You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Megan I Mitchell.
The indolent nature of some cancers makes early detection challenging, as such significant effort is placed on identifying circulating cancer biomarkers using minimally invasive, highly sensitive diagnostic assays. Biological fluids contain small extracellular vesicles including exosomes, which have many tissue origins. Cancer cells increase production and release of exosomes in the circulation to deliver biologically active compounds that can reprogram recipient cells, which potentially represent a valuable source of biomarkers.
- Extracellular Vesicles
- Exosomes
- Circulating Biomarkers
- Next Generation Sequencing
- Mass Spectrometry
- Lipidomics
- Proteomics
- Transcriptomics
- Cancer
- Liquid Biopsy
1. Extracellular Vesicles (EVs): A Journey from Discovery to Clinical Utility
The first description of EVs can be traced back to 1946, to the studies of Erwin Chargaff and Randolph West on blood coagulation [72,73]. They were the first to describe a platelet-free coagulation component from plasma which could be sedimented into a pellet by high-speed centrifugation (31,000× g) and could inhibit blood clotting [74].
The definition of exosomes was introduced in complementary seminal papers from Philip Stahl and Rose Johnstone’s laboratories in 1983 [75,76]. Stahl published stunning electron microscopic images which demonstrated the mechanical process of exosome release from the lumen of MVBs upon fusion with the plasma membrane, which helped define this novel intracellular sorting pathway then referred to as the exosome secretion pathway [75]. Functionally, Rose Johnstone and co-workers suggested that exosomes served as a cellular waste disposal mechanism, wherein they showed that under different cellular stressors the presence of the transferrin receptor on exosomes was altered at different times, thus suggesting that exosomes provided a major route for the shedding of obsolete membrane proteins [77]. However, many contradicting papers were published demonstrating the lateral cell–cell diffusion/transfer of proteins and lipids within the membranes of exosomes (i.e., membrane fluidity) [78], along with the identification of functional enzymes [79] and other active components (i.e., the tetraspanins, Rab4, and ARF [80]), suggestive of their active biological function. Additionally, several papers demonstrated that the production of exosomes and their composition could be altered at different stages of disease, being increased during transient brain ischemia, myocardial infarctions, angina, and Crohn’s disease, wherein exosomes were shown to act as functional activators [81,82,83].
Functional studies, many of which were conducted in the early 2000s, have highlighted the importance of the exosome cargoes and their functions as unique biological devices. Exosome proteomics, lipidomics, and transcriptomics analyses have led to the discovery of unique exosome cell-specific cargoes [84,85,86,87], confirming that they transport active proteins and also, importantly, carry unique and intact RNA molecules (i.e., mRNAs, miRNAs, and lncRNAs) that can post-transcriptionally modify the fate and behavior of recipient cells [88,89,90]. The study by Skog et al. demonstrated that the RNA found in glioblastoma-derived exosomes contains a “snapshot” of the cellular transcriptome of their cells of origin at a specific point in time [91]. With an ever-growing number of studies demonstrating the powerful and oncogenic effects of tumor exosomes, important breakthroughs such as the mechanistic description of metastatic dissemination have highlighted the unique intercellular communication properties of exosomes [92,93,94,95]. Jang et al. demonstrated in their study that the enhanced release of breast tumor-derived exosomes is critical for the education of pre-metastatic niche cells, which accommodate the colonization of circulating cancer cells from primary tumors [96,97]. This has in part been explained by the “seed and soil” hypothesis proposed by Stephen Paget [98], wherein exosomes carry oncogenic signals (i.e., mRNA, miRNA, and protein) specifically packaged by primary tumors’ (i.e., the “seed”) to prime target sites (i.e., the “soil”) prior to the dissemination of tumor cells, a process by which secondary tumors can prosper in a suitable microenvironment [99,100] (Figure 1).
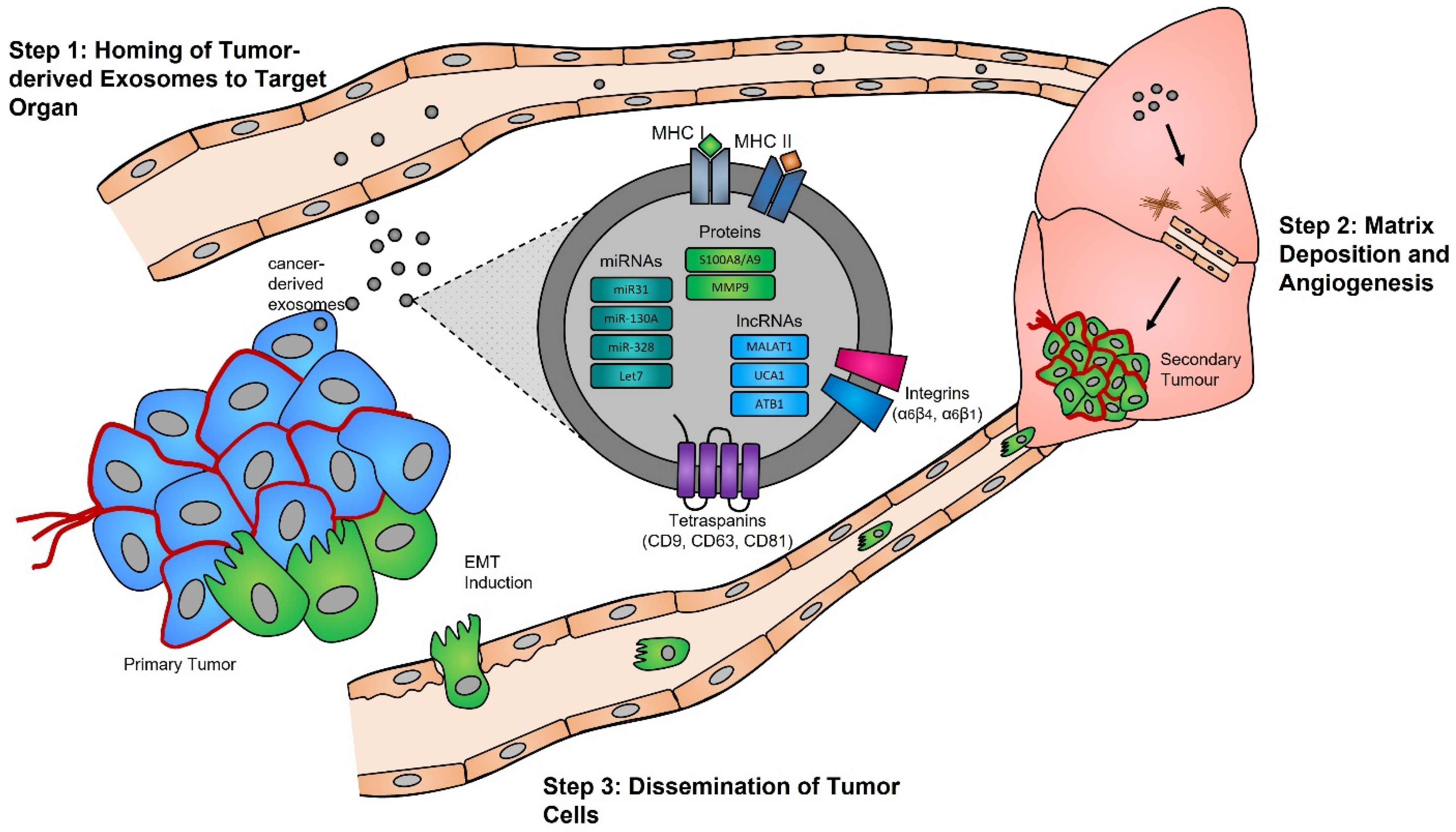
Figure 1. Exosomes and pre-metastatic niche formation. Exosomes released from primary tumors into the circulatory system specifically home to distant target organs (step 1). Upon their arrival, tumor-derived exosomes actively prepare the pre-metastatic niche through myofibroblast activation, induction of angiogenesis, and ECM remodeling (step 2). Local invasion of the primary tumor by cancer cells is followed by their intravasation into the tumor vasculature. These cancer cells survive and travel within the circulatory system, and upon their arrest in capillaries at distant sites, they extravasate into the parenchyma of target organs to commence metastatic colonization (step 3).
