Colorectal cancer (CRC) is a global health problem responsible for 10% of all cancer incidences and 9.4% of all cancer deaths worldwide. The number of new cases increases per annum, whereas the lack of effective therapies highlights the need for novel therapeutic approaches. Conventional treatment methods, such as surgery, chemotherapy and radiotherapy, are widely applied in oncology practice. Their therapeutic success is little, and therefore, the search for novel technologies is ongoing. Many efforts have focused recently on the development of safe and efficient cancer nanomedicines. Nanoparticles are among them. They are unique with their properties on a nanoscale and hold the potential to exploit intrinsic metabolic differences between cancer and healthy cells. This feature allows them to induce high levels of toxicity in cancer cells with little damage to the surrounding healthy tissues.
- colorectal cancer
- nanoparticles
- drug
- carbon-based NPs
- targeted therapy
- antitumor effects
- graphene oxide
1. Nanomaterials
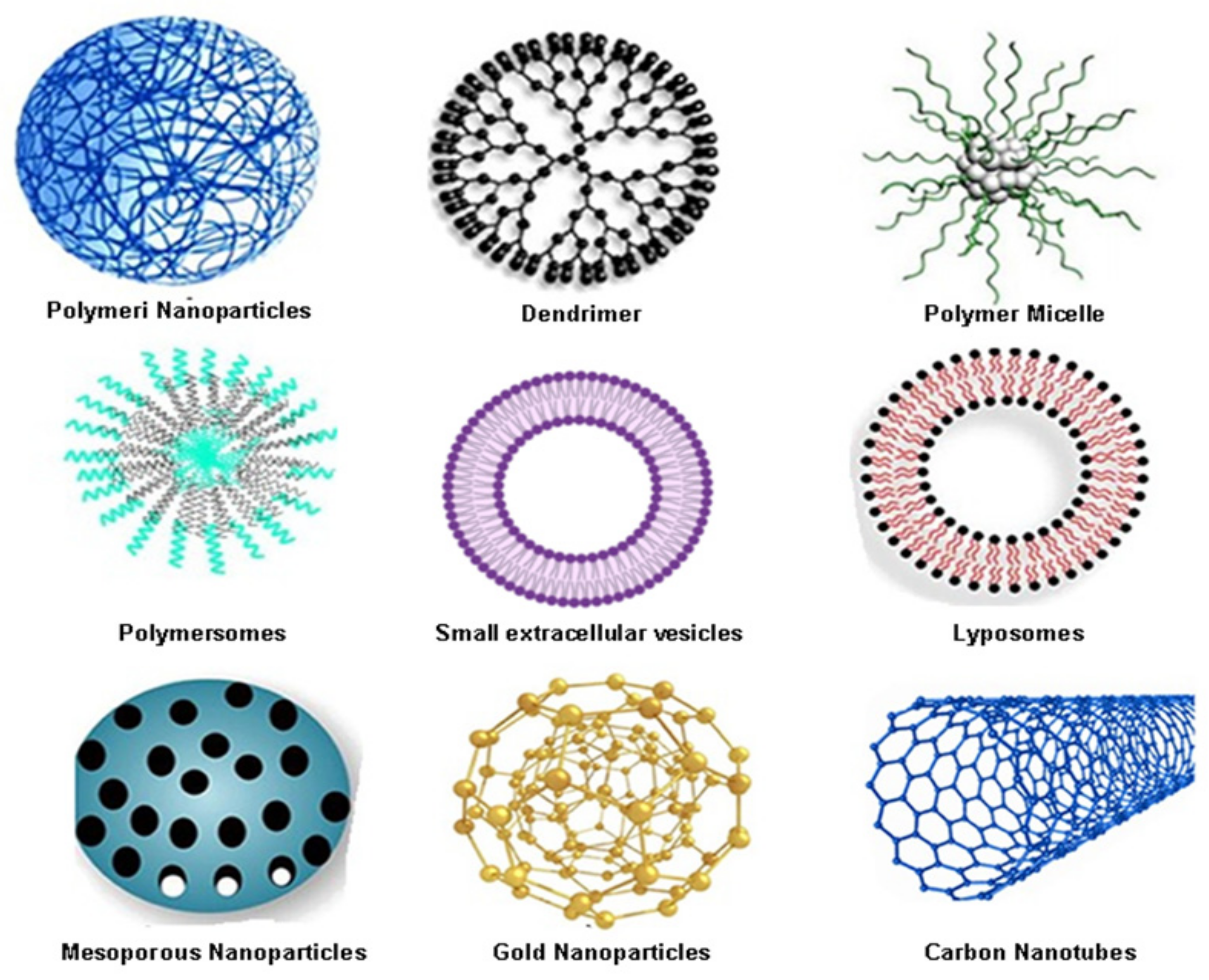
| Nanoparticles Type | Advantages | Disadvantages |
|---|---|---|
| Organic nanoparticles | ||
| Polymeric nanoparticles | Biocompatible and biodegradable; ability to entrap both hydrophilic and hydrophobic drugs; easy to modify; controlled drug release; protect the drug from metabolic degradation; prolonged residence time—bio-adhesive properties; good tissue penetration; easy manipulation; stability of drug; delivery of a higher concentration of drug to the desired location; easy merged into other activities associated to a drug delivery | Burst effect; limited drug loading capacity; high cost; low cell affinity; toxicity of degradation products; non-degradable polymers tend to accumulate in tissue; promote allergic reaction; in vivo metabolism and elimination is not elucidated; rapid clearance out of the abdominal cavity; toxic, reactive residues, unreacted monomers increase the risk of chemical reactions and the formation of unwanted oligomers |
| Dendrimers | Lower polydispersity index; the outer surface of dendrimer has multiple functional groups; they can be designed and synthesised for a specific application | High cost of synthesis process; non-specific toxicity; low loading capacity |
| Polymeric micelles | Prolonged retention time; easily synthesis; can be coupled with targeting ligands to increase accessibility to tumour sites, reduce the side effects; ability to control drug dissemination over a long period | Increased systemic toxicity |
| Polymersomes | Chemical versatility; an ability for controlled release and improved cellular uptake of anti-cancer molecules; low toxicity | Toxicity risk of polymers or metabolites; Polymer aggregation; hydration may be a challenge |
