Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Christos Kourek and Version 2 by Dean Liu.
Heart failure (HF) is a major public health issue worldwide with increased prevalence and a high number of hospitalizations. Patients with chronic HF and either reduced ejection fraction (HFrEF) or mildly reduced ejection fraction (HFmrEF) present vascular endothelial dysfunction and significantly decreased circulating levels of endothelial progenitor cells (EPCs).
- heart failure
- exercise training
- acute exercise
- endothelial progenitor cells
1. Introduction
Heart failure (HF) presents a major public health issue worldwide with a tremendous burden on healthcare systems and their resources due to the high number of hospitalizations and readmissions among diagnosed adults and the elderly [1][2][1,2]. It is estimated that approximately 20% of HF patients are readmitted in the US hospitals within the first 30 days, while the respective number in several European countries is lower [2][3][4][2,3,4]. The annual incidence of HF presents a linear increase with age and ranges widely from 1 to 9 cases per 1000 person-years in both Europe and the US, with the median number rising to 3.20 (IQR 2.66–4.17) cases [4][5][4,5]. The median length of stay is 8.50 (IQR 7.38–10) days [4]. Moreover, the prevalence of HF according to the 2021 American Heart Association Statistical Update is estimated between 1.5% and 1.9% of the total US and Canadian population, while in Europe it ranges between 1% and 2% [4][6][7][4,6,7].
Patients with HF usually present with impaired endothelium-dependent vasodilation, endothelial nitric oxide synthases (eNOS) uncoupling, and reduced availability of nitric oxide (NO) [8][9][10][8,9,10]. Vascular endothelial dysfunction caused by increased formation of superoxide radicals and other oxidant species, and “oxidative stress” result in reduced exercise capacity and, thus, in worse quality of life [8][9][10][8,9,10]. Exercise has been shown to have beneficial effects in vasodilation and, therefore, endothelial function resulting in higher exercise capacity and better quality of life between HF patients [11][12][13][14][11,12,13,14]. In addition, exercise is a strong recommendation (Class IA) of treatment in heart failure according to the latest ESC [15][16][15,16] and AHA Guidelines [17][18][17,18].
Endothelial progenitor cells (EPCs) are bone marrow-derived cells involved in endothelium regeneration, homeostasis, and neovascularization [19][20][19,20]. They either transform in mature circulating endothelial cells (CECs) or remain as precursor cells restoring the dysfunctional and injured endothelium and promoting vasculogenesis and angiogenesis [19][20][19,20].
2. Effects of Exercise on Circulating Endothelial and Progenitor Cells in Heart Failure
2.1. Acute Exercise
Acute exercise has been shown to increase EPCs and/or CECs in healthy volunteers [21][22][77,78], patients with cardiovascular diseases [23][24][79,80] and risk factors [25][26][27][81,82,83], and patients with chronic HF [28][29][30][31][32][33][84,85,86,87,88,89]. There are variables such as intensity of exercise, duration, patient’s medical history, and subgroup of endothelial population defined that determine the acute mobilization and the increase of EPCs number in circulation. In a previous study held in theour Institute, Kourek C. et al. [28][84] evaluated the effect of acute exercise in 49 consecutive patients with stable chronic HF and a reduced or mid-ranged EF. Most specifically, all patients underwent a ramp incremental symptom-limited maximal cardiopulmonary exercise testing (CPET) on a cycle ergometer and five endothelial cellular populations were identified and quantified by flow cytometry; three subgroups of EPCs (CD34+/CD45−/CD133+, CD34+/CD45−/CD133+/VEGFR-2+, and CD34+/CD133+/VEGFR-2+) and two subgroups of CECs (CD34+/CD45−/CD133− and CD34+/CD45-/CD133-/VEGFR-2). All EPCs and CECs subgroups increased statistically significantly after a single bout of maximal exercise [28][84]. The same results were repeated a few months later by the same Institution in 44 chronic HF patients following similar methodology [29][85]. Interestingly, in a post-hoc analysis of the previous study [30][86], it was shown that exercise-mediated EPCs and CECs mobilization was not associated with the severity of HF (based on cardiopulmonary exercise testing and echocardiographic indices) [30][86]. The acute effect of a single exercise bout on EPCs in patients with HF was also examined previously by another Institute in Belgium. Specifically, Van Craenenbroeck E.M. et al. [31][87] performed a symptom-limited CPET in 41 sedentary chronic HF patients on a graded bicycle ergometer and identified two EPCs subgroups defined as CD34+/KDR+/CD32− and CD34+/CD32− progenitor cells via flow cytometry. Patients were divided into two groups of HF