An important potential application of circulating tumor exosomes that has been extensively studied is their targeted purification for analysis of their content and identification of tumor biomarkers, including exosome studies of breast cancer [101], lung cancer [102], colon cancer [103,104], and pancreatic cancer [105,106]. Although many options are available for the purification of exosomes from biofluids, very few methods have been proven to provide both the sensitivity and specificity required for detecting low abundance tumor-specific circulating exosomes. Technological advancements aimed at enhancing tissue/cell-specific purification of exosomes from biofluids has significant potential to provide ultra-sensitive molecular assays for detection and monitoring of all human diseases, in particular cancer.
2. Biogenesis and Function of Exosomes in Normal and Pathological Processes
2.1. Biogenesis
Evidence supporting the endosomal origin of exosomes stems from observations that they contain proteins derived exclusively from the cytosol and are devoid of any nuclear proteins [188]. Initial proteomic analyses revealed that while exosomes harbor proteins specific to their cell and tissue of origin, for example integrins (e.g., α6β4, αvβ5, and α6β1 on breast cancer exosomes; αvβ3 and αvβ6 on prostate cancer exosomes; and β4 on pancreatic cancer exosomes), MHC class I and II on immune cell-derived exosomes, as shown for B lymphocytes and dendritic cells [189,190,191], prostate specific antigen (PSA) from prostate cells, asialoglycoprotein receptor 1 (ASGR1) from liver cells [192], microglial proteins (CD11b and CD45) from brain cells [193], placental alkaline phosphatase (PLAP) from placental cells [194,195], and Clara cell protein 16 (CC16) for exosomes produced and released by deep lung cells [196], they also contain proteins common to all exosomes irrespective of their cellular origin, which reflects a highly regulated sorting mechanism [186,197]. For instance, adapter protein ALG-2-interacting protein X (ALIX) and tumor susceptibility gene 101 (TSG101), two proteins extensively characterized for their roles in HIV budding (i.e., release of viral particles from the cell) and MVB formation [198,199], and several tetraspanins (CD63, CD81, CD9, and CD37) are present on all exosomes. The incorporation of specific tetraspanins into exosomes is dependent on cell-type specific needs, as exosomes are involved in a multitude of biological processes, including cell adhesion, motility, invasion, membrane fusion, intracellular signaling, and protein trafficking [200]. To better understand how cancer cells hijack the exosome machinery to regulate the immune response locally, for cell-to-cell communication between neighboring cells, and, as described by Lyden et al. [201], for the successful dissemination of tumor cells, wpeople have divided the endosomal pathway for exosome biogenesis into three major sections (Figure 2).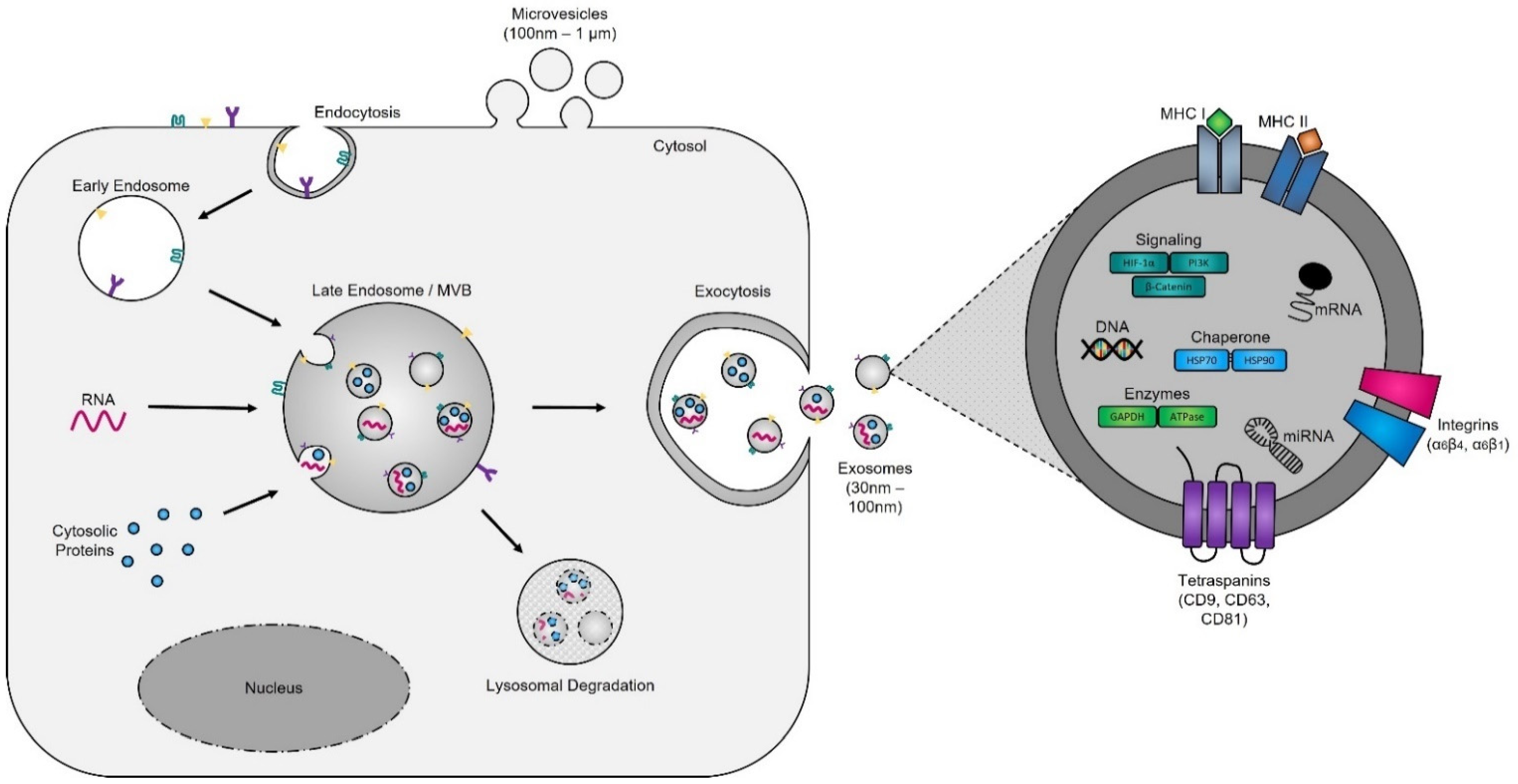
Figure 2. Exosome biogenesis. Exosomes originate from multivesicular bodies (MVBs) (also referred to as late endosomes). The inward budding of the late endosomal membrane around selectively packaged cargo results in the formation of exosomes. The selective packaging of proteins (e.g., tetraspanins, cytoplasmic proteins, and enzymes), nucleic acids (e.g., DNA, RNA, and miRNAs), and lipids (e.g., cholesterol) into exosomes is cell-type dependent and reflects the metabolic status of originating cells. Fusion of MVBs with either lysosomes or the plasma membrane results in either degradation or the release of exosomes into the extracellular matrix, respectively.