| Small extracellular vesicles | Biocompatible; safe degradation products; non-toxic; non-immunogenic; possibility for cell targeting | Low water solubility |
| Liposomes | Increase the efficacy and therapeutic index of drugs; biocompatible and completely biodegradable; low toxicity; flexible; improved pharmacokinetics of cargo; able to entrap both hydrophilic and hydrophobic drugs; controlled release protects the drug from metabolic degradation prolonged residence time—precorneal and vitreous; decreased the exposure of sensitive tissue to toxic drugs | Poor stability; could crystallise after prolonged storage conditions; difficult to prepare and sterilise; high cost; poor or moderate drug loading capacity; immunogenicity; low solubility; short half-time; leakage and fusion of encapsulated drug/molecules |
| Inorganic nanoparticles | ||
| Mesoporous silica nanoparticles | High drug and genes loading capacity; tunable pore size; large surface area; biocompatible and biodegradable; controlled porosity; versatility; non-toxic; easy endocytosis, and resistance to heat and pH | Expensive; not enough information about cytotoxicity, biodistribution, biocompatibility’ low stability formation of aggregates, haemolysis |
| Metallic and magnetic nanomaterials | Easy preparation and functionalisation; large surface area; multimodal application; high surface area; multiple forms (spherical, nanorod, triangles); biocompatibility; tuneable size; easy functionalisation excellent biodegradability in vivo; no leakage of encapsulated drugs | Low stability and storage; not enough information about uptake, biocompatibility, and low cytotoxicity in vivo |
| Carbon-based nanomaterials | ||
| Carbon nanotubes | Water-soluble; multifunctional; less toxic; biocompatibility; biodegradability; able to entrap both hydrophilic and hydrophobic drugs; high loading capacity; a high number of possibilities for surface modification; high surface area, needle-like structure, heat conductivity, and chemical stability | Expensive to produce; low degradation; not enough in vivo studies |
2. Organic Nanocarriers for Colorectal Cancer Drug Delivery
2.1. Polymeric Nanoparticles (PNPs) as One of the Most Widely Used Organic Nanocarriers in Colorectal Cancer Therapy
2.2. Dendrimers as Other Promising Colorectal Cancer Drug Nano-Delivery Systems
2.3. Polymeric Micelles (PMs) as Favourable Organic Nanocarriers in Colon Cancer Therapy
2.4. Polymeric-Based Nanocarriers as an Emerging Opportunity for Successful Drugs and Nucleic Acids Delivery in Colorectal Cancer
2.5. Small Extracellular Vesicles (sEVs)—The Trojan Horse for Many Cancers
2.6. Liposomes as Colorectal Therapeutics
3. Colorectal Cancer Drug Delivery Based on Inorganic Nanocarriers
3.1. Mesoporous Silica Nanoparticles (MSNs) as Carriers of Large Amounts of Biomolecules
