years (range of 6 months to 16 years). Of the total number of patients with CD, none had COVID-19 confirmed by laboratory testing, so the prevalence of COVID-19 was 0/387 (95% confidence interval, 0.0000–0.0095). 3.9% of patients (n = 15) had a fever but had no other symptoms associated with COVID-19. 5.9% of patients (n = 23) were included in the COVID-19 like- group (nine with fever and cough; two with fever, vomiting and diarrhea; ten with diarrhea and/or vomiting; two with cough), but none did not have respiratory failure or pneumonia and did not need oxygen administration or hospitalization. In that region, the confirmed prevalence of COVID-19 at 0–16 years was 155/199,289 (0.08%; 95% confidence interval, 0.0007–0.0009). Thus, the reseauthorchers concluded that children with CD did not significantly increase the prevalence of COVID-19 compared to the general population, and children in the COVID-19 group did not develop the severe or complicated disease. As an observation, the authoresearchers noted that the number of infections could be underestimated in the CD group because they could not assess the number of all asymptomatic carriers of SAR-CoV-2, but the same limitation should be considered in the general reference population. The findings of this studyresearch are consistent with the conclusions of other studies that have shown that pediatric patients with CD do not have an increased risk of SARS-CoV-2 infection. However, COVID-19 has caused an unprecedented crisis in the global health system, forced the rethinking of the management of patients with acute or chronic diseases and opened wide the doors to telemedicine, which is very suitable for tracking CD patients. Although these findings are not of particular concern to CD, compliance with prevention measures in the general population is also necessary for this chronic disease, and other long-term studies may imply a better understanding of the risk of contracting COVID-19 in pediatric patients with CD [109][98].
In correspondence to the publisher of Digestive and Liver Diseases, Catassi et al. wrote about life-threatening delays in diagnosing CD due to COVID-19 lockdown in Italy as a very dark side of pandemics. Forced lockdown of the COVID-19 pandemic has had major consequences for primary care, even for habitual GI disorders, but sometimes severe or critical. For example, the reseauthorchers succinctly outlined the history of one critical clinical case admitted to their regional medical academic center. A 17-month-old girl (breastfed only for four months and afterwards weaned with formula, cereals, meat and vegetables) was admitted during the Italian lockdown in March 2020 for abdominal pain, distention, and widespread edema. The weight and height were 8.0 Kg (below 3rd centile) and 70 cm (below 3rd centile), respectively. Laparotomy was applied for reduction of involved intestinal segments, but the day after surgery, the child was irritated and with significant edema of the face, abdomen, and upper and lower limbs. Laboratory data were normal, except for low serum albumin (2.8 g/dL) and total calcium (8 mg/dL). Because the clinical history suggested CD, the serum CD autoantibodies were measured, as suggested by the ESPGHAN diagnostic guidelines, and a GFD was immediately started before the results were obtained due to the gravity of the clinical manifestations. Diagnosis of CD was strongly suggested by high-level positivity (>10× UNL) of anti-DGP IgG and uncertain levels (1× UNL) of anti-tTG IgA. After 10 days of GFD, the edema had disappeared. CD diagnostic was clearly certified by the intestinal biopsy highlighting drastic villous atrophy and many intramembranous lymphocytes. After a month of GFD, the little girl continued to show signs of obvious improvement in her health. The authoresearchers concluded that this example is just one of many potentially life-threatening delays in the diagnosis and treatment of CD in children during the COVID-19 pandemic [110][99].
3. Molecular Mechanisms of SARS-CoV-2 Infection and How CD Led to an Adjuvant Drug for MIS-C
Management of COVID-19 is a constant challenge in the presence of Omicron’s mutations, which made it the most infectious coronavirus variant yet, and because effective treatments are not yet available worldwide, especially in severe forms, for example, in MIS-C. Coherent strategies are still needed to support, predict results and deal with new cases around the world. Therefore, it is vital to understand the complex molecular mechanisms of COVID-19 pathogenesis, especially in children and adolescents who developed fulminant cases of MIS-C, as well as in other autoimmune diseases, such as CD. It was noticed that a few weeks after contacting the SARS-CoV-2 virus responsible for triggering COVID-19 disease, even asymptomatic, some children or adolescents develop MIS-C if they have the virus that causes COVID-19 or they have been in contact with someone diagnosed with COVID-19. This disease, initially also called pediatric inflammatory, multisystem syndrome (PIMS), temporally associated with SARS-CoV-2 infection (PIMS-TS) or systemic inflammatory syndrome in COVID19 (SISCoV), is a systemic disease with incessant fever and maximum inflammation, which can be life-threatening because it can trigger multiple organ failure, even cardiogenic shock with ventricular dysfunction, and the family must seek medical attention immediately as most children will need intensive care. MIS-C is a severe consequence of COVID-19 in children or adolescents, connected with important hemodynamic, cardiovascular and other organs’ inflammation and damage, such as the lungs, kidneys, brain, skin, eyes, and marked GI symptoms.
The molecular mechanisms involved in the activation of the zonulin pathway and the pathophysiology of CD compared to the molecular aspects of the fulminant forms of MIS-C that appeared in children a few weeks after infection or contact with the SARS-CoV-2 virus, are presented in the figure below. The patient presents with alarming symptoms that must be recognized immediately and usually requires emergency hospitalization in intensive care units, as they are life-threatening. The mastery of these intrinsic molecular mechanisms and the hypothesis of activation of the zonulin pathway and loss of the intestinal mucosal barrier have led to the proposal of an adjuvant drug for MIS-C in this pandemic.