2.1.1. The Formation of Early Endosomes
Early endosomes were initially defined as the first cellular compartment to receive incoming endocytic vesicle cargoes [202] but are now recognized to be the main sorting station for initiation of the cellular endocytic pathway [203]. The exact mechanisms underlying the initiation of early endosome formation remain unclear; however, it is thought that their membrane is derived from the fusion of primary endocytic vesicles taken up from the extracellular space [204]. The function of early endosomes (i.e., their role in cargo sorting and delivery of vesicles to the plasma membrane) is heterogenous, cell-type dependent, and defined by the proteins present in their cytosol and on their surface [205]. Individually, early endosomes are complex in structure, with their membrane being comprised of a mosaic of protein subdomains, enriched in Rab5, Rab4, Rab11, Arf1/COPI, retromer, and caveolae of cellular origin [206]. Studies of these proteins have demonstrated that they are responsible for the molecular sorting and the direct transport of endosomes once matured to distinct organelles, including the trans-Golgi network and the plasma membrane [207].2.1.2. The Maturation of Late Endosomes and Formation of Exosomes
Late endosomes (also termed MVBs) are described as having a multivesicular morphology because they contain intraluminal vesicles (ILVs) [208]. The membrane of these ILVs contains the majority of V-ATPases (i.e., protein pumps responsible for controlling intracellular and extracellular pH), cholesterol, sphingolipid-rich lipid rafts, clathrin coats, and most importantly the endosomal sorting complexes required for transport (ESCRT) machinery [209,210]. This ESCRT machinery sequesters and sorts cytosolic ubiquitinated proteins into ILVs and is the most well-defined and widely understood system for biogenesis of normal and cancer exosomes. The ESCRT complex is comprised of a series of sub-complexes (i.e., ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III) that are sequentially activated by binding of cytosolic ubiquitinated proteins (Figure 3), which are then internalized as early endosomes mature [211].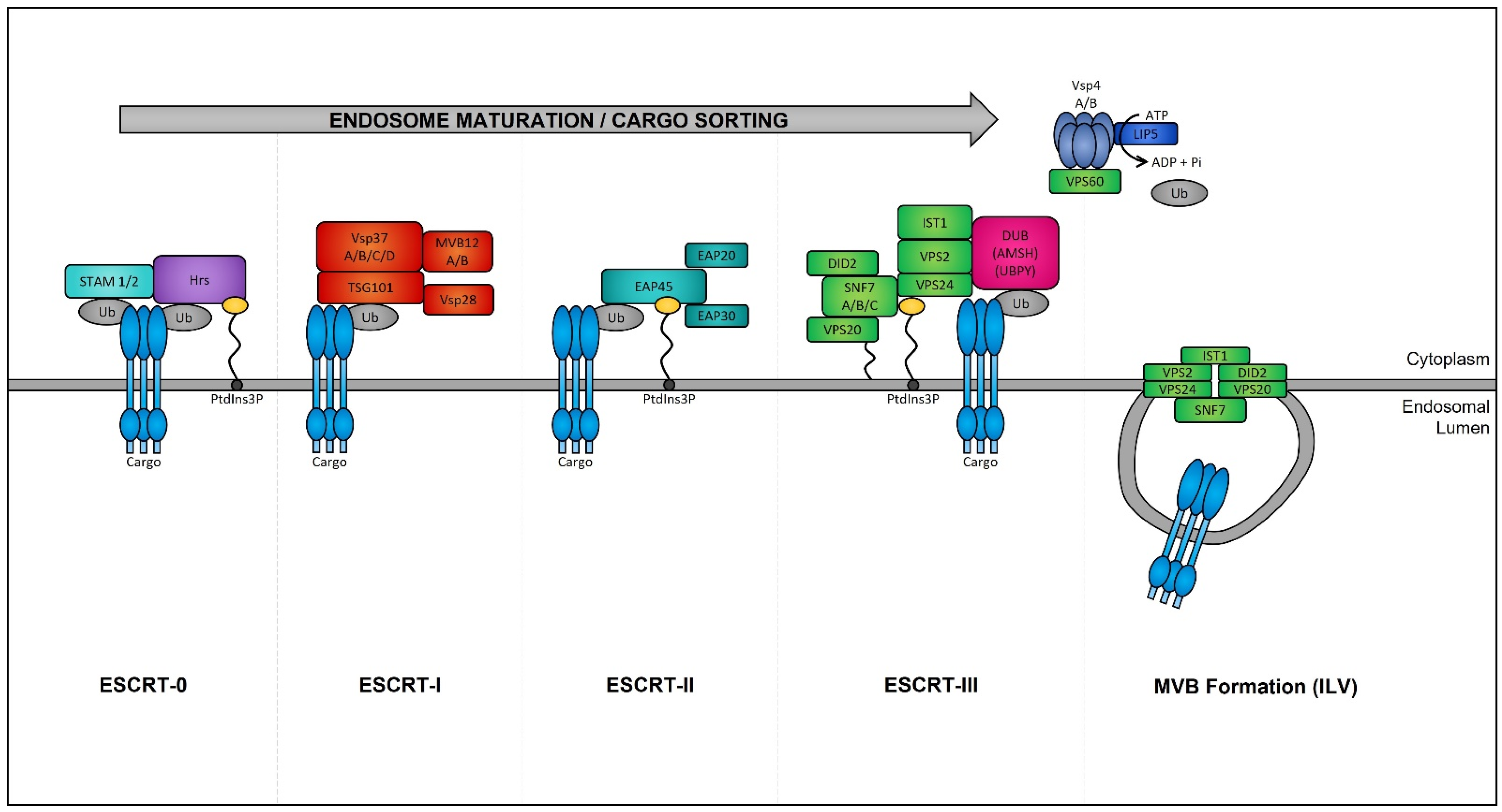
Figure 3. Endosomal sorting complexes required for transport (ESCRT)-dependent MVB formation. ESCRT-dependent MVB formation control the internalization of ubiquitinated proteins into the intraluminal vesicles (ILVs) of MVBs. The ESCRT complex is comprised of a series of sub-complexes which function uniformly during ILV production. ESCRT-0, -I, -II, and -III complexes function consecutively in a stepwise manner to control the selective sorting of ubiquitinated proteins into exosomes.