3.2. Metallic and Magnetic Nanomaterials as Photosensitizers in Colorectal Cancer Treatment
3.3. Carbon-Based Nanomaterials
References
- Brar, B.; Ranjan, K.; Palria, A.; Kumar, R.; Ghosh, M.; Sihag, S.; Minakshi, P. Nanotechnology in Colorectal Cancer for Precision Diagnosis and Therapy. Front. Nanotechnol. 2021, 3, 699266.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71.
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Ibrahim, M.N.M. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341.
- Gonçalves, G.; Vila, M.; Portolés, M.-T.; Vallet-Regí, M.; Grácio, J.; Marques, P.A.A.P. Nano-Graphene Oxide: A Potential Multifunctional Platform for Cancer Therapy. Adv. Health Mater. 2013, 2, 1072–1090.
- Barui, S.; Cauda, V. Multimodal Decorations of Mesoporous Silica Nanoparticles for Improved Cancer Therapy. Pharmaceutics 2020, 12, 527.
- Martinho, N.; Damgé, C.; Reis, C.P. Recent Advances in Drug Delivery Systems. J. Biomater. Nanobiotechnol. 2011, 2, 510–526.
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978.
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19.
- Gregoriadis, G.; Ryman, B.E. Liposomes as carriers of enzymes or drugs: A new approach to the treatment of storage diseases. Biochem. J. 1971, 124, 58P.
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387.
- Zielińska, A.; Carreiró, F.; Oliveira, A.; Neves, A.; Pires, B.; Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731.
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309.
- Wang, X.; Wang, Y.; Chen, Z.G.; Shin, D.M. Advances of Cancer Therapy by Nanotechnology. Cancer Res. Treat. 2009, 41, 1.
- Mishra, B.; Patel, B.B.; Tiwari, S. Colloidal nanocarriers: A review on formulation technology, types and applications toward targeted drug delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 9–24.
- Sah, H.; Thoma, L.A.; Desu, H.R.; Sah, E.; Wood, G.C. Concepts and practices used to develop functional PLGA-based nanoparticulate systems. Int. J. Nanomed. 2013, 8, 747–765.
- Akl, M.A.; Kartal-Hodzic, A.; Oksanen, T.; Ismael, H.R.; Afouna, M.M.; Yliperttula, M.; Samy, A.M.; Viitala, T. Factorial design formulation optimization and in vitro characterization of curcumin-loaded PLGA nanoparticles for colon delivery. J. Drug Deliv. Sci. Technol. 2016, 32, 10–20.
- Tummala, S.; Satish Kumar, M.N.; Prakash, A. Formulation and characterization of 5-Fluorouracil enteric coated nanoparticles for sustained and localized release in treating colorectal cancer. Saudi Pharm. J. 2015, 23, 308–314.
- Essa, S.; Daoud, J.; Lafleur, M.; Martel, S.; Tabrizian, M. SN-38 active loading in poly(lactic-co-glycolic acid) nanoparticles and assessment of their anticancer properties on COLO-205 human colon adenocarcinoma cells. J. Microencapsul. 2015, 32, 784–793.
