Flavonoids have been shown to be excellent natural agents useful in the treatment of various skin lesions with minimal side effects. Topical application is the best option for targeted use due to their lipophilic nature. On the other hand, the polyhydroxyl structure determines their antibacterial, antifibrotic, antioxidant, and anti-inflammatory properties. Prenylated Flavonoids are a subclass of flavonoids modified with at least one lipophilic side chain of varying length. They attract the attention of scientists because of their promising biological activities, such as antibacterial, antifungal, estrogenic, immunosuppressive, anticancer, anti-inflammatory, antioxidant, antiviral, larvicidal, osteogenic, antiallergic, and cytotoxic.
- antibacterial
- anti-inflammatory
- prenylated flavonoids
- skin
- wound healing
1. Flavonoids as Effective Anti-Infective and Wound Healing Agents
2. Flavonoids as Candidates for Therapy of Skin Lesions
3. Prenylated Flavonoids
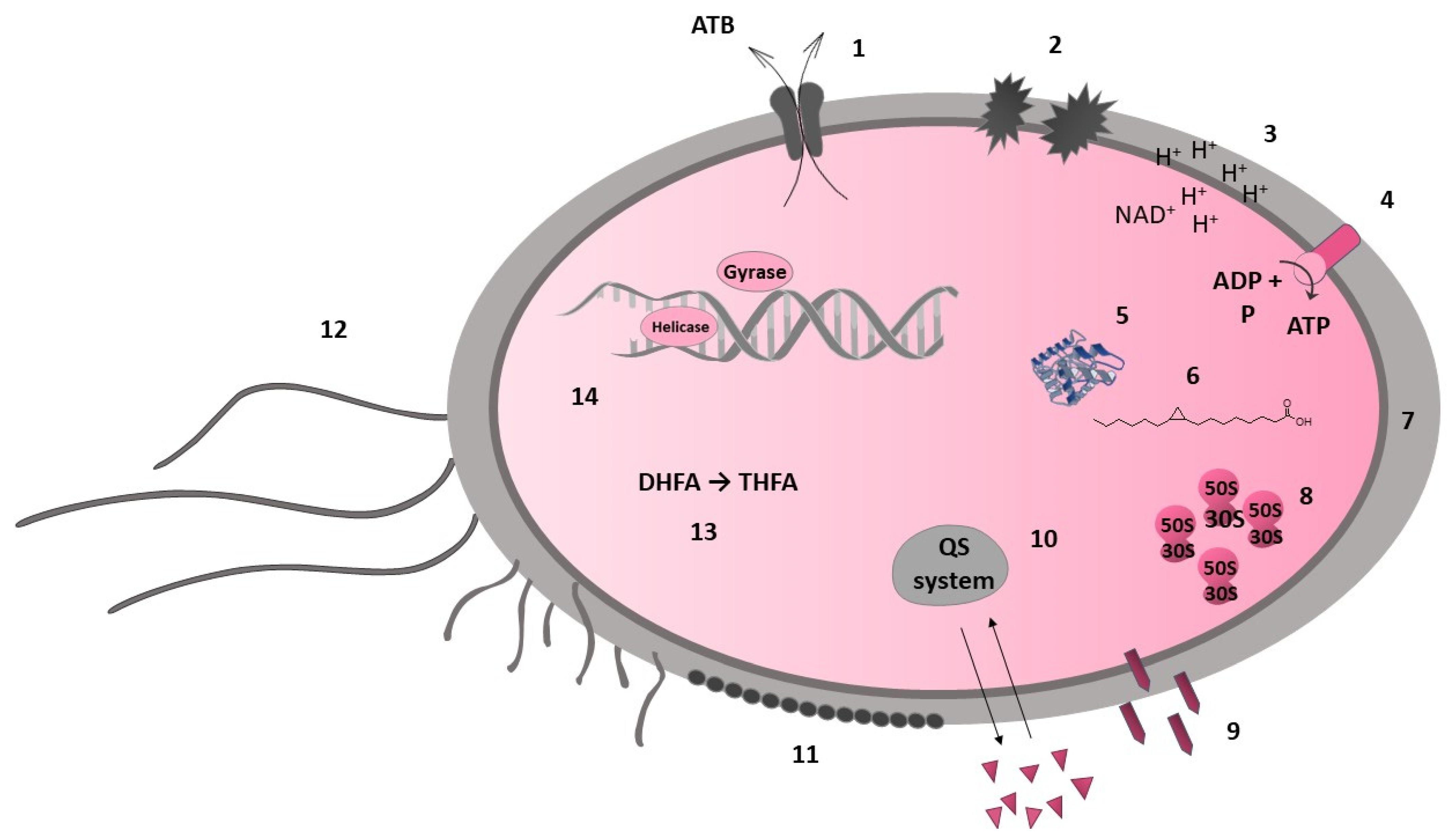
4. Mechanisms of Antibacterial Activity
4.1. Direct Interaction with Bacterial Cell
4.2. Indirect Antimicrobial Activity
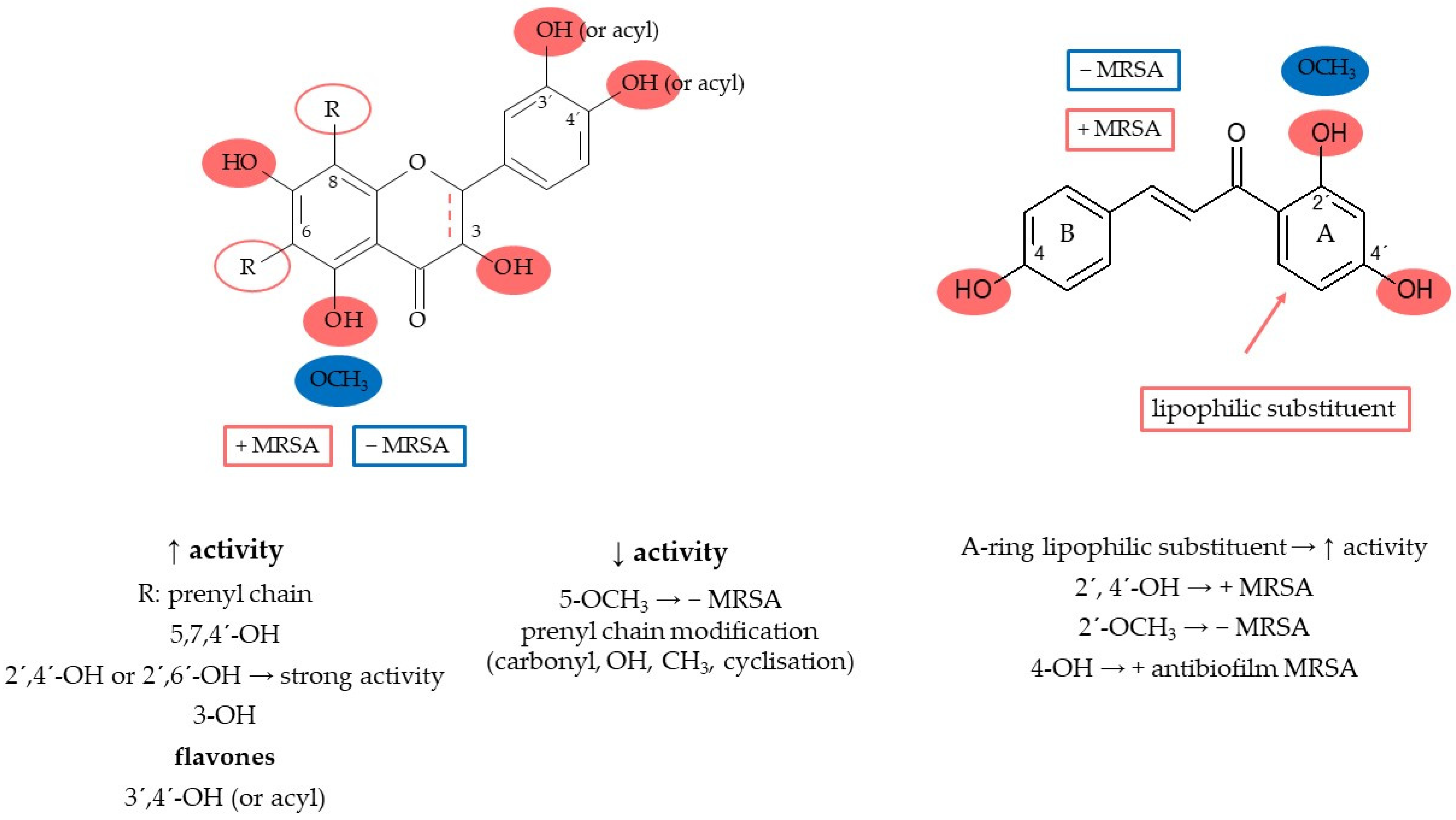
5. Structure–Activity Relationship
References
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, Biological Functions, and Biotechnological Applications. Front. Plant Sci. 2012, 3, 222.
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272.