2.1.3. MVB Trafficking and Exosome Release
The biogenesis of mature MVBs terminates with their progression through one of two pathways: (1) they fuse with lysosomes, resulting in the rapid and irreversible degradation of their contents, or (2) they fuse with the plasma membrane, resulting in the release of their ILV content into the extracellular environment, at which point they are referred to as exosomes [212]. The mechanisms underlying this intracellular switch, which determines the fate of MVBs, is complex and to peourple's knowledge remains unclear. However, recent studies suggest that both paths are interconnected and that decisions affecting one result in alterations of the other [213]. For instance, two studies showed that inhibition of lysosome fusion with bafilomycin A1 results in the increased secretion of exosomes [214,215]. The release of exosomes by fusion of the MVB with the plasma membrane involves two sequential steps: (i) The targeted movement of MVBs to the plasma membrane, which is reliant on the structure of the microtubule cytoskeleton and the dynamic mechanisms of molecular motors, including dynein, kinesins, and actin-based myosin [216,217], and is regulated by several small Ras-like GTPases (including Rab27 A/B, Rab11, and Rab35), and soluble NSF attachment protein receptor (SNARE) (i.e., small, abundant tail-anchored proteins which are post-translationally inserted into the plasma membrane) proteins [218,219], which have been shown to differ between different cell types [220,221]. (ii) The fusion with the plasma membrane, wherein Rab11 and Rab35 facilitate MVB and plasma membrane fusion [222,223] through the assembly and functionalization of the “SNAREpin” complex. This complex is comprised of one vesicle-SNARE protein (located on MVBs) and three target membrane SNARE proteins (located on the plasma membrane) and is responsible for bringing the two membranes in close apposition resulting in the opening of a fusion pore and the merging of the MVB and plasma membrane [224] and their releasing their exosome cargo into the intercellular space.2.2. Biological Functions of Exosomes
Exosomes are the most extensively characterized type of extracellular vesicles (typically 30–150 nm in size) [225], which, as described above, are produced via the endosomal pathway (Figure 1) [226]. The release of exosomes is an evolutionary process that has been conserved across all archaea, bacteria, and eukaryotic cells [227]. In humans, exosomes have been shown to regulate cellular functions of neighboring/target cells either by: (1) extracellular interactions of their membrane proteins and lipids with cell-surface receptors and membranes of recipient cells, respectively, to induce signaling cascades [228] or (2) cellular uptake and intracellular pre- or post-transcriptional reprogramming, via the uptake of transcription factors, microRNAs, and long non-coding RNAs, or via the import of foreign messenger RNAs, viral RNAs, and other RNA species [229,230]. Although initially thought to be responsible for the removal of unnecessary material (proteins, lipids, nucleic acids, etc.) [76], exosomes have been shown to play critical roles in a broad range of essential regulatory cellular functions, including the modulation of immunity [231] by regulation of immune cells, particularly by the inhibition of natural killer cells [232] or by acting as antiviral and antibacterial beacons [149,233]. Such activities have also been described with seminal fluid exosomes (originating from seminal glands, prostate, etc.), which protect (i.e., have antimicrobial activities) and provide energy (i.e., produce ATP) to sperm during fertilization [234]. Other functional studies of exosomes have identified their important roles in the maintenance of cellular stemness [235], coagulation of blood and repair of tissues [236], and cardiac and skeletal muscle repair [237,238]. Studies of the function of exosomes highlight the essential roles they play in maintaining the homeostasis of human tissues, which is perturbed during disease [239,240]. Unfortunately, the biological functions of exosomes are “hijacked” in human pathologies, including cardiovascular physiological and pathological disorders (e.g., cardiomyocyte hypertrophy, peripartum cardiomyopathy, and sepsis-induced cardiomyopathy) [241], where exosomes appear to contain erroneous cargoes. Furthermore, exosomes containing aggregation-prone proteins have been found in the cerebral spinal fluid and blood of Parkinson’s disease (PD), Alzheimer’s disease (AD), Creutzfeldt–Jakob disease (CJD), and amyotrophic lateral sclerosis (ALS) patients and may play roles in the propagation of these diseases [242,243,244,245]. Many studies have also highlighted the critical role that tumor exosomes play during the progression of cancer [246]. The best example on the functional diversion of exosomes has been described by the group of David Lyden, which showed that circulating tumor exosomes educate host cells within a secondary organ to prime that environment for establishment of the metastatic niche [247]. In their studies, they demonstrated that exosomes from mouse or human lung, liver, and brain tumor cells preferentially fuse with specific recipient cells (i.e., those at predicted target sites), namely lung epithelial and fibroblast cells, brain endothelial cells, and Kupffer cells in the liver [248].2.3. Alteration of Exosome Biogenesis in Tumor Cells
Numerous studies have demonstrated that the presence of tumors is associated with a significant increase in the levels of circulating exosomes when compared to healthy individuals [249]. In aggressive brain cancer, glioma cells carrying the EGFRvIII overexpressing mutation exhibit increased secretion of exosomes harboring EGFRvIII [250], and when transferred to astrocytes can confer their oncogenic activity (i.e., ability of exosomes to convey their tumorigenic signals to recipient cells) [251]. ESCRT protein silencing: In order to elucidate the mechanisms underlying the increase in exosome biogenesis observed in cancer cells, Colombo et al. performed a set of iconic experiments to evaluate the role of the different ESCRT components in exosome biogenesis using Hela-CIITA-OVA ovarian cancer cells, which were modified to express MHC-II molecules and allowed for monitoring of exosome secretion [252]. Using this modified cell line, they carried out experiments to silence all twenty-three ESCRT proteins [252]. The silencing of ESCRT-0/I (STAM1, Hrs, and TSG101) resulted in decreased production of CD63, CD81, and MHC-II in exosomes, whereas silencing of ESCRT-III (Vps4B) or ALIX led to increased production and secretion of all exosomes [253]. Three other independent studies, where Hrs (an ESCRT-0 component) was silenced, also led to alterations in exosome secretion [254]. Specifically, the depletion of Hrs in non-cancerous dendritic cells led to a reduction in exosome secretion as measured by the detection of ubiquitinated proteins [254], whereas knockdown of Hrs in HEK293 kidney cancer cells and in head and neck squamous cell carcinoma led to increased exosome secretion and exosomal Wnt3a levels [255]. These studies demonstrated that alterations in ESCRT proteins directly influence exosome production, which may partially help explain their increased production in cancer cells (Figure 4).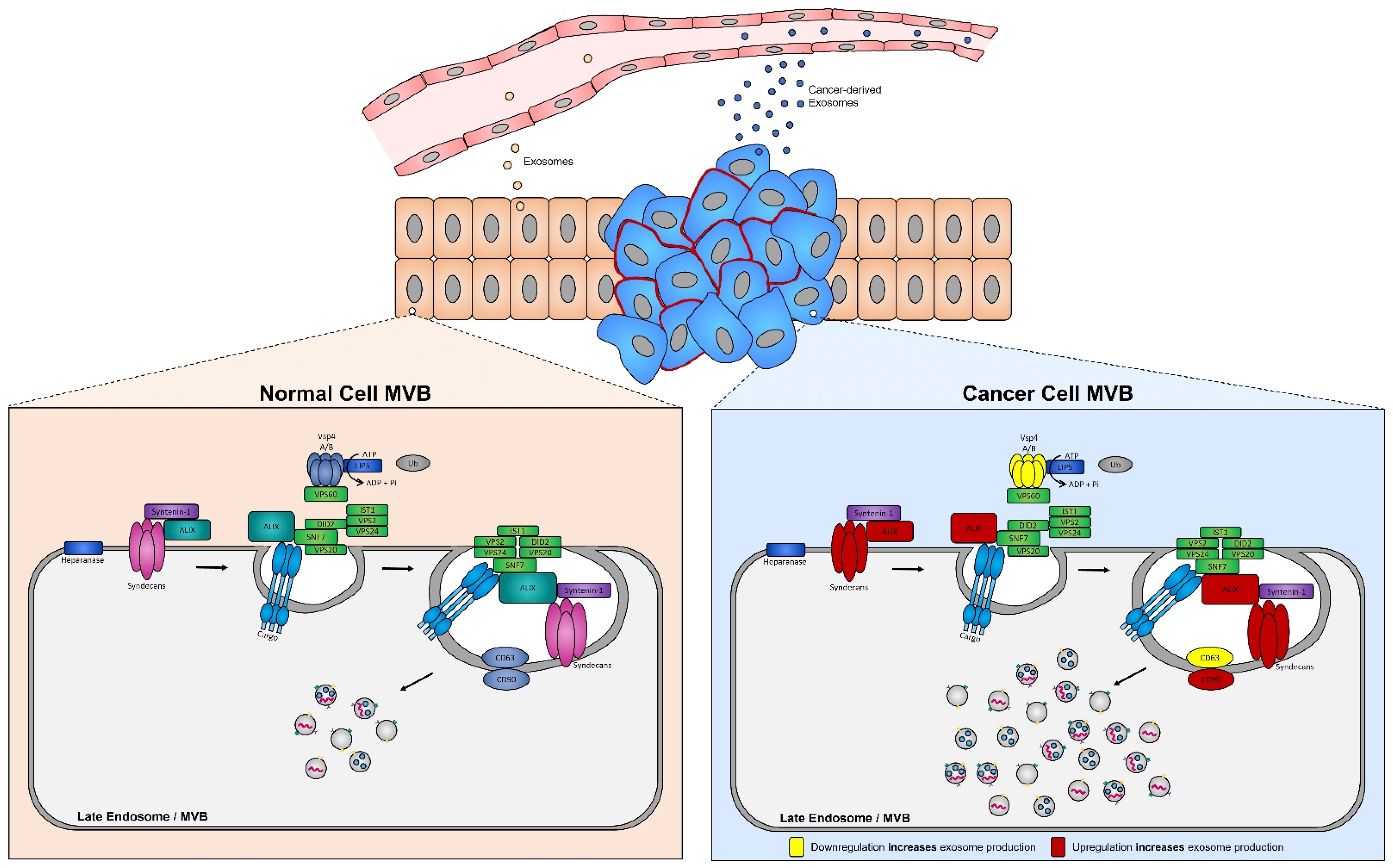