severity according to NT-proBNP levels: the group of mild and the group of severe chronic HF. There was also a group of 13 healthy volunteers as control group. They found that CD34+/KDR+/CD32− and CD34+/CD32− cell numbers remained unchanged after a single bout of maximal exercise. However, there was a potent stimulus to reverse circulating angiogenic cells dysfunction by improving their migration in severe (+52%, p < 0.05) and mild chronic HF (+31%, p < 0.05) and restoring it to levels similar to controls [31][87]. The same investigators tried to investigate whether the absent immediate effect of acute exercise on EPCs is due to attenuation or delayed mobilization in chronic HF [32][88]. In HF patients, the initial increase of EPCs was smaller and returned faster to baseline compared to healthy controls. They concluded that the immediate effect of acute exercise on EPCs numbers is not delayed, but significantly attenuated in CHF patients compared to healthy subjects [32][88].2.2. Exercise Training
The effect of exercise training on EPCs and CECs has been previously assessed in patients with chronic HF. During the last two decades many researchers have investigated the impact of a multi-session exercise training program on EPCs in HF [29][34][35][36][37][38][39][40][41][85,90,91,92,93,94,95,96,97]. The first investigators who evaluated the effect of a regular aerobic exercise training program were Sarto P. et al. [34][90]. They performed an 8-week supervised aerobic training program in 22 patients with stable chronic HF and evaluated the number of EPCs at the beginning of the study, after 8 weeks of the supervised training program and 8 weeks of the subsequent discontinued supervised aerobic training phase. EPCs were defined as CD34+/KDR+ circulating cells. They also measured plasma concentration of VEGF and stromal-derived factor 1 (SDF-1). Levels of EPCs, VEGF, and SDF-1 increased statistically significantly after the exercise training program but returned to the baseline levels after the discontinuation phase [34][90]. A couple of years later, Erbs S. et al. [35][91] enrolled 37 patients with chronic HF either into a 12-week exercise training program or sedentary lifestyle as control group. They defined EPCs as CD34+/KDR+ cells and quantified them by flow cytometry. EPCs were increased in patients who performed exercise training compared to controls [35][91]. Additional parameters including flow-mediated dilation, skeletal muscle neovascularization, and LV function were also improved after the aerobic training program. This data was confirmed the same year by Van Craenenbroeck E.M. et al. [36][92] who investigated the impact of exercise training on circulating angiogenic cells function and number of CD34+ and CD34+/KDR+ EPCs in 21 patients with chronic HF. These patients underwent 6-month exercise training and were compared to a non-trained control group of 17 patients and 10 healthy age-matched subjects. Authors showed that exercise training reversed circulating angiogenic cells dysfunction by increasing their migration by 77% and also increased the number of CD34+ and CD34+/KDR+ EPCs in chronic HF [36][92]. In the contrary, there were no differences in the control group and healthy subjects. The following years, six more studies were performed in HF patients. Gatta L. et al. [37][93] evaluated the effect of a 3-week exercise training program on CD34/KDR+ EPCs, MMPs, TIMP-1, and TNF-α in 14 chronic HF patients. Number of circulating CD34+/KDR+ EPCs, as well as MMP-2/TIMP-1 and MMP-9/TIMP-1 ratios increased after exercise training, while a decrease in serum concentration of MMP-1 and TIMP-1 was also observed, indicating their potential role in vascular remodeling [37][93]. Eleuteri E. et al. [38][94] performed five sessions of 30-min cycle ergometry (60 rev/min) per week, for 3 months, in 11 chronic HF patients and assessed EPCs (defined as CD45dim/CD34+/KDR+ cells), angiogenetic markers including angiogenin, angiopoietin-1 and -2, VEGF, Tie-2 and SDF-1a, and inflammatory markers including IL-6 and CRP in comparison with ten non-trained HF patients. After the 3-month program, EPCs and angiopoietin-serum levels significantly increased in the HF patients compared to the non-exercised group [38][94]. Mezzani A. et al. [39][95] presented similar results with the previous investigators, showing that a 3-month light-to-moderate-intensity aerobic exercise training program of five sessions a week of 30-min cycling (60 rpm) increased significantly the EPCs number (identified as CD45dim/CD34+/KDR+ cells) in trained patients, reaching values similar to those of normal subjects, whereas it remained unchanged in control patients [39][95]. Interestingly, a few years later, Sandri M. et al. [40][96] assessed whether disease and aging have additive effects on EPCs or whether beneficial effects of exercise