- Sarbu, L.G.; Bahrin, L.G.; Babii, C.; Stefan, M.; Birsa, M.L. Synthetic Flavonoids with Antimicrobial Activity: A Review. J. Appl. Microbiol. 2019, 127, 1282–1290.
- Tsala, D.E.; Amadou, D.; Habtemariam, S. Natural Wound Healing and Bioactive Natural Products. Phytopharmacology 2013, 4, 532–560.
- Carvalho, M.T.B.; Araújo-Filho, H.G.; Barreto, A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S.; Barreto, R.S.S. Wound Healing Properties of Flavonoids: A Systematic Review Highlighting the Mechanisms of Action. Phytomedicine 2021, 90, 153636.
- Nagula, R.L.; Wairkar, S. Recent Advances in Topical Delivery of Flavonoids: A Review. J. Control. Release 2019, 296, 190–201.
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial Activity and Mechanism of Plant Flavonoids to Gram-Positive Bacteria Predicted from Their Lipophilicities. Sci. Rep. 2021, 11, 10471.
- Yang, X.; Jiang, Y.; Yang, J.; He, J.; Sun, J.; Chen, F.; Zhang, M.; Yang, B. Prenylated Flavonoids, Promising Nutraceuticals with Impressive Biological Activities. Trends Food Sci. Technol. 2015, 44, 93–104.
- Chen, X.; Mukwaya, E.; Wong, M.S.; Zhang, Y. A Systematic Review on Biological Activities of Prenylated Flavonoids. Pharm. Biol. 2014, 52, 655–660.
- Barron, D.; Ibrahim, R.K. Isoprenylated Flavonoids—A Survey. Phytochemistry 1996, 43, 921–982.
- Šmejkal, K. Cytotoxic potential of C-prenylated flavonoids. Phytochem. Rev. 2014, 13, 245–275.
- Chen, Y.; Zhao, J.; Qiu, Y.; Yuan, H.; Khan, S.I.; Hussain, N.; Iqbal Choudhary, M.; Zeng, F.; Guo, D.A.; Khan, I.A.; et al. Prenylated Flavonoids from the Stems and Roots of Tripterygium wilfordii. Fitoterapia 2017, 119, 64–68.
- Al-Rehaily, A.J.; Albishi, O.A.; El-Olemy, M.M.; Mossa, J.S. Flavonoids and Terpenoids from Helichrysum forskahlii. Phytochemistry 2008, 69, 1910–1914.
- Sun, Q.; Wang, D.; Li, F.F.; Yao, G.D.; Li, X.; Li, L.Z.; Huang, X.X.; Song, S.J. Cytotoxic Prenylated Flavones from the Stem and Root Bark of Daphne giraldii. Bioorganic Med. Chem. Lett. 2016, 26, 3968–3972.
- Chang, S.K.; Jiang, Y.; Yang, B. An Update of Prenylated Phenolics: Food Sources, Chemistry and Health Benefits. Trends Food Sci. Technol. 2021, 108, 197–213.
- Mukai, R. Prenylation Enhances the Biological Activity of Dietary Flavonoids by Altering Their Bioavailability. Biosci. Biotechnol. Biochem. 2018, 82, 207–215.
- Hatano, T.; Shintani, Y.; Aga, Y.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Phenolic Constituents of Licorice. VIII. Structures of Glicophenone and Glicoisoflavanone, and Effects of Licorice Phenolics on Methicillin-Resistant Staphylococcus aureus. Chem. Pharm. Bull. 2000, 48, 1286–1292.
- Dong, W.K.; Yeon, S.C.; Son, K.H.; Hyeun, W.C.; Ju, S.K.; Sam, S.K.; Hyun, P.K. Effects of Sophoraflavanone G, a Prenylated Flavonoid from Sophora flavescens, on Cyclooxygenase-2 and in Vivo Inflammatory Response. Arch. Pharm. Res. 2002, 25, 329–335.
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356.
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. D-Alanine:D-Alanine Ligase as a New Target for the Flavonoids Quercetin and Apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426.
- Sirk, T.W.; Brown, E.F.; Sum, A.K.; Friedman, M. Molecular Dynamics Study on the Biophysical Interactions of Seven Green Tea Catechins with Lipid Bilayers of Cell Membranes. J. Agric. Food Chem. 2008, 56, 7750–7758.
- Kusuda, M.; Inada, K.; Ogawa, T.O.; Yoshida, T.; Shiota, S.; Tsuchiya, T.; Hatano, T. Polyphenolic Constituent Structures of Zanthoxylum piperitum Fruit and the Antibacterial Effects of Its Polymeric Procyanidin on Methicillin-Resistant Staphylococcus aureus. Biosci. Biotechnol. Biochem. 2006, 70, 1423–1431.