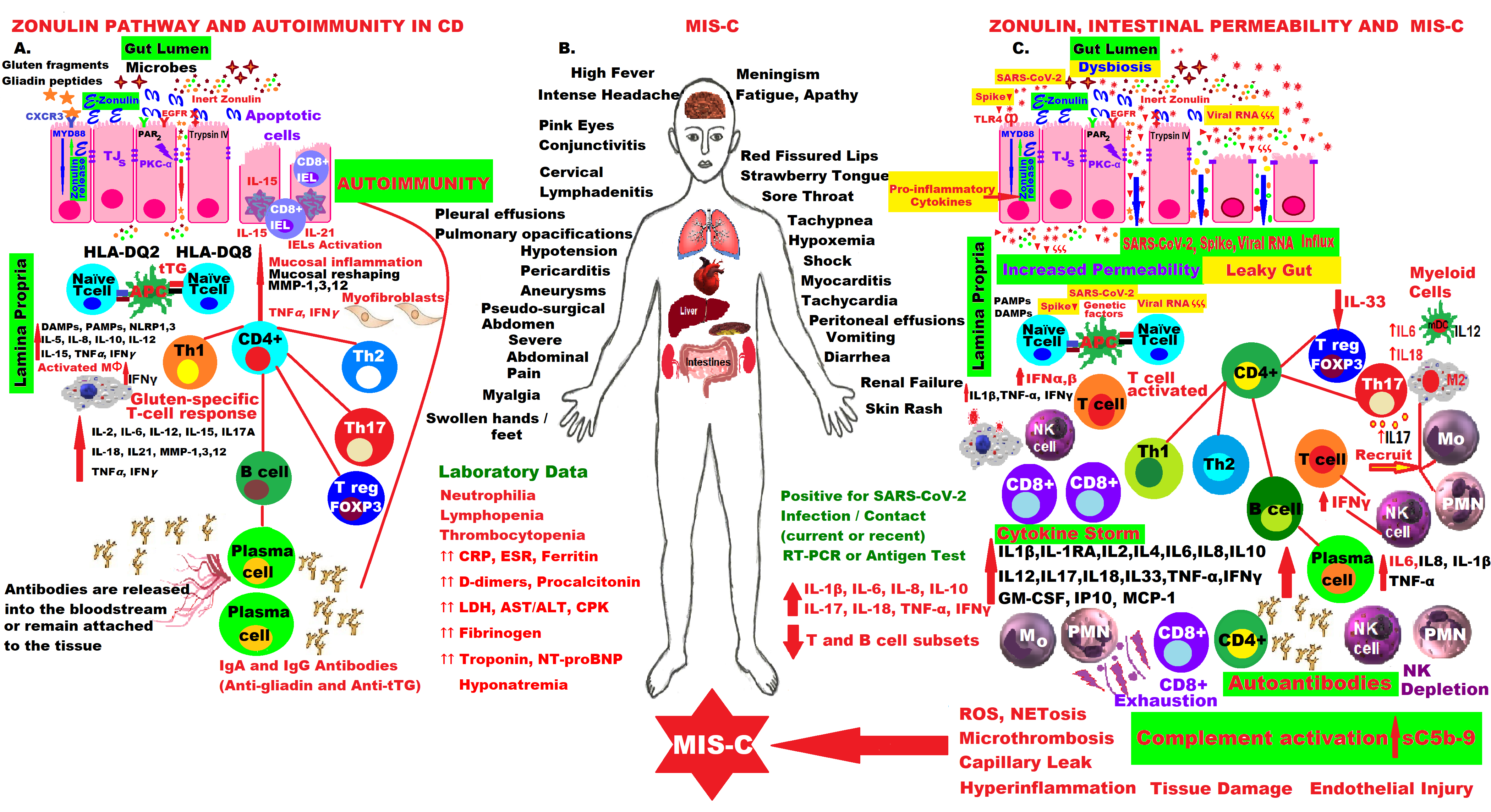 Comparat (Five molecgular mechanisms re 1).
Comparat (Five molecgular mechanisms re 1).
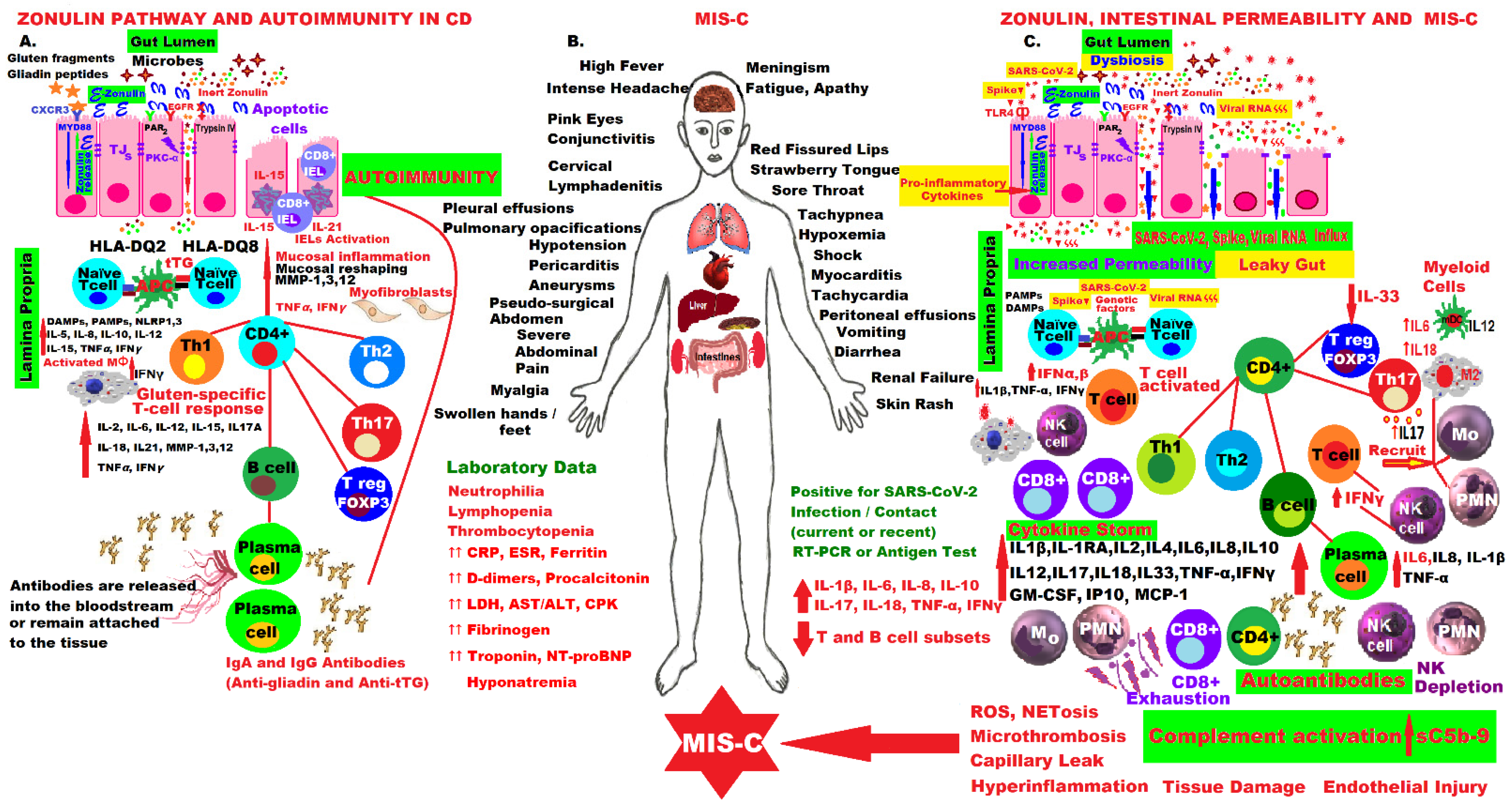 Fin the pathogenesis of CD and MIS-C by activating the zonulin pathway, increasing intestinalure 1. permeability, hyperinflammation and immune dysregulation.Comparative molecular mechanisms in the pathogenesis of CD and MIS-C by activating the zonulin pathway, increasing intestinal permeability, hyperinflammation and immune dysregulation.