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247.
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85.
- Huang, W.; Wang, X.; Shi, C.; Guo, D.; Xu, G.; Wang, L.; Bodman, A.; Luo, J. Fine-Tuning Vitamin E-Containing Telodendrimers for Efficient Delivery of Gambogic Acid in Colon Cancer Treatment. Mol. Pharm. 2015, 12, 1216–1229.
- Wu, L.; Ficker, M.; Christensen, J.B.; Trohopoulos, P.N.; Moghimi, S.M. Dendrimers in Medicine: Therapeutic Concepts and Pharmaceutical Challenges. Bioconjugate Chem. 2015, 26, 1198–1211.
- Lai, P.-S.; Lou, P.-J.; Peng, C.-L.; Pai, C.-L.; Yen, W.-N.; Huang, M.-Y.; Young, T.-H.; Shieh, M.-J. Doxorubicin delivery by polyamidoamine dendrimer conjugation and photochemical internalization for cancer therapy. J. Control. Release 2007, 122, 39–46.
- Malik, N.; Evagorou, E.G.; Duncan, R. Dendrimer-platinate: A novel approach to cancer chemotherapy. Anti-Cancer Drugs 1999, 10, 767–776.
- Zhuo, R.X.; Du, B.; Lu, Z.R. In vitro release of 5-fluorouracil with cyclic core dendritic polymer. J. Control. Release 1999, 57, 249–257.
- Lee, C.C.; Gillies, E.R.; Fox, M.E.; Guillaudeu, S.J.; Fréchet, J.M.J.; Dy, E.E.; Szoka, F.C. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc. Natl. Acad. Sci. USA 2006, 103, 16649–16654.
- Tunki, L.; Kulhari, H.; Sistla, R.; Pooja, D. 5-Dendrimer-Based Targeted Drug Delivery. In Pharmaceutical Applications of Dendrimers; Chauhan, A., Kulhari, H., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 107–129. ISBN 978-0-12-814527-2.
- Marzbali, M.Y.; Khosroushahi, A.Y. Polymeric micelles as mighty nanocarriers for cancer gene therapy: A review. Cancer Chemother. Pharmacol. 2017, 9, 637–649.
- Bhadra, D.; Bhadra, S.; Jain, S.; Jain, N. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int. J. Pharm. 2003, 257, 111–124.
- Amin, M.C.I.M.; Butt, A.M.; Amjad, M.W.; Kesharwani, P. Polymeric micelles for drug targeting and delivery. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Academic Press: Cambridge, MA, USA; pp. 167–202.
- Ameli, H.; Alizadeh, N. Targeted delivery of capecitabine to colon cancer cells using nano polymeric micelles based on beta cyclodextrin. RSC Adv. 2022, 12, 4681–4691.
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921–2942.
- Sharma, A.K.; Prasher, P.; Aljabali, A.A.; Mishra, V.; Gandhi, H.; Kumar, S.; Mutalik, S.; Chellappan, D.K.; Tambuwala, M.; Dua, K.; et al. Emerging era of “somes”: Polymersomes as versatile drug delivery carrier for cancer diagnostics and therapy. Drug Deliv. Transl. Res. 2020, 10, 1171–1190.
- Yin, H.; Kang, S.-W.; Bae, Y.H. Polymersome Formation from AB2 Type 3-Miktoarm Star Copolymers. Macromolecules 2009, 42, 7456–7464.
- Hu, Y.; Qiu, L. Polymersomes: Preparation and Characterization. Pharm. Nanotechnol. 2019, 2000, 247–265.
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640.
- Alibolandi, M.; Shahriari, M.; Ramezani, M. Smart Polymersomes as Intelligent Nanomedicines in Cancer Treatment. In Polymeric Nanoparticles as a Promising Tool for Anti-Cancer Therapeutics; Academic Press: Amsterdam, The Netherlands; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 343–371.