- Arakawa, H.; Kanemitsu, M.; Tajima, N.; Maeda, M. Chemiluminescence Assay for Catechin Based on Generation of Hydrogen Peroxide in Basic Solution. Anal. Chim. Acta 2002, 472, 75–82.
- Arakawa, H.; Maeda, M.; Okubo, S.; Shimamura, T. Role of Hydrogen Peroxide in Bactericidal Action of Catechin. Biol. Pharm. Bull. 2004, 27, 277–281.
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal Catechins Damage the Lipid Bilayer. BBA-Biomembr. 1993, 1147, 132–136.
- Miura, Y.H.; Tomita, I.; Watanabe, T.; Hirayama, T.; Fukui, S. Active Oxygens Generation by Flavonoids. Biol. Pharm. Bull. 1998, 21, 93–96.
- Cushnie, T.P.T.; Lamb, A.J. Detection of Galangin-Induced Cytoplasmic Membrane Damage in Staphylococcus aureus by Measuring Potassium Loss. J. Ethnopharmacol. 2005, 101, 243–248.
- Mirzoeva, O.K.; Grishanin, R.N.; Calder, P.C. Antimicrobial Action of Propolis and Some of Its Components: The Effects on Growth, Membrane Potential and Motility of Bacteria. Microbiol. Res. 1997, 152, 239–246.
- Tsuchiya, H.; Iinuma, M. Reduction of Membrane Fluidity by Antibacterial Sophoraflavanone G Isolated from Sophora exigua. Phytomedicine 2000, 7, 161–165.
- Haraguchi, H.; Tanimoto, K.; Tamura, Y.; Mizutani, K.; Kinoshita, T. Mode of Antibacterial Action of Retrochalcones from Glycyrrhiza inflata. Phytochemistry 1998, 48, 125–129.
- Chinnam, N.; Dadi, P.K.; Sabri, S.A.; Ahmad, M.; Kabir, M.A.; Ahmad, Z. Dietary Bioflavonoids Inhibit Escherichia coli ATP Synthase in a Differential Manner. Int. J. Biol. Macromol. 2010, 46, 478–486.
- Bensasson, R.V.; Zoete, V.; Jossang, A.; Bodo, B.; Arimondo, P.B.; Land, E.J. Potency of Inhibition of Human DNA Topoisomerase i by Flavones Assessed through Physicochemical Parameters. Free Radic. Biol. Med. 2011, 51, 1406–1410.
- Ohemeng, K.A.; Schwender, C.F.; Fu, K.P.; Barrett, J.F. DNA Gyrase Inhibitory and Antibacterial Activity of Some Flavones(1). Bioorganic Med. Chem. Lett. 1993, 3, 225–230.
- Bernard, F.X.; Sablé, S.; Cameron, B.; Provost, J.; Desnottes, J.F.; Crouzet, J.; Blanche, F. Glycosylated Flavones as Selective Inhibitors of Topoisomerase IV. Antimicrob. Agents Chemother. 1997, 41, 992–998.
- Xu, H.; Ziegelin, G.; Schröder, W.; Frank, J.; Ayora, S.; Alonso, J.C.; Lanka, E.; Saenger, W. Flavones Inhibit the Hexameric Replicative Helicase RepA. Nucleic Acids Res. 2001, 29, 5058–5066.
- Mori, A.; Nishino, C.; Enoki, N.; Tawata, S. Antibacterial Activity and Mode of Action of Plant Flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry 1987, 26, 2231–2234.
- Navarro-Martínez, M.D.; Navarro-Perán, E.; Cabezas-Herrera, J.; Ruiz-Gómez, J.; García-Cánovas, F.; Rodríguez-López, J.N. Antifolate Activity of Epigallocatechin Gallate against Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2005, 49, 2914–2920.
- Havsteen, B.H. The Biochemistry and Medical Significance of the Flavonoids. Pharmacol. Ther. 2002, 96, 67–202.
- Tasdemir, D.; Lack, G.; Brun, R.; Rüedi, P.; Scapozza, L.; Perozzo, R. Inhibition of Plasmodium falciparum Fatty Acid Biosynthesis: Evaluation of FabG, FabZ, and FabI as Drug Targets for Flavonoids. J. Med. Chem. 2006, 49, 3345–3353.