COVID vaccination has opened a new chapter in this pandemic, and wmuch are still have muchbe needed to do in all areas, including medicine, to deepen ourthe understanding of all mechanisms, to better care for ourthe patients and protect usthem from future waves [186][100]. Some reseauthorchers suggest that MIS-C should be reinterpreted as a special macrophage activation syndrome, and long-term protection against SARS-CoV-2 infection can only be provided by the vaccine, but we do not yet have there is no sufficient data [187][101]. Vaccination provides the best solution for controlling the COVID-19 pandemic, and patients with chronic inflammatory and autoimmune diseases, including CD, need to be convinced of the necessity, safety and efficacy of vaccines, even if they have been produced in a very short time, generating high levels of risk perception, different attitudes, significant debates on acceptance and great hesitation worldwide [188,189,190,191][102][103][104][105].
Fin the pathogenesis of CD and MIS-C by activating the zonulin pathway, increasing intestinalure 1. permeability, hyperinflammation and immune dysregulation.Comparative molecular mechanisms in the pathogenesis of CD and MIS-C by activating the zonulin pathway, increasing intestinal permeability, hyperinflammation and immune dysregulation.
COVID vaccination has opened a new chapter in this pandemic, and wmuch are still have muchbe needed to do in all areas, including medicine, to deepen ourthe understanding of all mechanisms, to better care for ourthe patients and protect usthem from future waves [186][100]. Some reseauthorchers suggest that MIS-C should be reinterpreted as a special macrophage activation syndrome, and long-term protection against SARS-CoV-2 infection can only be provided by the vaccine, but we do not yet have there is no sufficient data [187][101]. Vaccination provides the best solution for controlling the COVID-19 pandemic, and patients with chronic inflammatory and autoimmune diseases, including CD, need to be convinced of the necessity, safety and efficacy of vaccines, even if they have been produced in a very short time, generating high levels of risk perception, different attitudes, significant debates on acceptance and great hesitation worldwide [188,189,190,191][102][103][104][105].
 Comparat (Five molecgular mechanisms re 1).
Comparat (Five molecgular mechanisms re 1).
 Fin the pathogenesis of CD and MIS-C by activating the zonulin pathway, increasing intestinalure 1. permeability, hyperinflammation and immune dysregulation.Comparative molecular mechanisms in the pathogenesis of CD and MIS-C by activating the zonulin pathway, increasing intestinal permeability, hyperinflammation and immune dysregulation.
COVID vaccination has opened a new chapter in this pandemic, and wmuch are still have muchbe needed to do in all areas, including medicine, to deepen ourthe understanding of all mechanisms, to better care for ourthe patients and protect usthem from future waves [186][100]. Some reseauthorchers suggest that MIS-C should be reinterpreted as a special macrophage activation syndrome, and long-term protection against SARS-CoV-2 infection can only be provided by the vaccine, but we do not yet have there is no sufficient data [187][101]. Vaccination provides the best solution for controlling the COVID-19 pandemic, and patients with chronic inflammatory and autoimmune diseases, including CD, need to be convinced of the necessity, safety and efficacy of vaccines, even if they have been produced in a very short time, generating high levels of risk perception, different attitudes, significant debates on acceptance and great hesitation worldwide [188,189,190,191][102][103][104][105].
Fin the pathogenesis of CD and MIS-C by activating the zonulin pathway, increasing intestinalure 1. permeability, hyperinflammation and immune dysregulation.Comparative molecular mechanisms in the pathogenesis of CD and MIS-C by activating the zonulin pathway, increasing intestinal permeability, hyperinflammation and immune dysregulation.
COVID vaccination has opened a new chapter in this pandemic, and wmuch are still have muchbe needed to do in all areas, including medicine, to deepen ourthe understanding of all mechanisms, to better care for ourthe patients and protect usthem from future waves [186][100]. Some reseauthorchers suggest that MIS-C should be reinterpreted as a special macrophage activation syndrome, and long-term protection against SARS-CoV-2 infection can only be provided by the vaccine, but we do not yet have there is no sufficient data [187][101]. Vaccination provides the best solution for controlling the COVID-19 pandemic, and patients with chronic inflammatory and autoimmune diseases, including CD, need to be convinced of the necessity, safety and efficacy of vaccines, even if they have been produced in a very short time, generating high levels of risk perception, different attitudes, significant debates on acceptance and great hesitation worldwide [188,189,190,191][102][103][104][105].