- Pegoraro, C.; Cecchin, D.; Gracia, L.S.; Warren, N.; Madsen, J.; Armes, S.P.; Lewis, A.; MacNeil, S.; Battaglia, G. Enhanced drug delivery to melanoma cells using PMPC-PDPA polymersomes. Cancer Lett. 2013, 334, 328–337.
- Shahriari, M.; Taghdisi, S.M.; Abnous, K.; Ramezani, M.; Alibolandi, M. Synthesis of hyaluronic acid-based polymersomes for doxorubicin delivery to metastatic breast cancer. Int. J. Pharm. 2019, 572, 118835.
- Qin, H.; Jiang, Y.; Zhang, J.; Deng, C.; Zhong, Z. Oncoprotein Inhibitor Rigosertib Loaded in ApoE-Targeted Smart Polymersomes Reveals High Safety and Potency against Human Glioblastoma in Mice. Mol. Pharm. 2019, 16, 3711–3719.
- Ge, X.; Zhang, Q.; Cai, Y.; Duan, S.; Chen, S.; Lv, N.; Jin, T.; Chen, Y.; Yuan, W. PEG–PCL–DEX polymersome–protamine vector as an efficient gene delivery system via PEG-guided self-assembly. Nanomedicine 2014, 9, 1193–1207.
- Kim, H.-O.; Lim, J.-W.; Choi, J.; Lee, H.; Son, H.Y.; Kim, J.; Park, G.; Chun, H.; Song, D.; Huh, Y.-M.; et al. Anchored protease-activatable polymersomes for molecular diagnostics of metastatic cancer cells. J. Mater. Chem. B 2017, 5, 9571–9578.
- Petersen, M.A.; Hillmyer, M.A.; Kokkoli, E. Bioresorbable Polymersomes for Targeted Delivery of Cisplatin. Bioconjugate Chem. 2013, 24, 533–543.
- Discher, D.E.; Ahmed, F. Annual Review of Biomedical Engineering. Polymerosomes 2006, 8, 323–341.
- He, X.; Zhong, X.; Hu, Z.; Zhao, S.; Wei, P.; Li, D. An insight into small extracellular vesicles: Their roles in colorectal cancer progression and potential clinical applications. Clin. Transl. Med. 2020, 10, e249.
- Zhao, X.; Wu, D.; Ma, X.; Wang, J.; Hou, W.; Zhang, W. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. Biomed. Pharmacother. 2020, 128, 110237.
- Hartman, Z.C.; Wei, J.; Glass, O.K.; Guo, H.; Lei, G.; Yang, X.-Y.; Osada, T.; Hobeika, A.; Delcayre, A.; Le Pecq, J.-B.; et al. Increasing vaccine potency through exosome antigen targeting. Vaccine 2011, 29, 9361–9367.
- André, F.; Chaput, N.; Schartz, N.E.C.; Flament, C.; Aubert, N.; Bernard, J.; Lemonnier, F.; Raposo, G.; Escudier, B.; Hsu, D.-H.; et al. Exosomes as Potent Cell-Free Peptide-Based Vaccine. I. Dendritic Cell-Derived Exosomes Transfer Functional MHC Class I/Peptide Complexes to Dendritic Cells. J. Immunol. 2004, 172, 2126–2136.
- Romagnoli, G.G.; Zelante, B.B.; Toniolo, P.A.; Migliori, I.K.; Barbuto, J.A.M. Dendritic Cell-Derived Exosomes may be a Tool for Cancer Immunotherapy by Converting Tumor Cells into Immunogenic Targets. Front. Immunol. 2014, 5, 692.
- Kooijmans, S.A.A.; Aleza, C.G.; Roffler, S.R.; Van Solinge, W.; Vader, P.; Schiffelers, R.M. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles 2016, 5, 31053.
- Suntres, Z.E. Liposomal Antioxidants for Protection against Oxidant-Induced Damage. J. Toxicol. 2011, 2011, 152474.
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252, IN26–IN27.