training are diminished in old age. Sixty patients with stable chronic HF and 60 referent controls were randomized either to a training or a control group and exercised four times daily at 60% to 70% of max VO2 under supervision for a month [40][96]. CD34+/KDR+ EPCs and CD133+/KDR+ EPCs were quantified by flow cytometry and factors such as VEGF, SDF-1, soluble intercellular adhesion molecule (sICAM-1), soluble vascular cell adhesion molecule (sVCAM-1), and asymmetric dimethylarginine (ADMA) were also measured by highly sensitive ELISA [40][96]. The authors found that the EPCs function improved significantly by 24% in older referent controls above 65 years, while it remained unchanged in young training referent controls below 55 years and controls respectively [40][96]. Moreover, in both young and older patients with chronic HF, 4 weeks of exercise training resulted in a significant improvement in EPCs numbers and EPCs function (young: number +66% function +43%; p < 0.05; older: number +69% function +36%; p < 0.05), highlighting the benefits of rehabilitation in HF patients of older age [40][96]. In a recent study from theour Institute, Kourek C. et al. [29][85] provided further scientific knowledge investigating the effects of different exercise training regimens on EPCs and CECs. Specifically, 44 patients with stable chronic HF were randomized in either a high intensity interval training (HIIT) or a HIIT combined with muscle strength (COM) program and underwent 36 sessions of exercise training [29][85]. All patients underwent maximum CPET before and after the rehabilitation program and five endothelial populations were quantified by flow cytometry: CD34+/CD45−/CD133+, CD34+/CD45−/CD133+/VEGFR-2+, CD34+/CD133+/VEGFR-2+ (EPCs subgroups) and CD34+/CD45−/CD133−, CD34+/CD45−/CD133−/VEGFR-2+ (CECs subgroups) [29][85]. Authors demonstrated that all EPCs and CECs populations increased after the program (p < 0.01) while there were no differences between HIIT and COM groups. The beneficial effects of both aerobic and muscle strength protocols were similar for all patients, independently of HF severity [29][85]. Functional capacity assessed by peak VO2 and angiogenetic markers such as VEGF were also improved after the training program [29][85]. In another interesting study, Chen J. et al. [41][97] evaluated the effects of exercise training on cardiac function, B-natriuretic peptide (BNP) levels, cell viability, proliferation, apoptosis, and invasion ability of EPCs, eNOS, and VEGF in 80 elderly patients with chronic HF. The training group performed cardiac exercise rehabilitation for 12 weeks, 3–5 times a week while the control group only performed simple exercises at the bedside or indoors and walked freely for 30–60 min per day [41][97]. Through their results, it was shown that exercise training improved myocardial function and promoted angiogenesis and endothelial function via the improvement of the vitality, proliferation, and invasion of peripheral blood EPCs, and the expression of eNOS and VEGF through the upregulation of the PI3K/AKT pathway [41][97]. All studies come in agreement that exercise training has beneficial effects on EPCs and CECs by increasing their number in circulation and improving their functional possibilities in patients with HF.3. Physiology of Exercise on Circulating Endothelial and Progenitor Cells in Heart Failure
EPCs are a subtype of immature cells produced in the bone marrow and located between a large number of hematopoietic stem cells and bone marrow stromal cells. These conditions create an appropriate microenvironment which helps them to differentiate into different subsets of cells, mainly into mature endothelial cells. The circulating number of EPCs is low in normal conditions and consists of approximately 0.01% of monocytes. However, there are environmental or physiological factors including estrogens, statins, physical exercise, acute ischemia, and hypoxia that present a direct effect on these cellular populations by stimulating their mobilization from the bone marrow and their differentiation rates into mature endothelial cells. There are two main mechanisms of EPCs mobilization: shear stress and the hypoxic/ischemic stimulus [42][98]. The combination of both leads to the release of EPCs in circulation promoting their repairment properties on vascular endothelium’s barrier (Figure 13).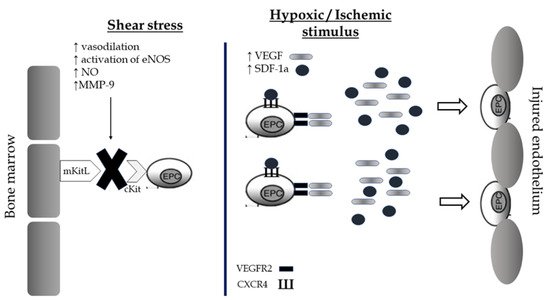
Figure 13. Shear stress and hypoxic/ischemic stimulus as potential mechanisms of mobilization of Endothelial Progenitor cells from the bone marrow and restoration of the endothelial barrier after exercise. EPCs, endothelial progenitor cells; NOS, nitric oxide synthase; NO, nitric oxide; VEGF, vascular endothelial growth factor; SDF, Stromal cell-derived factor; VEGFR, vascular endothelial growth factor receptor; CXCR, C-X-C chemokine receptor.