4. Conclusions
This revisewarch highlighted that the risk of infection and death due to COVID-19 was not higher in CD patients than in the general population. The highest risks of contracting the infection were observed in immunocompromised patients and in those with nutritional deficiencies, especially in patients with CD who did not comply with GFD. Incidence of CD diagnosis has increased, but especially in association with T1DM, although the number of intestinal biopsies has decreased. Long waiting lists for GI endoscopies have increased complications and caused life-threatening delays, especially in young children. COVID-19 pandemic caused shortcomings in GFD adherence due to high delivery prices, supply difficulties, long travel distances to obtain GFD, reduced family income, and decreased QOL through the lockdown. For patients with CD, the pandemic caused psychological distress, insomnia, irritability, anxiety, chronic fatigue, depression, decreased quality of life, low compliance with GFD and metabolic complications such as obesity and diabetes. Patients with CD can receive any of the vaccines available on the market that are safe and effective in preventing COVID-19, as none of the current vaccines contains gluten or prolamins. Introspection into the molecular pathophysiological mechanisms of SARS-CoV-2 infection and profound similarity in the disruption of mucosal integrity in CD led to the proposal of a CD-inspired drug for MIS-C, a zonulin antagonist. As the pandemic is not over and there are still cases of MIS-C, further studies are needed to pave the way for understanding the pathophysiological mechanisms of this fulminant disease. An ongoing challenge is to imagine new delivery platforms and new molecules as immunotherapies for resolving immune-related diseases and for balancing the response of the GI immune system as a multi-field sovereign system. Zonulin is widely studied in immunoengineering as an adjunct to improving the absorption of new oral drugs and vaccines. In the near future, scientists should develop innovative approaches to combat high rates of autoimmune diseases.References
- Sahin, Y. Celiac disease in children: A review of the literature. World J. Clin. Pediatr. 2021, 10, 53–71.
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European society Paediatric gastroenterology, hepatology and nutrition guidelines for diagnosing coeliac disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156.
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836.e2.
- Lindfors, K.; Ciacci, C.; Kurppa, K.; Lundin, K.E.A.; Makharia, G.K.; Mearin, M.L.; Murray, J.A.; Verdu, E.F.; Kaukinen, K. Coeliac disease. Nat. Rev. Dis. Prim. 2019, 5, 3.
- Laurikka, P.; Nurminen, S.; Kivelä, L.; Kurppa, K. Extraintestinal Manifestations of Celiac Disease: Early Detection for Better Long-Term Outcomes. Nutrients 2018, 10, 1015.
- Lebwohl, B.; Murray, J.A.; Verdú, E.F.; Crowe, S.E.; Dennis, M.; Fasano, A.; Green, P.H.; Guandalini, S.; Khosla, C. Gluten Introduction, Breastfeeding, and Celiac Disease: Back to the Drawing Board. Am. J. Gastroenterol. 2016, 111, 12–14.
- Jiang, L.L.; Zhang, B.L.; Liu, Y.S. Is adult celiac disease really uncommon in Chinese? J. Zhejiang Univ. Sci. B 2009, 10, 168–171.
- Wang, X.Q.; Liu, W.; Xu, C.D.; Mei, H.; Gao, Y.; Peng, H.M.; Yuan, L.; Xu, J.J. Celiac disease in children with diarrhea in 4 cities in China. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 368–370.
- Wang, M.; Kong, W.J.; Feng, Y.; Lu, J.J.; Hui, W.J.; Liu, W.D.; Li, Z.Q.; Shi, T.; Cui, M.; Sun, Z.Z.; et al. Epidemiological, clinical, and histological presentation of celiac disease in Northwest China. World J. Gastroenterol. 2022, 28, 1272–1283.
- Zhou, W.Y.; Liu, X.Y.; Wang, M.M.; Liang, L.P.; Liu, L.; Zheng, K.; Silvester, J.A.; Ma, W.J.; Wu, W.; Ji, G.Y.; et al. Prevalence of celiac disease in China: Meta-analysis and serological survey in high-risk populations. J. Dig. Dis. 2021, 22, 645–655.
- Tarar, Z.I.; Zafar, M.U.; Farooq, U.; Basar, O.; Tahan, V.; Daglilar, E. The Progression of Celiac Disease, Diagnostic Modalities, and Treatment Options. J. Investig. Med. High Impact Case Rep. 2021, 9, 23247096211053702.
- Abenavoli, L.; Dastoli, S.; Bennardo, L.; Boccuto, L.; Passante, M.; Silvestri, M.; Proietti, I.; Potenza, C.; Luzza, F.; Nisticò, S.P. The Skin in Celiac Disease Patients: The Other Side of the Coin. Medicina 2019, 55, 578.
- Hadithi, M.; von Blomberg, B.M.; Crusius, J.B.; Bloemena, E.; Kostense, P.J.; Meijer, J.W.; Mulder, C.J.; Stehouwer, C.D.; Peña, A.S. Accuracy of serologic tests and HLA-DQ typing for diagnosing celiac disease. Ann. Intern. Med. 2007, 147, 294–302.
- Hoffmanová, I.; Sánchez, D.; Szczepanková, A.; Tlaskalová-Hogenová, H. The Pros and Cons of Using Oat in a Gluten-Free Diet for Celiac Patients. Nutrients 2019, 11, 2345.
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142.
- Maglio, M.; Troncone, R. Intestinal Anti-tissue Transglutaminase2 Autoantibodies: Pathogenic and Clinical Implications for Celiac Disease. Front. Nutr. 2020, 7, 73.
- Lionetti, E.; Castellaneta, S.; Francavilla, R.; Pulvirenti, A.; Catassi, C. SIGENP Working Group of Weaning and CD Risk. Mode of Delivery and Risk of Celiac Disease: Risk of Celiac Disease and Age at Gluten Introduction Cohort Study. J. Pediatr. 2017, 184, 81–86.e2.