Figure 4. Syndecan–syntenin–ALIX couples to ESCRT-dependent MVB formation in healthy versus cancer cells. Syntenin-1 interacts directly with syndecans and ALIX, the interaction of ALIX with Snf7 of the ESCRT-III complex forms the syndecan–syntenin–ALIX pathway which is directly linked to exosome biogenesis. In cancer, several proteins in this pathway are altered, leading to enhanced exosome production. Alterations leading to the upregulation of either the syndecans, ALIX, and/or CD90 all result in enhanced exosome production, whereas any alterations leading to the downregulation of the ESCRT-III protein Vsp4 A/B and/or CD63 results in the increased production of exosomes seen in cancer cells.
3. Exosomes: A Source of Tumor Biomarkers
Valadi et al. were the first to determine that exosomes carry messenger RNAs (mRNAs) and microRNAs (miRNAs) [88], but since then many studies have demonstrated that exosomes contain a plethora of other RNA species (long-noncoding RNA (lncRNA), piwi RNAs, circular RNAs, etc.) [274]. Exosome whole-transcriptome studies have demonstrated that mRNA molecules are full length and well-protected from RNase activities within circulating exosomes [275]. Some studies have shown that exosomal mRNAs can be transcribed by recipient cells and are possibly used for reprogramming these cells [276]. However, the majority of exosome transcriptomic studies have been performed on microRNAs, because their function as post-transcriptional regulators allows them to control the recipient cells upon delivery [277]. Additional studies on the content of exosomes have also revealed the presence of fragmented genomic DNA, proteins, and lipids from both normal and cancer cells [278]. Fragmented genomic DNA, for example, which is more stable (i.e., protected from degradation) within circulating exosomes than in the circulation, can provide critical biomarkers for the detection of circulating tumor DNA mutations [279]. Castellanos-Rizaldos et al. recently showed that the detection of circulating genomic DNA carrying the EGFRT790M mutation in patients with non-small cell lung cancer (NSCLC) was superior when isolated from circulating exosomes rather than circulating free DNA (cfDNA), particularly in patients with metastatic stage 0/1a lung cancer [126,280]. Overall, several free databases, including Vesiclepedia [281], EVpedia [282], ExoCarta [283] exoRBase [284], and EVmiRNA [285], have been made available for screening the transcriptomes, proteomes and lipidomes of exosomes from different cell types and tissues. For the purposes of this review, we will concentrate our evaluation of exosome biomarkers for known microRNAs, proteins, and lipids.3.1. MicroRNA Biomarkers
MiRNAs represent a large class (~2600 identified in humans) of evolutionarily conserved small regulatory non-coding RNAs expressed from single genes, gene clusters, or intronic passengers, generally 19–25 nucleotides (nt) in length [277]. MiRNAs control gene expression by binding to imperfect complementary sites within the 3′ untranslated regions of their mRNA targets and orchestrate their degradation and/or post-transcriptional repression [286,287]. MiRNAs are involved in the control of all normal biological processes and their expression deregulation (i.e., increased or decreased expression) has been associated with the initiation and development of cancers [288,289,290,291]. Mature and functional cellular miRNAs can be detected in the blood and other biological fluids and can generally be found in two forms: either as cell/membrane-free molecules (i.e., free, bound to Argonaute (Argo) or to nucleophosmin proteins, complexed with high density lipoprotein (HDL)) or encapsulated within extracellular vesicles [274,291,292].3.1.1. Current Technologies for Quantification of Exosomal MicroRNAs
Since the cargo of exosomes is specifically tailored and to an extent reflects the state and condition of their cells of origin, circulating miRNAs have a potentially strong diagnostic and prognostic value as disease biomarkers [293]. Considering the low amount of material available from exosomes, which may be extracted from limited amounts of biofluids (saliva, sweat, urine, semen, exhaled breath condensates, etc.), current technologies and protocols have been optimized for the global quantification and detection of exosomal miRNAs [294].High-Throughput Expression Analyses
Next generation sequencing offers a unique unbiased opportunity to globally evaluate the content of exosomes [295,296]. It is important to note that miRNA quantification of cell specific exosomes, either from culture media or biofluids, requires highly purified exosomes [149], because miRNAs are extremely robust [297] and any external contamination can significantly affect quantification. Most next generation miRNA sequencing protocols require relatively high quantities of starting material; for instance, the Illumina TruSeq small-RNA sequencing platform requires a minimum of 10–50 ng of purified small-RNA (Illumina TruSeq small-RNA protocol) or 1000 ng of total RNA [298]. As such, many studies have been focused on global exosomal miRNA purifications from blood or biofluids, which provide workable amounts of total RNA [296,299]. In these studies, exosomes have been rarely isolated from plasma volumes less than 1 mL, and typically these isolations are carried out by means of ultracentrifugation, which yields higher exosome quantities [112]. However, currently many researchers are driving the development towards the utilization of optimized protocols adapted for NGS analysis of low miRNA abundant exosome sub-populations [300]. To this end, ourne laboratory developed and optimized