- Cushnie, T.P.T.; Lamb, A.J. Recent Advances in Understanding the Antibacterial Properties of Flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107.
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Suppression of Bacterial Cell-Cell Signalling, Biofilm Formation and Type III Secretion System by Citrus Flavonoids. J. Appl. Microbiol. 2010, 109, 515–527.
- Lee, J.H.; Regmi, S.C.; Kim, J.A.; Cho, M.H.; Yun, H.; Lee, C.S.; Lee, J. Apple Flavonoid Phloretin Inhibits Escherichia coli O157:H7 Biofilm Formation and Ameliorates Colon Inflammation in Rats. Infect. Immun. 2011, 79, 4819–4827.
- Budzyńska, A.; Rózalski, M.; Karolczak, W.; Wieckowska-Szakiel, M.; Sadowska, B.; Rózalska, B. Synthetic 3-Arylidenefl Avanones as Inhibitors of the Initial Stages of biofilm formation by Staphylococcus aureus and Enterococcus faecalis. Z. Naturforschung. C J. Biosci. 2011, 66, 104–114.
- Echeverría, J.; Opazo, J.; Mendoza, L.; Urzúa, A.; Wilkens, M. Structure-Activity and Lipophilicity Relationships of Selected Antibacterial Natural Flavones and Flavanones of Chilean Flora. Molecules 2017, 22, 608.
- Madan, S.; Singh, G.N.; Kohli, K.; Ali, M.; Kumar, Y.; Singh, R.M.; Prakash, O. Isoflavonoids from Flemingia strobilifera (L.) R. Br. Roots. Acta Pol. Pharm.-Drug Res. 2009, 66, 297–303.
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Curr. Med. Chem. 2014, 22, 132–149.
- Navrátilová, A.; Schneiderová, K.; Veselá, D.; Hanáková, Z.; Fontana, A.; Dall’acqua, S.; Cvačka, J.; Innocenti, G.; Novotná, J.; Urbanová, M.; et al. Minor C-Geranylated Flavanones from Paulownia tomentosa Fruits with MRSA Antibacterial Activity. Phytochemistry 2013, 89, 104–113.
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial Activity of Flavonoids and Their Structure–Activity Relationship: An Update Review. Phyther. Res. 2019, 33, 13–40.
- Alcaráz, L.E.; Blanco, S.E.; Puig, O.N.; Tomás, F.; Ferretti, F.H. Antibacterial Activity of Flavonoids against Methicillin-Resistant Staphylococcus aureus Strains. J. Theor. Biol. 2000, 205, 231–240.
- Tran, T.D.; Nguyen, T.T.N.; Do, T.H.; Huynh, T.N.P.; Tran, C.D.; Thai, K.M. Synthesis and Antibacterial Activity of Some Heterocyclic Chalcone Analogues Alone and in Combination with Antibiotics. Molecules 2012, 17, 6684–6696.
- Manner, S.; Skogman, M.; Goeres, D.; Vuorela, P.; Fallarero, A. Systematic Exploration of Natural and Synthetic Flavonoids for the Inhibition of Staphylococcus aureus Biofilms. Int. J. Mol. Sci. 2013, 14, 19434–19451.
- Oh, I.; Yang, W.Y.; Chung, S.C.; Kim, T.Y.; Oh, K.B.; Shin, J. In Vitro Sortase A Inhibitory and Antimicrobial Activity of Flavonoids Isolated from the Roots of Sophora flavescens. Arch. Pharm. Res. 2011, 34, 217–222.
- Tsuchiya, H.; Sato, M.; Miyazaki, T.; Fujiwara, S.; Tanigaki, S.; Ohyama, M.; Tanaka, T.; Iinuma, M. Comparative Study on the Antibacterial Activity of Phytochemical Flavanones against Methicillin-Resistant Staphylococcus aureus. J. Ethnopharmacol. 1996, 50, 27–34.
- Idowu, T.O.; Ogundaini, A.O.; Salau, A.O.; Obuotor, E.M.; Bezabih, M.; Abegaz, B.M. Doubly Linked, A-Type Proanthocyanidin Trimer and Other Constituents of Ixora coccinea Leaves and Their Antioxidant and Antibacterial Properties. Phytochemistry 2010, 71, 2092–2098.
- Mayer, R.; Stecher, G.; Wuerzner, R.; Silva, R.C.; Sultana, T.; Trojer, L.; Feuerstein, I.; Krieg, C.; Abel, G.; Popp, M.; et al. Proanthocyanidins: Target Compounds as Antibacterial Agents. J. Agric. Food Chem. 2008, 56, 6959–6966.