- Koletzko, S.; Lee, H.S.; Beyerlein, A.; Aronsson, C.A.; Hummel, M.; Liu, E.; Simell, V.; Kurppa, K.; Lernmark, Å.; Hagopian, W.; et al. TEDDY Study Group. Cesarean Section on the Risk of Celiac Disease in the Offspring: The Teddy Study. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 417–424.
- Zanoni, G.; Navone, R.; Lunardi, C.; Tridente, G.; Bason, C.; Sivori, S.; Beri, R.; Dolcino, M.; Valletta, E.; Corrocher, R.; et al. In celiac disease, a subset of autoantibodies against transglutaminase binds toll-like receptor 4 and induces activation of monocytes. PLoS Med. 2006, 3, e358.
- Silvester, J.A.; Leffler, D.A. Is Autoimmunity Infectious? The Effect of Gastrointestinal Viral Infections and Vaccination on Risk of Celiac Disease Autoimmunity. Clin. Gastroenterol. Hepatol. 2017, 15, 703–705.
- Hemming-Harlo, M.; Lähdeaho, M.L.; Mäki, M.; Vesikari, T. Rotavirus vaccination does not increase type 1 diabetes and may decrease celiac disease in children and adolescents. Pediatr. Infect. Dis. J. 2019, 38, 539–541.
- Bouziat, R.; Hinterleitner, R.; Brown, J.J.; Stencel-Baerenwald, J.E.; Ikizler, M.; Mayassi, T.; Meisel, M.; Kim, S.M.; Discepolo, V.; Pruijssers, A.J.; et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 2017, 356, 44–50.
- Thomas, K.E.; Sapone, A.; Fasano, A.; Vogel, S.N. Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: Role of the innate immune response in Celiac disease. J. Immunol. 2006, 176, 2512–2521.
- Neil, J.A.; Cadwell, K. The Intestinal Virome and Immunity. J. Immunol. 2018, 201, 1615–1624.
- Sánchez, D.; Hoffmanová, I.; Szczepanková, A.; Hábová, V.; Tlaskalová-Hogenová, H. Contribution of Infectious Agents to the Development of Celiac Disease. Microorganisms 2021, 9, 547.
- Simons, M.; Scott-Sheldon, L.A.J.; Risech-Neyman, Y.; Moss, S.F.; Ludvigsson, J.F.; Green, P.H.R. Celiac disease and increased risk of pneumococcal infection: A systematic review and meta-analysis. Am. J. Med. 2018, 131, 83–89.
- Canova, C.; Ludvigsson, J.; Baldo, V.; Barbiellini Amidei, C.; Zanier, L.; Zingone, F. Risk of bacterial pneumonia and pneumococcal infection in youths with celiac disease-A population-based study. Dig. Liver Dis. 2019, 51, 1101–1105.
- Mårild, K.; Kahrs, C.R.; Tapia, G.; Stene, L.C.; Størdal, K. Infections and risk of celiac disease in childhood: A prospective nationwide cohort study. Am. J. Gastroenterol. 2015, 110, 1475–1484.
- Van De Kamer, J.H.; Weijers, H.A.; Dicke, W.K. Coeliac disease. IV. An investigation into the injurious constituents of wheat in connection with their action on patients with coeliac disease. Acta Paediatr. 1953, 42, 223–231.
- Auricchio, S.; De Ritis, G.; De Vincenzi, M.; Silano, V. Toxicity mechanisms of wheat and other cereals in celiac disease and related enteropathies. J. Pediatr. Gastroenterol. Nutr. 1985, 4, 923–930.
- De Re, V.; Caggiari, L.; Tabuso, M.; Cannizzaro, R. The versatile role of gliadin peptides in celiac disease. Clin. Biochem. 2013, 46, 552–560.
- De Re, V.; Magris, R.; Cannizzaro, R. New Insights into the Pathogenesis of Celiac Disease. Front. Med. 2017, 4, 137.
- Biesiekierski, J.R. What is gluten? J. Gastroenterol. Hepatol. 2017, 32, 78–81.
- Sharma, N.; Bhatia, S.; Chunduri, V.; Kaur, S.; Sharma, S.; Kapoor, P.; Kumari, A.; Garg, M. Pathogenesis of Celiac Disease and Other Gluten Related Disorders in Wheat and Strategies for Mitigating Them. Front. Nutr. 2020, 7, 6.
- Silano, M.; Vincentini, O.; De Vincenzi, M. Toxic, immunostimulatory and antagonist gluten peptides in celiac disease. Curr. Med. Chem. 2009, 16, 1489–1498.
- Valenti, S.; Corica, D.; Ricciardi, L.; Romano, C. Gluten-related disorders: Certainties, questions and doubts. Ann. Med. 2017, 11, 569–581.
- Cebolla, Á.; Moreno, M.L.; Coto, L.; Sousa, C. Gluten Immunogenic Peptides as Standard for the Evaluation of Potential Harmful Prolamin Content in Food and Human Specimen. Nutrients 2018, 10, 1927.
- Clemente, M.G.; De Virgiliis, S.; Kang, J.S.; Macatagney, R.; Musu, M.P.; Di Pierro, M.R.; Drago, S.; Congia, M.; Fasano, A. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut 2003, 52, 218–223.
- Moreno, M.L.; Cebolla, Á.; Muñoz-Suano, A.; Carrillo-Carrion, C.; Comino, I.; Pizarro, Á.; León, F.; Rodríguez-Herrera, A.; Sousa, C. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut 2017, 66, 250–257.
- Palanski, B.A.; Weng, N.; Zhang, L.; Hilmer, A.J.; Fall, L.A.; Swaminathan, K.; Jabri, B.; Sousa, C.; Fernandez-Becker, N.Q.; Khosla, C.; et al. An efficient urine peptidomics workflow identifies chemically defined dietary gluten peptides from patients with celiac disease. Nat. Commun. 2022, 13, 888.
- Dunne, M.R.; Byrne, G.; Chirdo, F.G.; Feighery, C. Coeliac Disease Pathogenesis: The Uncertainties of a Well-Known Immune Mediated Disorder. Front. Immunol. 2020, 11, 1374.