- Abreu, A.S.; Castanheira, E.M.; Queiroz, M.-J.R.; Ferreira, P.M.; Vale-Silva, L.A.; Pinto, E. Nanoliposomes for encapsulation and delivery of the potential antitumoral methyl 6-methoxy-3-(4-methoxyphenyl)-1H-indole-2-carboxylate. Nanoscale Res. Lett. 2011, 6, 482.
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release Off. J. Control Release Soc. 2012, 160, 117–134.
- Lammers, T.; Hennink, W.E.; Storm, G. Tumour-targeted nanomedicines: Principles and practice. Br. J. Cancer 2008, 99, 392–397.
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48.
- Li, W.; Chen, H.; Yu, M.; Fang, J. Targeted Delivery of Doxorubicin Using a Colorectal Cancer-Specific ssDNA Aptamer. Anat. Rec. 2014, 297, 2280–2288.
- Sang, R.; Stratton, B.; Engel, A.; Deng, W. Liposome technologies towards colorectal cancer therapeutics. Acta Biomater. 2021, 127, 24–40.
- Clinical Trials Database: NCT00361842. Available online: https://clinicaltrials.gov/ct2/show/NCT00361842 (accessed on 2 July 2021).
- Lei, S.; Chien, P.-Y.; Sheikh, S.; Zhang, A.; Ali, S.; Ahmad, I. Enhanced therapeutic efficacy of a novel liposome-based formulation of SN-38 against human tumor models in SCID mice. Anti-Cancer Drugs 2004, 15, 773–778.
- ClinicalTrials.gov, Identifier: NCT00311610. Available online: https://clinicaltrials.gov/ct2/show/NCT00311610 (accessed on 29 June 2016).
- Chen, K.-J.; Chaung, E.-Y.; Wey, S.-P.; Lin, K.-J.; Cheng, F.; Lin, C.-C.; Liu, H.-L.; Tseng, H.-W.; Liu, C.-P.; Wei, M.-C.; et al. Hyperthermia-Mediated Local Drug Delivery by a Bubble-Generating Liposomal System for Tumor-Specific Chemotherapy. ACS Nano 2014, 8, 5105–5115.
- Celsion. Phase 2 Study of Thermodox as Adjuvant Therapy with Thermal Ablation (RFA) in Treatment of Metastatic Colorectal Cancer(mCRC) (ABLATE). 2011. Available online: https://clinicaltrials.gov/ct2/show/NCT01464593 (accessed on 29 June 2016).
- Yang, C.; Liu, H.Z.; Fu, Z.X.; Lu, W.D. Oxaliplatin long-circulating liposomes improved therapeutic index of colorectal carcinoma. BMC Biotechnol. 2011, 11, 21.
- Li, L.; Ahmed, B.; Mehta, K.; Kurzrock, R. Liposomal curcumin with and without oxaliplatin: Effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol. Cancer Ther. 2007, 6, 1276–1282.
- Cay, O.; Kruskal, J.B.; Nasser, I.; Thomas, P.; Clouse, M.E. Liver metastases from colorectal cancer: Drug delivery with liposome-encapsulated doxorubicin. Radiology 1997, 205, 95–101.
- Stang, J.; Haynes, M.; Carson, P.; Moghaddam, M. A Preclinical System Prototype for Focused Microwave Thermal Therapy of the Breast. IEEE Trans. Biomed. Eng. 2012, 59, 2431–2438.
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133.
- Radhakrishnan, K.; Gupta, S.; Gnanadhas, D.P.; Ramamurthy, P.C.; Chakravortty, D.; Raichur, A.M. Protamine-Capped Mesoporous Silica Nanoparticles for Biologically Triggered Drug Release. Part. Part. Syst. Charact. 2013, 31, 449–458.
- Yu, M.; Jambhrunkar, S.; Thorn, P.; Chen, J.; Gu, W.; Yu, C. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanoscale 2013, 5, 178–183.
- Gidding, C.E.; Kellie, S.J.; Kamps, W.A.; de Graaf, S.S. Vincristine Revisited. Crit. Rev. Oncol. Hematol. 1999, 29, 267–287.