a cDNA library preparation protocol which allows for 3′ barcoded miRNAs from up to 18 individual samples to be pooled prior to 5′ adapter ligation, reverse transcription, and PCR amplification (~15 to 20 cycles decided via a pilot PCR reaction), which increases the carrier effect of the combined RNA for subsequent enzymatic reactions and purifications [301,302]. This protocol was purposely designed for the analysis of samples with minute quantities of total RNA, and wthese researchers demonstrated its robustness for exosomal small-RNA analyses with total RNA input <1.5 ng [149]. Using the EV-CATCHER assay for highly pure selection of CD63 exosomes from the serum of patients infected with the SARS-CoV-2 virus and admitted to peourple's health network, we wereit is able to detect miRNA expression differences in miR-146a and miR-126-3p between COVID-19 patients hospitalized with mild symptoms that did not receive mechanical ventilation and COVID-19 patients hospitalized with severe respiratory symptoms that received mechanical ventilation, within levels estimated at ~25 zeptomoles (10 e-21 mole), which wethese researchers successfully validated by qPCR [149]. Although the detection miRNAs from low-input total RNA is challenging, outheir experiments strongly indicate that the specificity and purity of the exosome preparation is critical for the successful detection of unique and rare disease miRNA biomarkers. Of note, the Nanostring nCounter miRNA platform is an advancing technology for the sensitive, reproducible, and highly multiplexed analysis of up to 800 pre-selected miRNAs. The nCounter miRNA platform is based on a novel method of direct molecular barcoding and digital detection using color-coded probe-pairs [303]. This technology relies on the use of unique miRtags (i.e., oligonucleotide tags), which are ligated to the 3′ end of specific miRNAs. Sequence specificity during ligation is ensured through stepwise controlled annealing and ligation temperature in addition to the use of Tm-optimized bridging oligonucleotides complementary to a portion of the target miRNA and miRtag [304]. To date, a few studies have employed this technology for the quantification of miRNAs from plasma-derived ultracentrifuged exosomes [305]. This methodology is very attractive because it bypasses PCR amplification, which is required during the preparation of small-RNA cDNA libraries, but it may not offer the proper sensitivity yet for the analysis of molecules in the zeptamole concentration range.Quantitative PCR (qPCR) Analyses
Currently there are numerous commercially available platforms and qPCR-based assays for the analysis of miRNAs (i.e., TaqMan miRNA assays (Thermo Fisher), TaqMan Array microRNA 384-well cards (Thermo Fisher, Tokyo, Japan), OpenArray (Thermo Fisher), SmartChip (Takara, Kusatsu City, Japan), miScript miRNA PCR arrays (Qiagen, Hilden, Germany), miRCURY™ LNA™ Universal RT microRNA PCR (Exiqon, Vedbæk, Denmark), and miRNA Oligo chip (3D Gene)). Quantitative PCR technology is still considered the gold standard for validation of findings made during high-throughput analyses; however, it has inherent biological and experimental limitations for exosomal miRNA studies, which include: (1) The discovery of tumor miRNA panels specific to a tumor exosome remains limited to known or selected miRNAs (which can be multiplexed for up to 700 miRNAs using TaqMan card arrays) rather than global unbiased NGS analyses. In this fashion it does not allow discovery of IsomiR (i.e., miRNA variants of the same miRNA, which can differ in sequence and/or length composition and will bind to different mRNA targets than the original miRNA sequence [306]) expression ratio differences for a specific miRNA in exosome sub-populations. The study of isomiRs is relevant in cancer, as their expression ratio has been shown to be affected by and can elicit changes in tumor cell migration and invasion [307]. (2) qPCR assays typically require large inputs of RNAs for global evaluation and may still require a pre-amplification step (i.e., typically ~14 cycles) before the 40-cycle amplification for fluorescent quantification, which leaves room for potential amplification bias or errors. Most importantly, even when using single qPCR assays, the sensitivity of detection may not be sufficient for miRNA expression validation of total RNA extracted from a selected subpopulation of circulating exosomes. In fact, wea group haves previously demonstrated that the validation of differentially expressed exosome miRNAs, identified by NGS below 25 zeptomoles, using RT-qPCR is challenging as this quantification lies within the threshold of detection (Ct values of ~36–39) [149]. Studies that have been performed using total RNA extracted from globally circulating exosomes for NSCLC [308], prostate cancer [309], metastatic colorectal cancer [310], and gastric cancer [311] have all failed to provide robust, reproducible, and diagnostic cancer-specific miRNA signatures. The lack of technical standardization and the total RNA purification from globally purified exosomes from plasma (i.e., most of them likely released by blood cells) may have contributed to these limitations.Droplet Digital PCR (ddPCR) Analysis
Droplet digital PCR (ddPCR) technology has thus become very popular because of its inherent capability for the ultra-sensitive detection of rare molecules [312]. This technology enables absolute quantification by partitioning the reaction into individual droplets (i.e., using water-in-oil and microfluidic technology), wherein molecules are randomly distributed, with each droplet/compartment containing zero, one, or many molecules. In essence this allows for amplification of each compartmentalized PCR mix/target molecule(s) to occur independently of each other [313]. Following amplification, the absorbance of each droplet is counted [314] and using Poisson statistics and the number of positive reactions (i.e., compartments which contain molecules and thus positive signal) the initial copy number and target density are calculated. ddPCR relies on the analysis of individual droplet amplitude and scatter for the absolute quantification of nucleic acids [315]. BioRad has become the leader in ddPCR technology and currently markets their award-winning QX200 AutoDG ddPCR instrument for either EvaGreen or probe-based digital PCR applications. The instrument’s specifications indicate that in each 100 µL volume, up to 20,000 droplets may be generated; generally data from 12,000–16,000 droplets are used for