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Russel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology 2017, 152, 1100–1113.e12.
- Schumann, M.; Siegmund, B.; Schulzke, J.D.; Fromm, M. Celiac disease: Role of the epithelial barrier. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 150–162.
- Patel, N.; Robert, M.E. Frontiers in Celiac Disease: Where Autoimmunity and Environment Meet. Am. J. Surg. Pathol. 2022, 46, e43–e54.
- Kupfer, S.S.; Jabri, B. Pathophysiology of celiac disease. Gastrointest. Endosc. Clin. N. Am. 2012, 22, 639–660.
- Parzanese, I.; Qehajaj, D.; Patrinicola, F.; Aralica, M.; Chiriva-Internati, M.; Stifter, S.; Elli, L.; Grizzi, F. Celiac disease: From pathophysiology to treatment. World J. Gastrointest. Pathophysiol. 2017, 8, 27–38.
- Stamnaes, J.; Sollid, L.M. Celiac disease: Autoimmunity in response to food antigen. Semin. Immunol. 2015, 27, 343–352.
- Palová-Jelínková, L.; Dáňová, K.; Drašarová, H.; Dvořák, M.; Funda, D.P.; Fundová, P.; Fundová, P.; Kotrbová-Kozak, A.; Černá, M.; Kamanová, J.; et al. Pepsin Digest of Wheat Gliadin Fraction Increases Production of IL-1β via TLR4/MyD88/TRIF/MAPK/NF-κB Signaling Pathway and an NLRP3 Inflammasome Activation. PLoS ONE 2013, 8, e62426.
- Araya, R.E.; Gomez Castro, M.F.; Carasi, P.; McCarville, J.L.; Jury, J.; Mowat, A.M.; Verdu, E.F.; Chirdo, F.G. Mechanisms of innate immune activation by gluten peptide p31-43 in mice. Am. J. Physiol. Liver Physiol. 2016, 311, G40–G49.
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364.
- Patankar, J.V.; Becker, C. Cell death in the gut epithelium and implications for chronic inflammation. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 543–556.
- Perez, F.; Ruera, C.N.; Miculan, E.; Carasi, P.; Chirdo, F.G. Programmed Cell Death in the Small Intestine: Implications for the Pathogenesis of Celiac Disease. Int. J. Mol. Sci. 2021, 22, 7426.
- Kuja-Halkola, R.; Lebwohl, B.; Halfvarson, J.; Wijmenga, C.; Magnusson, P.K.; Ludvigsson, J.F. Heritability of non-HLA genetics in coeliac disease: A population-based study in 107000 twins. Gut 2016, 65, 1793–1798.
- Dubois, P.C.; Trynka, G.; Franke, L.; Hunt, K.A.; Romanos, J.; Curtotti, A.; Zhernakova, A.; Heap, G.A.; Adány, R.; Aromaa, A.; et al. Multiple common variants for celiac disease influencing immune gene expression. Nat. Genet. 2010, 42, 295–302.
- Itzlinger, A.; Branchi, F.; Elli, L.; Schumann, M. Gluten-Free Diet in Celiac Disease-Forever and for All? Nutrients 2018, 10, 1796.
- Crehuá-Gaudiza, E.; Barrés Fernández, A.; Jovaní Casano, C.; Latorre Tejerina, M.; Largo Blanco, E.M.; Moreno Ruiz, M.A.; Berghezan Suárez, A.; García-Peris, M.; Gil Piquer, R.; Coret Sinisterra, A.; et al. Diagnóstico de enfermedad celiaca en la práctica clínica: Presente y futuro. Diagnosis of celiac disease in clinical practice: Present and future. An. Pediatría 2021, 94, 223–229. (In Spanish)
- Parisi, P.; Pietropaoli, N.; Ferretti, A.; Nenna, R.; Mastrogiorgio, G.; Del Pozzo, M.; Principessa, L.; Bonamico, M.; Villa, M.P. Role of the gluten-free diet on neurological-EEG findings and sleep disordered breathing in children with celiac disease. Seizure 2015, 25, 181–183.
- Ben Houmich, T.; Admou, B. Celiac disease: Understandings in diagnostic, nutritional, and medicinal aspects. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211008709.
- Sabino, L.; Marinot, S.; Falsaperla, R.; Pisani, F.; Massimino, C.; Pavone, P. Celiac disease and headache in children: A narrative state of the art. Acta Biomed. 2020, 91, e2020056.
- Bao, F.; Green, P.H.; Bhagat, G. An update on celiac disease histopathology and the road ahead. Arch. Pathol. Lab. Med. 2012, 136, 735–745.
- Volta, U.; Granito, A.; Parisi, C.; Fabbri, A.; Fiorini, E.; Piscaglia, M.; Tovoli, F.; Grasso, V.; Muratori, P.; Pappas, G.; et al. Deamidated gliadin peptide antibodies as a routine test for celiac disease: A prospective analysis. J. Clin. Gastroenterol. 2010, 44, 186–190.
- Maheshwari, A.; He, Z.; Weidner, M.N.; Lin, P.; Bober, R.; Del Rosario, F.J. Influence of Age and Type 1 Diabetes Mellitus on Serological Test for Celiac Disease in Children. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 218–229.
- Kulkarni, A.; Patel, S.; Khanna, D.; Parmar, M.S. Current pharmacological approaches and potential future therapies for Celiac disease. Eur. J. Pharmacol. 2021, 909, 174434.
- Pais, W.P.; Duerksen, D.R.; Pettigrew, N.M.; Bernstein, C.N. How many duodenal biopsy specimens are required to make a diagnosis of celiac disease? Gastrointest. Endosc. 2008, 67, 1082–1087.
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The histopathology of coeliac disease: Time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194.
- Corazza, G.R.; Villanacci, V.; Zambelli, C.; Milione, M.; Luinetti, O.; Vindigni, C.; Chioda, C.; Albarello, L.; Bartolini, D.; Donato, F. Comparison of the interobserver reproducibility with different histologic criteria used in celiac disease. Clin. Gastroenterol. Hepatol. 2007, 5, 838–843.