- Hanafi-Bojd, M.Y.; Jaafari, M.R.; Ramezanian, N.; Xue, M.; Amin, M.; Shahtahmassebi, N.; Malaekeh-Nikouei, B. Surface functionalized mesoporous silica nanoparticles as an effective carrier for epirubicin delivery to cancer cells. Eur. J. Pharm. Biopharm. 2015, 89, 248–258.
- Bretin, L.; Pinon, A.; Bouramtane, S.; Ouk, C.; Richard, L.; Perrin, M.-L.; Chaunavel, A.; Carrion, C.; Bregier, F.; Sol, V.; et al. Photodynamic Therapy Activity of New Porphyrin-Xylan-Coated Silica Nanoparticles in Human Colorectal Cancer. Cancers 2019, 11, 1474.
- Advances in Colorectal Cancer Research. Nanotechnology to Improve Early Detection and Treatment of Colorectal Cancer. 2016. Available online: https://www.nih.gov/research-training/nanotechnology-improve-doneearly-detection-treatment-colorectal-cancer (accessed on 15 March 2016).
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446.
- Kuo, C.-Y.; Liu, T.-Y.; Chan, T.-Y.; Tsai, S.-C.; Hardiansyah, A.; Huang, L.-Y.; Yang, M.-C.; Lu, R.-H.; Jiang, J.-K.; Yang, C.-Y.; et al. Magnetically triggered nanovehicles for controlled drug release as a colorectal cancer therapy. Colloids Surf. B Biointerfaces 2016, 140, 567–573.
- Esmaelbeygi, E.; Khoei, S.; Khoee, S.; Eynali, S. Role of iron oxide core of polymeric nanoparticles in the thermosensitivity of colon cancer cell line HT-29. Int. J. Hyperth. 2015, 31, 489–497.
- Feng, S.-T.; Li, J.; Luo, Y.; Yin, T.; Cai, H.; Wang, Y.; Dong, Z.; Shuai, X.; Li, Z.-P. pH-Sensitive Nanomicelles for Controlled and Efficient Drug Delivery to Human Colorectal Carcinoma LoVo Cells. PLoS ONE 2014, 9, e100732.
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469.
- Rastogi, V.; Yadav, P.; Bhattacharya, S.S.; Mishra, A.K.; Verma, N.; Verma, A.; Pandit, J.K. Carbon Nanotubes: An Emerging Drug Carrier for Targeting Cancer Cells. J. Drug Deliv. 2014, 2014, 670815.
- Hampel, S.; Kunze, D.; Haase, D.; Krämer, K.; Rauschenbach, M.; Ritschel, M.; Leonhardt, A.; Thomas, J.; Oswald, S.; Hoffmann, V.; et al. Carbon nanotubes filled with a chemotherapeutic agent: A nanocarrier mediates inhibition of tumor cell growth. Nanomedicine 2008, 3, 175–182.
- Lee, Y.; Geckeler, K.E. Cellular Interactions of a Water-Soluble Supramolecular Polymer Complex of Carbon Nanotubes with Human Epithelial Colorectal Adenocarcinoma Cells. Macromol. Biosci. 2012, 12, 1060–1067.
- Zhou, M.; Peng, Z.; Liao, S.; Li, P.; Li, S. Design of microencapsulated carbon nanotube-based microspheres and its application in colon targeted drug delivery. Drug Deliv. 2014, 21, 101–109.
- Levi-Polyachenko, N.H.; Merkel, E.J.; Jones, B.T.; Carroll, D.L.; Stewart, I.J.H. Rapid Photothermal Intracellular Drug Delivery Using Multiwalled Carbon Nanotubes. Mol. Pharm. 2009, 6, 1092–1099.
- Zakaria, A.B.; Picaud, F.; Rattier, T.; Pudlo, M.; Dufour, F.; Saviot, L.; Chassagnon, R.; Lherminier, J.; Gharbi, T.; Micheau, O.; et al. Nanovectorization of TRAIL with Single Wall Carbon Nanotubes Enhances Tumor Cell Killing. Nano Lett. 2015, 15, 891–895.