accurate quantification of target molecules (https://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_6407.pdf (accessed on 25 May 2022)). Wang et al. recently demonstrated that the analysis of miRNA targets from exosomes (i.e., purified from urine), when compared with traditional TaqMan qPCR technology, was significantly more accurate and sensitive for the detection of low concentrations (i.e., <100 copies/µL) [316]. However, although Bio-Rad offers a large array of mRNA-based detection using their ddPCR instrument, they have not established a standardized protocol for the analysis of miRNAs, and the only available protocols are from published studies [317,318]. The protocol of Hindson et al. suggests that using ddPCR with TaqMan probes improved the day-to-day reproducibility by 7-fold, as seen by a 37–86% decrease in the coefficient of variation, compared to standard qPCR [315]. Considering that tumor exosome miRNA biomarkers may be within or below the zeptomole concentration in a given biofluid, ddPCR technology offers a unique opportunity for the development of ultra-low, -sensitive, and -specific clinical assays and the detection of rare tumor-specific circulating exosomal miRNAs. Standardization of both the tumor exosome isolation techniques (i.e., antigen-based capture, nanoparticle enumeration) and miRNA ddPCR assay preparation will be critical.3.1.2. Function of MicroRNA in Cancer Exosomes
Exosomal miRNAs from tumor exosomes have been shown to exert a multitude of biological effects during cancer progression which include: (1) the regulation of tumor growth, (2) The evasion from host immune responses, and (3) remodeling of the tumor microenvironment and metastasis.Regulation of Tumor Growth
Cellular proliferation is a critical aspect to tumor progression, which commonly is a result of the deregulation of cell-cycle related proteins [316], and is a known hallmark of cancer [22]. Research studies have demonstrated that tumor-derived exosomal miRNAs can regulate cancer cell proliferation by targeting cell cycle-associated proteins and signaling proteins [317]. For example, in colorectal cancer, miR-200b is transferred between adjacent cancer cells, where it directly targets 3′-UTRs of p27 and Rho Family GTPase 3 (RND3), two proteins involved in cell cycle regulation [318,319], leading to their downregulation, which in turn induces proliferation in recipient cells [320]. Contrarily, miR-6869-5p, known to act as a tumor suppressor [321], is significantly downregulated in serum-derived exosomes originating from colorectal cancer cells [322]. The downregulation of exosomal miR-6869-5p delivery is associated with enhanced cell proliferation and increased production of the inflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) [323]. In metastatic breast cancer, the transfer of exosomal miR-1246 to non-malignant breast cells promotes proliferation, migration, and drug resistance through the direct downregulation of cyclin-G2 (CCNG2) [321]. Moreover, exosomal miRNAs profoundly regulate the apoptotic signaling pathway in cancer cells; for example, Jing et al. demonstrated that the delivery of exosomal miR-769-5p to gastric cancer cells directly targets caspase-9, a cysteine-aspartic protease that serves as the initiator of the intrinsic apoptosis pathway [324], leading to the inhibition of the downstream caspase-dependent apoptotic pathway, and promoting the degradation of the tumor suppressor protein p53 through the ubiquitin–proteosome pathway [325]. Furthermore, in breast cancer, the uptake of exosomal miR-128 leads to a reduction in the expression of the pro-apoptotic protein Bcl-2-associated X protein (Bax) in recipient breast cancer cells [326].Evasion from Host Immune Responses
Exosomal miRNAs have been shown to function as critical mediators in the crosstalk between cancer cells and macrophages within the tumor microenvironment [327]. Macrophages can be divided into two classes: either M1-polarized or M2-polarized depending on their function [328]. M1 macrophages inhibit tumor growth whereas M2 macrophages have been demonstrated to provide immunosuppression around the tumor niche, while promoting tumor development and metastasis [329]. Exosomes secreted from the SKOV3 epithelial ovarian cancer cell line and containing miR-222-3p can induce macrophage polarization and differentiation to the M2 phenotype by suppression of the cytokine signaling 3 (SOCS3)/STAT3 pathway, which results in tumor growth and enhanced metastatic capability [330]. Similarly, exosomes from hypoxic ovarian cancer cells, present in the center of tumors, deliver miR-940 to macrophages and promote their polarization to the M2 phenotype, which results in tumor progression [331]. In a similar manner, hypoxic glioma-derived exosomes promote M2 macrophage polarization through the delivery of miR-1246, which targets TERF2 interacting protein (TER2IP) via the STAT3 and NF-κB pathways [332]. Dendritic cells are antigen presenting cells (APCs) linking innate and adaptive immunity which express a wide range of toll-like receptors (TLRs) and cytokines [333] that are critical for the activation of host immune responses to pathogens [334], including recognition of cancer cells [335]. In pancreatic cancer, the delivery of exosomes containing miR-203 can inhibit the expression of TLR4 and the production of cytokines, including TNF-α and interleukin-12 (IL-12) in dendritic cells [336], leading to dendritic cell dysfunction, which facilitates cancer cell evasion of the host’s immune response [337]. In addition to the effects that exosomal miRNAs have on macrophages and dendritic cells, they have also been shown to have profound effects on natural killer (NK) cells [338]. NK cells are a part of the innate immune system and can directly kill tumor cells [339] through the release of perforins and granzymes from their cytoplasm [340]. Briand et al. demonstrated that radiotherapy induced the release of exosomes containing increased levels of miR-378a-3p from a variety of cancer cell types, which resulted in the decreased release of granzyme-B from NK cells [341]. Natural killer group 2D receptor (NKG2D) is an activating receptor that is expressed on NK cells, which plays a pivotal role in tumor immunosurveillance [342]. Under hypoxic conditions, the IGR-Heu lung carcinoma and K562 myelogenous leukemia cell lines release exosomes containing increased levels of miR-23a, which when transferred to NK cells results in decreased expression of NKG2D, and thus reduced NK function [343].Tumor Microenvironment Remodeling and Metastasis