- Catassi, C.; Fasano, A. Celiac disease diagnosis: Simple rules are better than complicated algorithms. Am. J. Med. 2010, 123, 691–693.
- Raiteri, A.; Granito, A.; Giamperoli, A.; Catenaro, T.; Negrini, G.; Tovoli, F. Current guidelines for the management of celiac disease: A systematic review with comparative analysis. World J. Gastroenterol. 2022, 28, 154–175.
- Husby, S.; Koletzko, S.; Korponay-Szabo, I.R.; Mearint, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160, Erratum in J. Pediatr. Gastroenterol. Nutr. 2012, 54, 572.
- Calado, J.; Machado, M.V. Celiac Disease Revisited. GE Port. J. Gastroenterol. 2021, 29, 111–124.
- Wysocka-Mincewicz, M.; Groszek, A.; Ambrozkiewicz, F.; Paziewska, A.; Dąbrowska, M.; Rybak, A.; Konopka, E.; Ochocińska, A.; Żeber-Lubecka, N.; Karczmarski, J.; et al. Combination of HLA-DQ2/-DQ8 Haplotypes and a Single MSH5 Gene Variant in a Polish Population of Patients with Type 1 Diabetes as a First Line Screening for Celiac Disease? J. Clin. Med. 2022, 11, 2223.
- Valitutti, F.; Trovato, C.M.; Montuori, M.; Cucchiara, S. Pediatric celiac disease: Follow-up in the spotlight. Adv. Nutr. 2017, 8, 356–361.
- Bascuñán, K.A.; Vespa, M.C.; Araya, M. Celiac disease: Understanding the gluten-free diet. Eur. J. Nutr. 2017, 56, 449–459.
- Fueyo-Díaz, R.; Montoro, M.; Magallón-Botaya, R.; Gascón-Santos, S.; Asensio-Martínez, Á.; Palacios-Navarro, G.; Sebastián-Domingo, J.J. Influence of Compliance to Diet and Self-Efficacy Expectation on Quality of Life in Patients with Celiac Disease in Spain. Nutrients 2020, 12, 2672.
- Wagner, G.; Berger, G.; Sinnreich, U.; Grylli, V.; Schober, E.; Huber, W.D.; Karwautz, A. Quality of life in adolescents with treated coeliac disease: Influence of compliance and age at diagnosis. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 555–561.
- Dowhaniuk, J.K.; Mileski, H.; Saab, J.; Tutelman, P.; Thabane, L.; Brill, H. The Gluten Free Diet: Assessing Adherence in a Pediatric Celiac Disease Population. J. Can. Assoc. Gastroenterol. 2020, 3, 67–73.
- Wieser, H.; Ruiz-Carnicer, Á.; Segura, V.; Comino, I.; Sousa, C. Challenges of Monitoring the Gluten-Free Diet Adherence in the Management and Follow-Up of Patients with Celiac Disease. Nutrients 2021, 13, 2274.
- Stasi, E.; Marafini, I.; Caruso, R.; Soderino, F.; Angelucci, E.; Del Vecchio Blanco, G.; Paoluzi, O.A.; Calabrese, E.; Sedda, S.; Zorzi, F.; et al. Frequency and Cause of Persistent Symptoms in Celiac Disease Patients on a Long-term Gluten-free Diet. J. Clin. Gastroenterol. 2016, 50, 239–243.
- Truitt, K.E.; Daveson, A.J.M.; Ee, H.C.; Goel, G.; MacDougall, J.; Neff, K.; Anderson, R.P. Randomised clinical trial: A placebo-controlled study of subcutaneous or intradermal NEXVAX2, an investigational immunomodulatory peptide therapy for coeliac disease. Aliment. Pharmacol. Ther. 2019, 50, 547–555.
- Rubio-Tapia, A.; Kelly, D.G.; Lahr, B.D.; Dogan, A.; Wu, T.T.; Murray, J.A. Clinical staging and survival in refractory celiac disease: A single center experience. Gastroenterology 2009, 136, 99–353.
- Galli, G.; Carabotti, M.; Pilozzi, E.; Lahner, E.; Annibale, B.; Conti, L. Relationship between Persistent Gastrointestinal Symptoms and Duodenal Histological Findings after Adequate Gluten-Free Diet: A Gray Area of Celiac Disease Management in Adult Patients. Nutrients 2021, 13, 600.
- Sparks, B.; Hill, I.; Ediger, T. Going Beyond Gluten-Free: A Review of Potential Future Therapies for Celiac Disease. Curr. Treat. Options Pediatr. 2021, 7, 17–31.
- Syage, J.A.; Green, P.H.R.; Khosla, C.; Adelman, D.C.; Sealey-Voyksner, J.A.; Murray, J.A. Latiglutenase Treatment for Celiac Disease: Symptom and Quality of Life Improvement for Seropositive Patients on a Gluten-Free Diet. GastroHep 2019, 1, 293–301.
- Calitri, C.; Fumi, I.; Ignaccolo, M.G.; Banino, E.; Benetti, S.; Lupica, M.M.; Fantone, F.; Pace, M.; Garofalo, F. Gastrointestinal involvement in paediatric COVID-19-from pathogenesis to clinical management: A comprehensive review. World J. Gastroenterol. 2021, 27, 3303–3316.
- Cakir, M.; Guven, B.; Issi, F.; Ozkaya, E. New-onset celiac disease in children during COVID-19 pandemic. Acta Paediatr. 2022, 111, 383–388.
- Trovato, C.M.; Montuori, M.; Pietropaoli, N.; Oliva, S. COVID-19 and celiac disease: A pathogenetic hypothesis for a celiac outbreak. Int. J. Clin. Pract. 2021, 75, e14452.
- Asri, N.; Nazemalhosseini Mojarad, E.; Mirjalali, H.; Mohebbi, S.R.; Baghaei, K.; Rostami-Nejad, M.; Yadegar, A.; Rezaei-Tavirani, M.; Asadzadeh Aghdaei, H.; Rostami, K.; et al. Toward finding the difference between untreated celiac disease and COVID-19 infected patients in terms of CD4, CD25 (IL-2 Rα), FOXP3 and IL-6 expressions as genes affecting immune homeostasis. BMC Gastroenterol. 2021, 21, 462.
- Renzo, S.; Scarallo, L.; Antoniello, L.M.; Bramuzzo, M.; Chiaro, A.; Cisarò, F.; Contini, A.C.I.; De Angelis, G.L.; Angelis, P.; Nardo, G.D.; et al. Impact of COVID-19 pandemic on pediatric endoscopy: A multicenter study on behalf of the SIGENP Endoscopy Working Group. Dig. Liver Dis. 2022, 54, 572–579.
- Concas, G.; Barone, M.; Francavilla, R.; Cristofori, F.; Dargenio, V.N.; Giorgio, R.; Dargenio, C.; Fanos, V.; Marcialis, M.A. Twelve Months with COVID-19: What Gastroenterologists Need to Know. Dig. Dis. Sci. 2022, 67, 2771–2791.
- Uche-Anya, E.; Husby, S.; Kaplan, G.G.; Underwood, F.E.; Green, P.H.R.; Lebwohl, B. An International Reporting Registry of Patients With Celiac Disease and COVID-19: Initial Results From SECURE-CELIAC. Clin. Gastroenterol. Hepatol. 2021, 19, 2435–2437.e4.
- Mehtab, W.; Chauhan, A.; Agarwal, A.; Singh, A.; Rajput, M.S.; Mohta, S.; Jindal, V.; Banyal, V.; Ahmed, A.; Pramanik, A.; et al. Impact of Corona Virus Disease 2019 pandemic on adherence to gluten-free diet in Indian patients with celiac disease. Indian J. Gastroenterol. 2021, 40, 613–620.
- Falcomer, A.L.; Farage, P.; Pratesi, C.B.; Pratesi, R.; Gandolfi, L.; Nakano, E.Y.; Raposo, A.; Zandonadi, R.P. Health-Related Quality of Life and Experiences of Brazilian Celiac Individuals over the Course of the Sars-Cov-2 Pandemic. Nutrients 2021, 13, 1582.
- Monzani, A.; Lionetti, E.; Felici, E.; Fransos, L.; Azzolina, D.; Rabbone, I.; Catassi, C. Adherence to the Gluten-Free Diet during the Lockdown for COVID-19 Pandemic: A Web-Based Survey of Italian Subjects with Celiac Disease. Nutrients 2020, 12, 3467.
- Temsah, M.H.; Aljamaan, F.; Alhaboob, A.; Almosned, B.; Alsebail, R.; Temsah, R.; Senjab, A.; Alarfaj, A.; Aljudi, T.; Jamal, A.; et al. Enhancing parental knowledge of childhood and adolescence safety: An interventional educational campaign. Medicine 2022, 101, e28649.
- Barschkett, M.; Koletzko, B.; Spiess, C.K. COVID-19 Associated Contact Restrictions in Germany: Marked Decline in Children’s Outpatient Visits for Infectious Diseases without Increasing Visits for Mental Health Disorders. Children 2021, 8, 728.
- Dipasquale, V.; Passanisi, S.; Cucinotta, U.; Cascio, A.; Romano, C. Implications of SARS-COV-2 infection in the diagnosis and management of the pediatric gastrointestinal disease. Ital. J. Pediatr. 2021, 47, 71.
- Bükülmez, A.; Baş, M.T.; Çiftci, E. Evaluation of anti-COVID-19 measures taken by the parents of children with celiac disease: A cross-sectional study. Sao Paulo Med. J. 2021, 139, 201–209.
- Lionetti, E.; Fabbrizi, A.; Catassi, C. Prevalence of COVID-19 in Italian Children With Celiac Disease: A Cross-Sectional Study. Clin. Gastroenterol. Hepatol. 2021, 19, 1075.
- Catassi, G.N.; Vallorani, M.; Cerioni, F.; Lionetti, E.; Catassi, C. A negative fallout of COVID-19 lockdown in Italy: Life-threatening delay in the diagnosis of celiac disease. Dig. Liver. Dis. 2020, 52, 1092–1093.
- Lefthériotis, G.; Wray, S.; Girardi, A.C.C.; Vidal-Petiot, E.; Bailey, M.A.; Schechtman, D.; Ravi, N.; Noble, D. Editorial: The Tribute of Physiology for the Understanding of COVID-19 Disease. Front. Physiol. 2021, 12, 761644.
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Implications of SARS-CoV-2 Infection in Systemic Juvenile Idiopathic Arthritis. Int. J. Mol. Sci. 2022, 23, 4268.
- Costantino, A.; Topa, M.; Roncoroni, L.; Doneda, L.; Lombardo, V.; Stocco, D.; Gramegna, A.; Costantino, C.; Vecchi, M.; Elli, L. COVID-19 Vaccine: A Survey of Hesitancy in Patients with Celiac Disease. Vaccines 2021, 9, 511.
- Zhen, J.; Stefanolo, J.P.; Temprano, M.P.; Seiler, C.L.; Caminero, A.; de-Madaria, E.; Huguet, M.M.; Santiago, V.; Niveloni, S.I.; Smecuol, E.G.; et al. Risk perception and knowledge of COVID-19 in patients with celiac disease. World J. Gastroenterol. 2021, 27, 1213–1225.
- Aaron Lerner. The COVID-19 Vaccination Debate: CoV-2 in Celiac Disease: A Pathogen or just along for the Ride? Int. J. Celiac Dis. 2021, 9, 6–9.
- Cascini, F.; Pantovic, A.; Al-Ajlouni, Y.; Failla, G.; Ricciardi, W. Attitudes, acceptance and hesitancy among the general population worldwide to receive the COVID-19 vaccines and their contributing factors: A systematic review. eClinicalMedicine 2021, 40, 101113.
More