The initiation of metastasis typically occurs when cancer cells undergo a process of epithelial-to-mesenchymal transition (EMT), wherein non-motile epithelial cells transform to a motile/invasive mesenchymal cell type [344]. Once these cells detach from primary tumors and travel through blood vessels, they establish the metastatic niche. Significant evidence suggests that tumor exosomes with their miRNA cargoes play a profound regulatory role in the metastatic process by controlling EMT, angiogenesis (i.e., formation of new blood vessels), niche cell education [345], and tumor cell invasion (Figure 1). Cai et al. demonstrated that exosomes isolated from glioblastoma patients carried increased levels of miR-148a compared to exosomes from healthy volunteers [346]. Exosomal delivery of miR-148a to T98G glioblastoma cells results in cell proliferation and metastasis by downregulation of the cell adhesion molecule 1 (CADM1) and activation of the STAT3 pathway. In triple negative breast cancer, exosomal miR-122 released from breast cancer cells has been shown to reduce the glycolytic pyruvate kinase PKM, and thus decreases glucose uptake in non-tumorigenic cells within the pre-metastatic niche, allowing for increased nutrient availability within the pre-metastatic niche and promoting survival of breast cancer cells that have metastasized [347]. In melanoma, tumor cells exchange miR-222 via exosomes to enhance tumor malignancy by the activation of the phosphoinositide 3-kinase (PI3K)/Akt pathway, via the downregulation of its target gene p27Kip1 [348]. Bone marrow-derived stem cells (BMSCs) present in the hypoxic tumor microenvironment are known to contribute to cancer progression [349]. In lung cancer, hypoxic BMSC-derived exosomes shuttle miR-193a-3p, miR-210-3p, and miR-5100 to lung epithelial cancer cells, which promotes their metastasis via the activation of STAT3 pathway-mediated EMT [350]. Moreover, Yang et al. demonstrated that hepatocarcinoma (HCC) cells with high metastatic potential are able to transfer exosomes containing miR-92a-3p to HCC cells with low metastatic potential, by promoting the EMT-mediated PEN/Akt pathway, as demonstrated by the increased expression of mesenchymal biomarkers (N-cadherin, β-catenin, Snail) and decreased expression of E-cadherin [275]. Altogether, these experiments demonstrate the powerful role that exosomes play in the intercellular communication of cancer cells for the self-propagation of cancer. Altogether, these studies have demonstrated that cancer cells take advantage of exosomes to transfer tumorigenic miRNAs that can reprogram (i) adjacent tumor cells to promote tumor growth and (ii) a plethora of critical immune cells, to protect the tumor, and (iii) educate pre-metastatic niche cells for the formation of secondary tumor sites before metastasis. However, circulating cancer exosome studies conducted on the same human cancer types have not systematically identified the same deregulated miRNAs, globally suggesting that the miRNA packaging of exosomes may help differentiate healthy individuals from cancer patients (See Table 1). Along with the design of ultra-sensitive and specific cancer exosome purification assays, these studies highlight the potential clinical applications of exosome-based miRNA signatures for non-invasive diagnostic and prognostic evaluation of human cancers.Table 1. Published miRNA exosome cancer biomarkers identified in human biofluids. Lists of exosomal miRNAs circulating in biofluids of patients diagnosed with colorectal cancer, ovarian cancer, Glioblastoma, liver cancer, pancreatic cancer, lung cancer, extranodal natural killer/T-cell lymphoma, and prostate cancers.
| Cancer Type | Differentially Expressed Between Healthy and Cancer | Reference | |
|---|---|---|---|
| miRNA Biomarkers | |||
| Colorectal Cancer | ↑ | miR-224-5p, miR-548d-5p, miR-200a-3p, miR-320d, miR-200b-3p, miR-1246 | Tang et al., 2019 [310] |
| ↓ | novel_246, novel_301, miR-27a-5p | ||
| miR-135a-5p, miR-204-5p | Sun et al., 2021 [104] | ||
| ↓ | miR-6869-5p | Yan et al., 2018 [321] | |
| ↑ | miR-486-5p, miR-3180-5p | Yan et al., 2017 [322] | |
| ↓ | miR-638, miR-5787, miR-8075, miR-6869-5p, miR-548c-5p | ||
| Ovarian Cancer | ↑ | miR-21, miR-141, miR-200a, miR-200b, miR-200c, miR-203, miR-205, miR-214, miR-215 | Taylor and Taylor, 2008 [89] |
| ↑ | miR-940 | Chen et al., 2017 [331] | |
| ↑ | miR-222-3p | Ying et al., 2016 [330] | |
| Glioblastoma | ↑ | let-7a, miR-15b, miR-16, miR-19b, miR-21, miR-26a, miR-27a, miR-92, miR-93, miR-320, miR-20 | Skog et al., 2008 [91] |
| ↑ | miR-148a | Cai et al., 2018 [346] | |
| Liver Cancer | ↑ | miR-17, miR-18a, miR-19a, miR-19b, miR-20a, miR-92a-3p | Yang et al., 2020 [276] |
| ↑ | miR-193a-3p, miR-210-3p, miR-5100 | Zhang et al., 2019 [350] | |
| Pancreatic Cancer | ↑ | miR-21, miR-210 | Wu et al., 2020 [294] |
| ↑ | miR-193a-3p, miR-210-3p, miR-5100 | Zhang et al., 2019 [350] | |
| Lung Cancer | ↑ | miR-132-3p, miR-181b-5p, miR-27a-3p, miR-27b-3p, miR-320a, miR-361-5p, let-7b-5p, miR-24-3p, miR-3184-5p, miR-486-5p, miR-486-3p, miR-320b | Jin et al., 2017 [295] |
| ↓ | let-7a-5p, let-7d-5p, let-7f-5p, miR-26b-5p, miR-30a-3p, miR-30e-3p, miR-744-5p, miR-744-5p, let-7e-5p, miR-191-5p, miR-191-5p, miR-206, miR-21-5p, miR-23a-5p, miR-23b-5p, miR-10b-5p, miR-15b-5p | ||
| miR-30b, miR-30c, miR-103, miR-122, miR-195, miR-203, miR-221, miR-222 | Giallombardo et al., 2016 [308] | ||
| ↑ | miR-193a-3p, miR-210-3p, miR-5100 | Zhang et al., 2019 [350] | |
| Extranodal Natural Killer/T-Cell Lymphoma | ↑ | miR-320e, miR-4454, miR-4516, miR-630, miR-122-5p, miR-574-5p, miR-22-3p, miR-486-3p, miR-1915-5p, miR-1972, miR-1285-5p, miR-222-3p, miR-1305, miR-891b, miR-4455, miR-21-5p, miR-1258, let-7b-5p, miR-25-3p, miR-1268a | Ryu et al., 2020 [305] |
| ↓ | miR-564, miR-196a-5p, miR-520c-3p, let-7d-5p, let-7i-5p, miR-212-3p, miR-29a-3p, miR-608, miR-503-5p, miR-587, miR-548g-3p, miR-765, miR-34c-3p, miR-770-5p, miR-301a-5p, miR-526a, miR-340-5p, miR-325, miR-199a-3p+miR-199b-3p, miR-423-3p | ||
| Prostate Cancer | ↓ | miR-196a-5p, miR-34a-5p, miR-501-3p, miR-92a-1-5p | Rodríguez et al., 2017 [309] |
↑ = miRNA expression observed in circulating exosomes isolated from cancer patients as compared to circulating exosomal miRNAs isolated from healthy individuals and ↓ = decreased miRNA expression observed in circulating exosomes isolated from cancer patients as compared to circulating exosomal miRNAs isolated from healthy individuals.
